Abstract
Low-level laser therapy (LLLT) is known for its ability to induce a photochemical process, primarily targeting mitochondria, a process referred to as photobiomodulation (PBM). Recently, its use has been attributed as an adjunct in obesity treatment, to stimulate lipolysis and apoptosis. However, the pathway of stimulation remains uncertain. Thus, the objective of this study was to understand whether mitochondrial stimulation occurs in adipose tissue cells after PBM therapy, which could lead to the processes of lipolysis and apoptosis. A non-randomized clinical trial was conducted using a split abdomen design in obese women who received red and infrared LED photobiomodulation therapy (PBMT). The patients underwent bariatric surgery, and adipose tissue samples were collected for immunohistochemical analysis with primary mitochondrial antibodies. Adipose tissue samples subjected to LED intervention exhibited positivity in mitochondrial antibodies for cAMP, DRP1, FAS, FIS1, MFN2, and OPA1 (p<0.001) compared to the control group. In conclusion, we observed that PBMT was capable of generating mitochondrial stimulation in adipose tissue cells, as evidenced by the positive antibody signals. This finding suggests that mitochondrial stimulation could be the mechanism and action underlying adipose tissue lipolysis and apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-level laser therapy (LLLT) is known for its ability to induce a cellular-level photochemical process called photobiomodulation (PBM). For a significant period, it was believed that this PBM effect was achievable only through sources of low-intensity Laser Emission Stimulated Radiation (LASER), due to the unique properties of lasers such as coherence and monochromaticity [1,2,3].
However, with technological advancements, discoveries, and the high efficiency of light-emitting diodes (LEDs) pioneered by Dr. Nick Holonyak Jr. and his collaborators, it has become evident in recent years that non-coherent LEDs perform just as well as lasers, with the additional advantage of lower cost [2,3,4].
This advancement has also led to new indications for light therapy, supported by strong scientific evidence of its effects on tissue repair and regeneration. This has culminated in the recent hypothesis of correlating its use as a potential adjunct in obesity treatment. The purpose is to physiologically stimulate lipolysis, followed by apoptosis—cellular death in adipocytes. This approach holds the potential to reduce risk factors associated with obesity-related comorbidities and aid in the weight loss process [4, 5].
Our previous research has demonstrated the potential effects of LED-based photobiomodulation therapy (PBMT) on adipose tissue through histological and immunohistochemical analyses. It was revealed that LED-based PBM could stimulate both apoptosis and lipolysis in adipose tissue, as evidenced by increased expression of Cleaved Caspase 3 and Caspase 3—markers present in the apoptosis process—as well as CD68, HSL, and adipophilin, markers present in the lipolysis process [6,7,8].
Such evidence has strengthened the hypothesis of utilizing PBMT as an adjunct treatment for obesity. However, the results have unveiled a pathway that remains relatively unexplored. Despite the positive effects on adipose tissue lipolysis and apoptosis, the activation pathway for these stimuli remains unanswered. It is understood that PBMT triggers a photochemical process, primarily targeting mitochondria, stimulating the production of adenosine triphosphate (ATP), which can boost cellular metabolism and produce physiological effects [4, 5, 9].
Hence, the objective of this present study was to comprehend whether, after PBMT using light-emitting diodes, there is mitochondrial stimulation in adipose tissue cells that may lead to the processes of lipolysis and apoptosis, as indicated by histological and immunohistochemical analyses from previous studies [8].
Methods
Ethical considerations
A non-randomized split abdomen clinical trial was conducted by the 1964 Helsinki Declaration and was approved by the institutional ethics committee of the State University of Campinas (UNICAMP) under protocol number 3,286,896 and registration ClinicalTrials ID NCT05012514.
Study population
Convenience sampling included women from the preoperative preparation group for bariatric surgery at the Hospital de Clínicas da UNICAMP. Participants had Grade II or III obesity with indications for bariatric surgery according to National Institutes of Health (NIH) criteria, and a body mass index (BMI) ≥ 40 kg/m² or BMI ≥35 kg/m² combined with comorbidities. Due to the nature of the study, which involved laser therapy and the understanding that skin characteristics can significantly affect light absorption, a thorough examination was conducted to identify tattoos, scars, or lesions in the treatment area that could interfere with skin light absorption. If these conditions were present, the patient was excluded from the research [10, 11].
Exclusion criteria were as follows: individuals with skin lesions, a history of deep vein thrombosis, metabolic disorders, smoking habits, electronic implant devices such as cardiac pacemakers, or those who had lost less than 10% of their initial weight. All participants simultaneously belonged to both groups, with the left half-abdomen being the intervention group receiving photobiomodulation therapy (PBMT) using LED in red and infrared wavelengths, while the right half-abdomen served as the control group without any treatment. The abdominal split was based on the linea alba.
Photobiomodulation parameters
The therapy was performed using the Antares® (IBRAMED – Amparo - Brazil) in cluster mode, following the parameters described in Table 1. Seven treatment sessions were administered, twice a week, totaling six sessions before the seventh session conducted just before the bariatric surgery. This schedule was carefully planned according to the logistics of participant care, also taking into consideration the time required for surgical scheduling.
Sample collection
At the beginning of the bariatric surgery procedure, with participants under general venous anesthesia and mechanical ventilation, a surgical doctor collected two fragments of adipose tissue and skin with an average diameter of 5 cm from each participant. One sample was taken from the intervention site on the left side, and the other, the control, was taken from the right side.
Immunohistochemical procedure
The blocks are cut using a rotating microtome in sections of 3 μm thickness, the size of which is used in histological studies. The immunohistochemical reactions were carried out following the manufacturer’s protocol and standardized by the anatomopathological analysis laboratory of the Hospital de Clínicas da UNICAMP on marked sheets. The specifications of the primary mitochondrial antibodies used are described in Table 2.
After the immunohistochemical procedure, the tissues were analyzed using a DMR® microscope (Leica Microsystems, Wetzlar, Germany) and photographed at a magnification of 400×, following established protocols [8]. The quantity of positive cells for the utilized markers was analyzed using ImageJ® software (NIH, Bethesda, USA). Based on a pixel scale considering the number of positive cells and the intensity of staining, scores were created to determine antibody immunoreactivity. The final scores were obtained through a specific formula, ranging from 0 to 300 points.
Statistical analysis
The data were tabulated and subjected to tests of normality, showing a normal distribution. The two-tailed Student’s t-test was applied to identify differences between the control and treated groups, with values of p<0.05 considered statistically significant; for this purpose, the IBM-SPSS Statistics 20.0 software was used.
Results
Initially, ten volunteers were involved in the study, but two were excluded for not adhering to the internal protocol of the preoperative program for bariatric surgery at the Hospital de Clínicas da UNICAMP. Consequently, only eight participants completed the treatment and underwent the surgical procedure.
The participants had a mean age of 35.0±8.6 years, initial weight (kg) of 110±5.2, final weight of 95.2±6.3, height (cm²) of 1.63±0.05, initial body mass index (BMI) of 41.4±2.2 kg/cm², and final BMI of 35.8±2.1 kg/cm², classified as Grade II and III obesity. None of the participants had associated comorbidities such as diabetes and hypertension.
Immunohistochemical analysis
Samples were marked with antibodies related to cellular metabolism signaling pathways (cAMP), mitochondrial division (DRP1), cellular apoptosis (FIS1, FAS, and OPA), and cellular proliferation regulation (Anti-MFN1 and Anti-MFN2). The blocks are cut with a rotating microtome in 3-μm-thick sections, the size of which is used in histological studies. Analyses were carried out in 3-μm blocks by optical microscopy with a binocular microscope (Nikon YS 100, Japan), adapted with a WSCF 10X/18 eyepiece and Nikon 4X/0.10 objective lenses (Table 2).
Control group samples showed that adipose tissue maintained normal morphology, with consistent size and regular distribution of adipocytes. Conversely, adipose tissue samples that underwent LED intervention appeared disorganized and had higher scores of staining for cellular metabolism activation, indicated by cAMP positivity, showing a significant difference between groups (p<0.05). This result demonstrates a likely alteration in the cellular metabolism of adipocytes in the tissue (Fig. 1).
Protein DRP1 is essential for proper cellular distribution of mitochondria and was prominently expressed in the LED group, indicating increased mitochondrial division compared to the control group with p < 0.05 (Fig. 2).
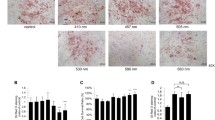
Samples subjected to LED PBMT intervention exhibit higher staining scores for FAS (p < 0.05), FIS1 (p < 0.05), and OPA1 (p < 0.001) compared to the control group. These results indicate a probable process of apoptosis and degeneration of adipocytes in adipose tissue (Fig. 3)
Qualitative and quantitative analysis of immunoreactivity for (A) FAS in the control group and (B) FAS in the LED group, demonstrating positivity for cellular apoptosis. (C) Comparative graph of FAS positivity between groups. (D) FIS1 in the control group and (E) FIS1 in the LED group, showing positivity for cellular apoptosis. (F) Comparative graph of FIS1 positivity between groups. (G) OPA1 in the control group and (H) OPA1 in the LED group, demonstrating positivity for cellular apoptosis. (I) Comparative graph of OPA1 positivity between groups. Scale bar length (3 μm)
MFN1 did not show significant differences, and MFN2 exhibited a higher immunopositivity score in the intervention sample compared to the control (p < 0.05), indicating cell proliferation regulation (Fig. 4).
Qualitative and quantitative analysis of immunoreactivity for (A) MFN1 in the control group and (B) MFN1 in the LED group. (C) Comparative graph of MFN1 between groups did not show significant differences. (D) MFN2 in the control group and (E) MFN2 in the LED group, demonstrating diffuse positivity in cell proliferation regulation. (F) Comparative graph of MFN2 between groups. Scale bar length (3 μm)
Discussion
PBMT has become a common therapeutic modality in cellular photochemical therapy. Although the use of light for various treatments emerged with the studies of Ender Mester [12], research on its mitochondrial action in adipose tissue cells that could lead to the processes of lipolysis and apoptosis is still limited. Therefore, this study investigated possible mechanisms of action of LED PBMT using red (630 nm) and infrared (850 nm) wavelengths through histological and immunohistochemical analyses.
Mitochondria are responsible for cellular respiration and metabolism, playing important roles in oxidative stress regulation, calcium metabolism, apoptosis, and signaling pathways [13]. Chromophores within mitochondria, especially cytochrome C oxidase (CCO), absorb photon energy and participate in light-tissue interaction through PBM effects [14]. Adenosine triphosphate (ATP) production and cellular oxygen consumption increase, which can lead to changes in nitric oxide (NO) levels, activation of secondary messenger pathways, activation of transcription factors, and production of growth factors [15].
Cell death can occur due to various factors, such as depletion of ATP reserves within the cell from high doses of light, excessive production of reactive oxygen species (ROS), excessive release of free NO, and activation of a cytotoxic mitochondrial signaling pathway. Mitochondrial dynamics are a crucial point for regulating cellular lipolysis and apoptosis, and the imbalance between fission and fusion contributes to mitochondrial and cellular dysfunction [16].
Cyclic AMP (cAMP) is responsible for regulating mitochondrial metabolism and cell cycle progression. In the present study, the LED-irradiated group exhibited a higher amount of cAMP in adipose tissue, which could be attributed to the stress induced by light, with an attempt to normalize cellular metabolism [17].
Mitochondrial fission is mediated by the protein DRP1, which triggers the apoptotic pathway [18]. Mitochondrial oxidative stress regulates the expression of DRP1, disrupting the balance between mitochondrial fission and fusion, leading to mitochondrial dysfunction, breakdown, and cell death [16]. With the increased cAMP due to photon absorption–induced stress, there was an elevation of DRP1 in the mitochondria during cell death, leading to an increase in mitochondrial fission and a decrease in fusion [19]. Results from the current study support this hypothesis, as the LED-irradiated group showed a higher quantity of DRP1 compared to the control group.
Markers of cellular apoptosis, FIS1, FAS, and OPA1, obtained higher staining scores in the LED-irradiated groups compared to the control group. FIS1 plays a crucial role in activating DRP1 in mitochondria and promotes mitochondrial fission. According to its function, overexpression of FIS1 blocks fusion activity and causes extensive mitochondrial fragmentation. FAS is a cell surface receptor that transduces critical apoptotic signals for immune homeostasis and tolerance [20], characteristic of the tumor necrosis factor receptor (TNFR) family. During apoptosis, OPA1 is cleaved, and cytochrome c is released into the intermembrane space [21].
MFN1 and MFN2 primarily function as essential components of the fusion machinery. Cells lacking Myofusins are highly susceptible to apoptotic death, indicating the importance of maintaining membrane morphology [21, 22]. MFN2 overexpression regulates respiratory complexes [23, 24], suggesting that MFN2 may play a role in metabolic regulation. The significant increase of MFN2 in the current study indicates that LED is capable of enhancing the fusion process, leading to increased proliferation and consequently stabilizing cellular metabolism.
The results of this study demonstrate that LED photobiomodulation therapy (PBMT) was capable of generating mitochondrial stimulation in adipose tissue cells, as evidenced by the positive antibody signals for cAMP, DRP1, FIS1, FAS, OPA, and MFN2.
It is believed that after irradiation with red and infrared light wavelengths, the mitochondrial molecule cytochrome C oxidase became electronically excited, leading to increased levels of cAMP in adipose tissue. This, in turn, initiates a process of mitochondrial oxidative stress, disrupting the balance of mitochondrial fission and fusion, as indicated by the increased expression of DRP1. This leads to mitochondrial dysfunction, breakdown, and cell death, supported by elevated levels of apoptotic markers FIS1, FAS, and OPA1. This cascade of cellular events ultimately resulted in the positivity of MFN2, suggesting possible induction in the respiratory chain with the regulation of respiratory complexes.
The physiological associations found in these results emphasize that LED PBMT acts through the mitochondrial pathway, stimulating a cascade of cellular events capable of promoting lipolysis and apoptosis in superficial adipose tissue. This corroborates with our previous study by Modena et al. [8], which showed positive expression of apoptotic markers Caspase 3 and Cleaved Caspase in the group treated with red and infrared LED. This suggests that the therapy was able to induce autophagic lipolysis through the apoptosis of adipocyte cells in the subcutaneous tissue of obese individuals.
In our study, the correlation with apoptosis can be observed through the absorption of photons of light at high doses, resulting in increased cAMP, DRP1, FIS1, FAS, and OPA. Given that all mitochondrial molecules do not absorb energy equally, lipolysis could be mediated by the mitochondrial molecules of surrounding adipocytes that absorb lower energy. In other words, the cascade of physiological events had lower intensity but was capable of inducing the respiratory chain and consequent metabolic increase.
This is one of the few studies that evaluated the mechanism of action of PBMT on the mitochondrial pathway of adipose tissue in human individuals. However, it presents limitations concerning sample size and lack of randomization in distinct groups of individuals. Nevertheless, the research demonstrated relevant and innovative results regarding mitochondrial stimulation in adipose tissue lipolysis and apoptosis, thus fostering the future hypothesis of possible PBMT utilization as an adjunct in obesity treatment. To explore this further, new randomized clinical studies must be conducted.
Conclusion
LED photobiomodulation therapy with red and infrared light was able to generate mitochondrial stimulation in adipose tissue cells, as evidenced by the positive antibody signals for cAMP, DRP1, FIS1, FAS, OPA, and MFN2.
References
Mansano BSDM, da Rocha VP, Antonio EL, Peron DF, do Nascimento de Lima R, Tucci PJF, Serra AJ. (2021) Enhancing the therapeutic potential of mesenchymal stem cells with light-emitting diode: implications and molecular mechanisms. Oxidative Med Cell Longev 3:6663539
Huang YY, Sharma SK, Carroll J, Hamblin MR (2011) Biphasic dose response in low level light therapy - an update. Dose-Response 9(4):602–618
Huang A, Nguyen JK, Jagdeo J (2020) Light-emitting diode-based photodynamic therapy for photoaging, scars, and dyspigmentation: a systematic review. Dermatol Surg 46(11):1388–1394
Jagdeo J, Austin E, Mamalis A, Wong C, Ho D, Siegel DM (2018) Light-emitting diodes in dermatology: a systematic review of randomized controlled trials. Lasers Surg Med 50(6):613–628
Nassab R (2015) The evidence behind noninvasive body contouring devices. Aesthet Surg J 35(3):279–293
Campos RMS, Dâmaso AR, Masquio DCL et al (2018) The effects of exercise training associated with low-level laser therapy on biomarkers of adipose tissue transdifferentiation in obese women. Lasers Med Sci 33(6):1245–1254
Liu PF, Hu YC, Kang BH, Tseng YK, Wu PC, Liang CC, Hou YY, Fu TY, Liou HH, Hsieh IC, Ger LP, Shu CW (2017) Expression levels of cleaved caspase-3 and caspase-3 in tumorigenesis and prognosis of oral tongue squamous cell carcinoma. PLoS One 12(7):e0180620
Modena DAO, Soares CD, Martignago CCS, Almeida S, Cazzo E, Chaim EA (2022) Effects of LED photobiomodulation therapy on the subcutaneous fatty tissue of obese individuals - histological and immunohistochemical analysis. s 8:1–7
Xie D, Li YL, Wang GF, Jiang J, Sun LR (2021) Ultraviolet light-emitting diode irradiation induces reactive oxygen species production and mitochondrial membrane potential reduction in HL-60 cells. J Int Med Res 49(5):3000605211016623
Chaim EA, Pareja JC, Gestic MA et al (2017) Programa multidisciplinar pré-operatório de cirurgia bariátrica: uma proposta para o Sistema Único de Saúde. Arq Gastroenterol 54(1):70–74
National Institutes of Health (1992) Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference statement. Am J Clin Nutr 55(l):615S–619S
Mester E, Mester AE, Mester A (1985) The biomedical effect of laser application. Lasers Surg Med 5(1):31–39
Nicholls DG (2002) Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int J Biochem Cell Biol 34(11):1372–1381
Karu TI (2010) Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 62(8):607–610
de Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22(3):348–364
Wu S, Zhou F, Zhang Z, Xing D (2011a) Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission–fusion proteins. FEBS J 278(6):941–954
Gwinn DM, Shackelford DB, Egan DF et al (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30(2):214–226
Suen DF, Norris KL, Youle RJ (2008) Mitochondrial dynamics and apoptosis. Genes Dev 22(12):1577–1590
Chang CR, Blackstone C (2010) Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci 1201:34–39
Nomura J, Matsumoto K, Iguchi-Ariga SMM et al (2005) Positive regulation of Fas gene expression by MSSP and abrogation of Fas-mediated apoptosis induction in MSSP-deficient mice. Exp Cell Res 305(2):324–332
Olichon L, Baricault N, Gas E et al (2003) Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome-c release and apoptosis. J Biol Chem 278:7743–7746
John GB, Shang Y, Li L et al (2005) The mitochondrial inner membrane protein mitofilin controls cristae morphology. Mol Biol Cell 16:1543–1554
Bach D, Pich S, Soriano FX et al (2003) Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem 278:17190–17197
Pich S, Bach D, Briones P et al (2005) The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through the expression of OXPHOS system. Hum Mol Genet 14:1405–1415
Author information
Authors and Affiliations
Contributions
PhD. D. A. O. M., data collection and analysis, writing of the article. MSc. A. P. F., data collection and analysis, writing of the article. PhD. E. C. de O. G., contributions in the writing of the article. PhD. E. C., data collection, joint supervision of the research and contributions in the writing of the article. PhD. E. A. C., data collection, supervision of the research and contributions in the writing of the article.
Corresponding author
Ethics declarations
Ethical approval
The research was not submitted to an ethics committee, as it was a review study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Modena, D.A.O., Ferro, A.P., de Oliveira Guirro, E.C. et al. Photobiomodulation therapy with light-emitting diode in stimulating adipose tissue mitochondria. Lasers Med Sci 38, 238 (2023). https://doi.org/10.1007/s10103-023-03906-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-023-03906-y