Abstract
Microangiopathy should be noted in diabetes with subclinical vascular diseases. Little is known about whether various surrogate markers of systemic arterial trees exacerbate simultaneously in preclinical atherosclerosis. To clarify the association of skin microvascular reactivity with arterial stiffness is essential to elucidating early atherosclerotic changes. The post-occlusive reactive hyperemia of skin microcirculation was evaluated in 27 control and 65 type 2 diabetic subjects, including 31 microalbuminuria (MAU) and 34 normoalbuminuria (NAU) patients. The laser Doppler skin perfusion signals were transformed into three frequency intervals for the investigation of endothelial, neurogenic, and myogenic effects on basal and reactive flow motion changes. The analysis of spectral intensity and distribution provided insight into potential significance of microvascular regulation in subclinical atherosclerotic diseases. Systemic arterial stiffness was studied by the brachial ankle pulse wave velocity (baPWV). Following occlusive ischemia, the percent change of endothelial flow motion was lower in MAU than in NAU and control groups. The MAU group revealed a relative increase in myogenic activity and a decrease in endothelial activity in normalized spectra. The baPWV showed more significant associations with reactive endothelial change (r = − 0.48, P < 0.01) and normalized myogenic value (r = − 0.37, P < 0.05) than diabetes duration and HbA1c. By multivariate regression analysis, only endothelial vasomotor changes independently contributed to the decreased baPWV (OR 3.47, 95% CI 1.63–7.42, P < 0.05). Impaired microcirculatory control is associated with increased arterial stiffness in preclinical atherosclerosis. To identify the early manifestations is necessary for at-risk patients to prevent from further vascular damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is associated with widespread atherosclerosis and subsequent cardiovascular complications. Apart from glycemic and comorbidity control, it is crucial in pre-clinical evaluation before the at-risk patients develop further vascular damage [1]. The brachial ankle pulse wave velocity (baPWV) is well established as a marker for arterial stiffness in systemic atherosclerotic diseases. Previous studies have demonstrated a significant association between baPWV and vascular structure changes and known risk factors for cardiovascular diseases [2, 3]. Microvascular function is believed to be impaired in early stage of atherosclerosis. However, only a limited number of studies have investigated the mutual relationship among various surrogate markers in systemic arterial trees such as the baPWV and microvascular indicators. Little is known about whether the levels of these functional markers exacerbate simultaneously in subclinical atherosclerosis and whether the cardiovascular risk factors contribute to their deterioration.
Skin perfusion can be evaluated by laser Doppler technique and coupled with various reactivity tests to challenge hyperemia response [4, 5]. A near-infrared laser beam is delivered to the target skin area via an optical-fiber probe. The light will be scattered from moving erythrocytes within the upper horizontal vascular plexus of the skin. The reflected light will undergo a frequency shift. The cutaneous microcirculation can be characterized by the amplitude and distribution of the frequency spectrum. The spectral analysis of basal and stimulated flow motion changes can be a functional indicator of microvascular regulation. The study of spectral intensity and distribution may provide insight into relative contributions of endothelial, neural and myogenic activity on skin perfusion changes [6, 7]. Impaired skin flow motion should be related to systemic vascular dysfunction, accounting in part for the greater cardiovascular risk in diabetes [7]. It is essential to identifying the association between microvascular functional markers and arterial stiffness, in order to elucidate early atherosclerotic changes and prevent the at risk patients from further vascular injury.
This study investigated the relationship between microvascular components and baPWV in type 2 diabetes with preclinical atherosclerosis. The post-occlusive vasomotor changes in skin perfusion were studied in type 2 diabetes with and without microalbuminuria. The diabetes with elevated urinary albumin excretion reflects microvascular deterioration predisposing an individual to atherosclerotic changes [1, 8]. The association between conduit artery function and microcirculatory control was assessed using baPWV and laser Doppler flow motion changes. It was hypothesized that reactive vasomotor changes might be impaired in type 2 diabetes with early stage atherosclerosis and its severity might be related to systemic arterial stiffness.
Methods
Study population
Among the diabetic outpatients receiving recent annual survey in a veterans’ affair medical center, a total of 65 type 2 diabetic patients were included in this study. A control group of 27 non-diabetic volunteers was recruited from the community. All participants gave their written informed consent to this study, which had been approved by the ethics committee of the hospital. None of the subjects had a history of skin ulceration or abnormal ankle brachial pressure index (< 0.9 or > 1.4) in their feet. The patients receiving α-, β-adrenergic blocker, direct-acting vasodilator, or calcium antagonists were excluded from this study. However, the use of angiotensin converting enzyme inhibitor, angiotensin II receptor blocker, or diuretics was allowed in this study.
The diabetic patients were evaluated and categorized based on the ratio of urine albumin-to-creatinine (UACR). The subjects with UACR < 30 mg/ g Cr were included in the normoalbuminuria (NAU) group. The UACR in the microalbuminuria (MAU) group was between 30 and 300 mg/g Cr. The three groups were matched for age (within 5 years), gender composition, and body mass index (BMI) (within 3 kg/m2). The clinical characteristics of the studied groups are shown in Table 1.
baPWV
The participants were requested to assume supine position for at least 10 min in the air-conditioned room with a constant temperature of 25 ± 1 °C. The baPWV was measured using an automatic oscillometric device (VP-1000, Omron Healthcare Company, Kyoto, Japan) with pressure cuffs wrapped on the brachium and ankles. Repeated measurements were performed at 10-s intervals via automatic gain analysis. The left and right baPWV were calculated for the final mean values. Details of the method have been described previously [9].
Post-occlusive reactive hyperemia by laser Doppler flowmetry
Post-occlusive reactive hyperemia was assessed at the dorsum of right big toe using a laser Doppler flowmetry (MSP310XP; Oxford Optronix, Oxford, UK) with a sampling rate of 100 Hz. Occlusive ischemia was produced by a pneumatic cuff positioned at the right ankle and inflated up to 30 mm Hg above the systolic blood pressure. After 3 min of arterial occlusion, the pneumatic cuff was deflated. Skin perfusion was measured for 10 min under basal conditions, during 3 min of ischemia compression, and for 10 min following the pneumatic cuff release.
The laser Doppler flux signals were expressed as arbitrary units (AU) and transformed for subsequent analysis with validated custom-built computer programs [7]. Spectral amplitude was shown as a function of time and frequency after the wavelet transformations of skin perfusion signals (Fig. 1A). The three-dimensional time–frequency plot was then projected into two dimensions as averaged within a given period of time (Fig. 1B). The low-frequency interval was divided into three subintervals: 0.0095–0.021, 0.021–0.052, and 0.052–0.145 Hz corresponding to endothelial, neurogenic, and myogenic related activities [10]. The absolute spectral amplitudes of each frequency subinterval were averaged during 5 min before ischemia and during 5 min after the peak of post-ischemic reactive hyperemia. To obtain the relative contribution of a particular spectral subinterval within the full frequency range, the normalized or relative amplitude was defined as the ratio of the sum of spectral amplitudes within a given subinterval to the total spectral amplitudes of all sampled frequency contents based on the published method [11].
Statistical analysis
The SPSS Statistics 21.0 (IBM Corporation, USA) was used for data analysis. The spectral amplitudes of skin perfusion were not normally distributed, and all values were log transformed before analysis. One-way ANOVA with post hoc analysis by Tukey’s least significant difference test was performed to compare the spectral parameters among three groups. Pearson’s correlation coefficients were utilized to assess the degree of association between multiple variables and baPWV. The independent contributions of clinical and vasomotor factors to baPWV were explored by stepwise regression analysis. A double-sided P-value of less than 0.05 was considered statistically significant.
Results
The clinical characteristics are shown in Table 1. The patients with incipient nephropathy were characterized by longer duration of diabetes. Although the two diabetic groups had similar HbA1c and systemic blood pressure, a higher proportion of MAU patients used concomitant medications for comorbidity such as hypertension or dyslipidemia (P < 0.01). The baPWV values were not significantly different between MAU and NAU groups.
The average amplitudes of the three divided subintervals were obtained for the frequency range from 0.0095 to 0.145 Hz in Table 2. No significant difference was noted in the mean spectral amplitude in each frequency interval between groups under basal conditions. Following occlusive ischemia, there was a significant increase in absolute spectral amplitude within the three sub-intervals (P < 0.01). The post-ischemic change divided by basal flow motion was presented as the percentage changes in Fig. 2. The MAU patients showed a lower post-ischemic percent change than the other groups (183% vs. 212% and 225%, P < 0.05) within the 0.0095–0.021 Hz frequency range related to endothelial activity. The other subintervals did not differ statistically in vasomotor change after ischemia among the three groups.
For further comparison among three frequency subintervals, the spectral amplitude of a subinterval was normalized by the spectral amplitude of the full frequency range. The normalized spectral values of the three subintervals are shown for each group in Table 3. Under basal conditions, the mean normalized amplitudes of the three frequency intervals were not significantly different among three groups. Following ischemia, the MAU patients showed a lower normalized value of endothelial interval compared to the NAU subjects, together with a higher normalized value of myogenic interval (P < 0.05). However, the difference was not significant when looking at the absolute frequency spectrum.
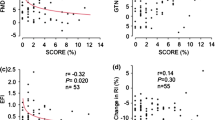
Among the aforementioned vasomotor components, only the post-ischemic endothelial percent change and normalized myogenic value revealed significant differences between groups. The two vasomotor variables were taken for further correlation and regression analysis. Correlation coefficients were obtained between vasomotor and clinical variables and baPWV. Among conventional risk factors, only the diabetes duration (r = 0.35, P < 0.05) and HbA1C (r = 0.29, P < 0.05) had a significant positive correlation with the baPWV. No significant correlation was noted between baPWV and BMI, systolic blood pressure, and comorbidity treatment. The baPWV was negatively correlated to the changes in reactive flow motion of the endothelial interval (r = − 0.48, P < 0.01) and normalized myogenic value (r = − 0.37, P < 0.05). The microvascular variables showed more significant associations with baPWV than conventional risk factors.
Stepwise multiple regression analysis revealed that when endothelial flow motion change, normalized myogenic value, diabetes duration, glycosylated hemoglobin value, and the uses of comorbidity medication were included as the determinants of baPWV, only reactive endothelial vasomotor changes independently contributed to the variance of baPWV [OR 3.47 (95% CI 1.63–7.42), P < 0.05].
Discussion
Previous studies have revealed that the diabetes per se accelerate atherosclerotic changes in the arterial wall. Some diabetic patients have changed extracellular matrix composition in capillary basement membranes [12]. Vessel function could be influenced by the structural changes in atherosclerotic diseases. The functional deterioration has been quantified by prior investigators but the results varied [13, 14]. Systemic microangiopathy can be studied in patients with concomitant cardiovascular diseases. Widespread atherosclerosis seemed to play a certain role in the pathogenesis of diabetic vascular diseases or as a consequence of these complications [15]. Numerous studies have found possible link between albuminuria and enhanced cardiovascular risks in diabetes [16, 17]. This association is independent of conventional risk factors. This study evaluated the diabetic patients with microalbuminuria as a model to assess microvascular function in early stage of atherosclerosis.
The skin offers an alternative approach to the microcirculatory changes in patients with diabetes. The altered skin perfusion response to ischemia is a complex phenomenon, which includes local NO-mediated endothelial activity, the influence of perivascular nerve, and smooth muscle responsiveness [18]. Previous studies revealed that reactive skin perfusions in response to various stimuli were preserved in chronic nephropathy patients [19, 20]. By measuring post-occlusive perfusion response, our study did not find significant differences between diabetes with and without incipient nephropathy. However, Schmiedel et al. indicated that diabetic microalbuminuria patients have impaired vasodilatory response to endothelium dependent stimuli [21]. These findings suggested that the stimulated perfusion varied from one subject to another even if they had similar medical condition. The degree of impairment depended not only on the severity of the cardiovascular diseases but also on their associated microangiopathy.
Previous studies have found the uremic patients to be with impaired low-frequency oscillations in response to endothelium-dependent stimuli [11, 22, 23]. Our research extended prior observations of reactive hyperemia by wavelet analysis into more refined frequency range of interest. This study revealed that diabetic patients with microalbuminuria had reduced flow motion changes in very low frequency interval. Our findings were consistent with impaired flow motion in corresponding endothelial range in previous uremic patients [11, 23, 24]. The dysregulated skin microcirculation could be related to the endothelial dysfunction in either overt or subclinical atherosclerosis. Our study gave some insights into the extent of the possible mechanisms involved in disordered reactive hyperemia. It is interesting to note that a relative change was present in spectral distribution of neurogenic and myogenic flow motion following ischemia. Our results implied that microvascular perfusion might be disturbed with additional endothelial-independent components in patients with incipient nephropathy.
This study found that post-ischemic increase in the normalized myogenic component showed significant inverse association with the arterial stiffness. The smooth muscle dysfunction should be related to the severity of atherosclerosis. Previous study has indicated that the severity of postural hypotension was negatively correlated with myogenic vasomotor amplitude [7]. Our findings were consistent with the established close relation between smooth muscle dysfunction and severe cardiovascular complications [25, 26]. This study revealed that various microcirculatory markers would not deteriorate to the same degree in subclinical atherosclerosis. The endothelial control seemed to be dysfunctional first, leaving the neurogenic and myogenic regulation intact in microvasculature [27, 28]. Therefore, impaired systemic vascular tone was likely to develop in advanced atherosclerotic diseases, leading to symptomatic vascular insufficiency.
The vessel diameter changes are related to rhythmic constriction and dilation of arteriolar smooth muscles. The vasomotor responses contribute to lowering the resistance and increasing the perfusion pressure in microvasculature [29, 30]. Diabetes involving peripheral vascular structure could reduce the magnitude of the locally driven vessel diameter changes. Our study indicated that there was impaired microvascular flow motion in patients with early atherosclerosis even in the absence of large vessel disease. Increased vasomotor output could be a part of the normal physiological response to compensate for impaired tissue perfusion [31, 32]. The microalbuminuria patients are at risk of systemic vascular insufficiency as a result of their inability to mount this response. Our data can be interpreted as the functional alteration within small arteriole such as diminished endothelial activity together with increased myogenic activity in preserved reactive hyperemia perfusion. These results suggested that spectral distribution changes preceded the development of disturbed skin perfusion or spectral power. That could be the reason why the structural perfusion changes did not occur in the early stage of atherosclerosis.
Impaired vascular function has been studied in both microvascular and macrovascular tissues and possible association could be estimated among various surrogate markers. Tao et al. indicated that systemic arterial elasticity deteriorated parallel with cutaneous microvascular flow changes [33]. However, lack of correlation was shown between conduit vessel response and laser Doppler measures of skin perfusion by previous studies [34, 35]. The various tests characterized distinct vascular pathologies in patients at cardiovascular risks. The use of different vascular bed may account for the aforementioned heterogeneous findings. This study advocated the pulse wave analysis to investigate the entire vascular pathway including central elastic and peripheral muscular arteries. This post-ischemic increase in vasomotor component was included as a surrogate marker as well for microcirculatory function.
Reactive vasomotion and pulse wave velocity represented different clinical implications in patients with preclinical atherosclerosis. The distinct vascular assessment can be integrated in this study using the regression model. Multiple regression analysis revealed that reduced endothelial flow motion changes independently contributed to increased pulse wave velocity. This study indicated that the impairment degree of reactive flow motion could reflect increased baPWV to some extent. These findings may underlie the association between diabetic microangiopathy and arterial stiffness. The associations were independent of other clinical covariates. This study highlighted the importance of early detection of subclinical atherosclerotic diseases in diabetic patients.
The limitation of this study was that the analysis of various degrees of vasculopathy might not be conclusive enough owing to a small sample size in each subgroup. The regression model could only be used to predict the patients with minor cardiovascular risks. The skin perfusion could only be expressed as arbitrary units, and it was difficult to define the threshold level for the normality and to be compared with other relevant reports. Our findings demonstrated a less robust but still significant relationship between arterial stiffness and reactive vasomotor changes. Further investigations are needed to clarify whether these microvascular changes are present in some target organs other than cutaneous tissues. The pathological basis and the temporal relationships of these associations must be explored by additional mechanistic and prospective studies to understand the clinical significance of these findings.
Conclusion
The spectral analysis of laser Doppler skin perfusion provided insights into potential significance of microcirculatory control in the development of macrovascular dysfunction. Various microvascular markers would not deteriorate to the same degree in subclinical atherosclerosis. Reduced endothelial related change was noted first, followed by frequency spectrum redistribution. Impaired reactive flow motion and arterial stiffness correlated with each other. The associations were possibly related to subclinical atherosclerosis and independent of conventional risk factors. The functional adjustment could precede structural perfusion insufficiency in incipient vasculopathy patients.
Abbreviations
- MAU:
-
Diabetes with microalbuminuria
- NAU:
-
Diabetes with normoalbuminuria
- baPWV:
-
Brachial ankle pulse wave velocity
References
Strain WD, Paldánius PM (2018) Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol 17:57
Tomiyama H, Matsumoto C, Shiina K, Yamashina A (2016) Brachial-Ankle PWV: current status and future directions as a useful marker in the management of cardiovascular disease and/or cardiovascular risk factors. J Atheroscler Thromb 23:128–146
Sougawa Y, Miyai N, Utsumi M, Miyashita K, Takeda S, Arita M (2020) Brachial-ankle pulse wave velocity in healthy Japanese adolescents: reference values for the assessment of arterial stiffness and cardiovascular risk profiles. Hypertens Res 43:331–341
Sorelli M, Francia P, Bocchi L (2019) Assessment of cutaneous microcirculation by laser Doppler flowmetry in type 1 diabetes. Microvasc Res 124:91–96
Fuchs D, Dupon PP, Schaap LA, Draijer R (2017) The association between diabetes and dermal microvascular dysfunction non-invasively assessed by laser Doppler with local thermal hyperemia: a systematic review with meta-analysis. Cardiovasc Diabetol 16:11
Hu HF, Hsiu H, Sung CJ, Lee CH (2017) Combining laser-Doppler flowmetry measurements with spectral analysis to study different microcirculatory effects in human prediabetic and diabetic subjects. Lasers Med Sci 32:327–334
Sun PC, Chen CS, Kuo CD, Lin HD, Chan RC, Wei SH (2012) Impaired microvascular flow motion in subclinical diabetic feet with sudomotor dysfunction. Microvasc Res 83:243–248
Abdelhafiz AH, Ahmed S, El Nahas M (2011) Microalbuminuria: marker or maker of cardiovascular disease. Nephron Exp Nephrol 119:e6-10
Chang LH, Lin HD, Kwok CF et al (2014) The combination of the ankle brachial index and brachial ankle pulse wave velocity exhibits a superior association with outcomes in diabetic patients. Intern Med 53:2425–2431
Kvandal P, Stefanovska A, Veber M, Kvermmo HD, Kirkeboen KA (2003) Regulation of human cutaneous circulation evaluated by laser Doppler flowmetry, iontophoresis, and spectral analysis: importance of nitric oxide and prostaglandins. Microvasc Res 61:160–171
Rossi M, Cupisti A, Di Maria C, Galetta F, Santoro G (2008) Blunted post-ischemic increase of the endothelial skin blood flow motion component as early sign of endothelial dysfunction in chronic kidney disease patients. Microvasc Res 75:315–322
Hayden MR, Sowers JR, Tyagi SC (2005) The central role of vascular extracellular matrix and basement membrane remodeling in metabolic syndrome and type 2 diabetes: the matrix preloaded. Cardiovasc Diabetol 4:9
Zhang Y, Lacolley P, Protogerou AD, Safar ME (2020) Arterial Stiffness in Hypertension and Function of Large Arteries. Am J Hypertens 33:291–296
Sawada S, Tsuchiya S, Kodama S, Kurosawa S, Endo A, Sugawara H (2020) Vascular resistance of carotid and vertebral arteries is associated with retinal microcirculation measured by laser speckle flowgraphy in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 165:108–240
Safar ME, Struijker-Boudier HA (2010) Cross-talk between macro- and microcirculation. Acta Physiol (Oxf) 198:417–430
Jonasson H, Bergstrand S, Nystrom FH, Länne T, Östgren CJ, Bjarnegård N (2017) Skin microvascular endothelial dysfunction is associated with type 2 diabetes independently of microalbuminuria and arterial stiffness. Diab Vasc Dis Res 14:363–371
Zandbergen AA, Sijbrands EJ, Lamberts SW, Bootsma AH (2006) Normotensive women with type 2 diabetes and microalbuminuria are at high risk for macrovascular disease. Diabetes Care 29:1851–1855
Roustit M, Cracowski JL (2012) Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation 19:47–64
Cupisti A, Rossi M, Placidi S, Caprioli R, Morelli E, Vagheggini G (2000) Response of the skin microcirculation to acetylcholine and sodium nitroprusside in chronic uremic patients. Int J Clin Lab Res 30:157–162
Babos L, Járai Z, Nemcsik J (2013) Evaluation of microvascular reactivity with laser Doppler flowmetry in chronic kidney disease. World J Nephrol 2:77–83
Schmiedel O, Schroeter ML, Harvey JN (2007) Microalbuminuria in type 2 diabetes indicates impaired microvascular vasomotion and perfusion. Am J Physiol Heart Circ Physiol 293:H3424–H3431
Houben AJHM, Martens RJH, Stehouwer CDA (2017) Assessing microvascular function in humans from a chronic disease perspective. J Am Soc Nephrol 28:3461–3472
Farkas K, Nemcsik J, Kolossváry E, Járai Z, Nádory E, Farsang C (2005) Impairment of skin microvascular reactivity in hypertension and uraemia. Nephrol Dial Transplant 20:1821–1827
Smogorzewski M (2017) Skin blood flow and vascular endothelium function in uremia. J Ren Nutr 27:465–469
Lacolley P, Regnault V, Segers P, Laurent S (2017) Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev 97:1555–1617
Lacolley P, Regnault V, Avolio AP (2018) Smooth muscle cell and arterial aging: basic and clinical aspects. Cardiovasc Res 114:513–528
Vinik AI, Erbas T, Park TS, Stansberry KB, Scanelli JA, Pittenger GL (2001) Dermal neurovascular dysfunction in type 2 diabetes. Diabetes Care 24:1468–1475
Lespagnol E, Dauchet L, Pawlak-Chaouch M, Balestra C, Berthoin S, Feelisch M (2020) Early endothelial dysfunction in type 1 diabetes is accompanied by an impairment of vascular smooth muscle function: A Meta-Analysis. Front Endocrinol (Lausanne) 11:203
Durand S, Zhang R, Cui J, Wilson TE, Crandall CG (2004) Evidence of a myogenic response in vasomotor control of forearm and palm cutaneous microcirculations. J Appl Physiol 97:535–539
Tuna BG, Bakker EN, VanBavel E (2012) Smooth muscle biomechanics and plasticity: relevance for vascular calibre and remodelling. Basic Clin Pharmacol Toxicol 110:35–41
Leo F, Krenz T, Wolff G, Weidenbach M, Heiss C, Kelm M (2020) Assessment of tissue perfusion and vascular function in mice by scanning laser Doppler perfusion imaging. Biochem Pharmacol 176:1138–1193
Sun PC, Kuo CD, Chi LY, Lin HD, Wei SW, Chen CS (2013) Microcirculatory vasomotor changes are associated with severity of peripheral neuropathy in patients with type 2 diabetes. Diab Vasc Dis Res 10:270–276
Tao J, Jin YF, Yang Z, Wang LC, Gao XR, Lui L (2004) Reduced arterial elasticity is associated with endothelial dysfunction in persons of advancing age: comparative study of noninvasive pulse wave analysis and laser Doppler blood flow measurement. Am J Hypertens 17:654–659
Shamim-Uizzaman Q, Pfenninger D, Kehrer C (2002) Altered cutaneous microvascular responses to reactive hyperemia in coronary artery disease: a comparative study with conduit vessel responses. Clin Sci 103:267–273
Çekiç EG, Başaran Ö, Filiz Başaran N, Elmas O, Doğan V, Mert GÖ (2017) Cutaneous microvascular reactivity and aortic elasticity in coronary artery disease: Comparison of the laser Doppler flowmetry and echocardiography. Microvasc Res 109:19–25
Author information
Authors and Affiliations
Contributions
Study concept and design: Drs Sun, Wei. Recruitment of patients, acquisition of data: Drs Wei, Lin. Analysis and interpretation of data: Drs Sun, Kuo. Draft of the manuscript: Drs Sun, Kuo. Administrative, technical or material support: Drs Kuo, Lin.
Corresponding author
Ethics declarations
Informed consent
Yes.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, PC., Kuo, CD., Wei, SH. et al. Microvascular reactivity using laser Doppler measurement in type 2 diabetes with subclinical atherosclerosis. Lasers Med Sci 38, 80 (2023). https://doi.org/10.1007/s10103-023-03737-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-023-03737-x