Abstract
This study aimed to determine the inhibitory effects of green tea (Gt), EGCG, and nanoformulations containing chitosan (Nchi) and chitosan+green tea (Nchi+Gt) against Streptococcus mutans and Lactobacillus casei. In addition, the antibacterial effect of nanoformulations was evaluated directly on dentin after the selective removal of carious lesion. At first, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) against S. mutans and L. casei isolates were investigated. In parallel, dentin specimens were exposed to S. mutans to induce carious lesions. Soft dentin was selectively removed by Er:YAG laser (n=33) or bur (n=33). Remaining dentin was biomodified with Nchi (n=11) or Gt+Nchi (n=11). Control group (n=11) did not receive any treatment. Dentin scraps were collected at three time points. Microbiological analyses were conducted and evaluated by agar plate counts. Gt at 1:32 dilution inhibited S. mutans growth while 1:16 was efficient against L. casei. EGCG at 1:4 dilution completely inhibited S. mutans and L. casei growth. Independently of the association with Gt, Nchi completely inhibited S. mutans at 1:4 dilution. For L. casei, different concentrations of Nchi (1:32) and Nchi+Gt (1:8) were required to inhibit cell growth. After selective carious removal, viability of S. mutans decreased (p<0.001), without difference between bur and Er:YAG laser (p>0.05). Treatment with Nchi and Nchi+Gt did not influence the microbial load of S. mutans on dentin (p>0.05). Although variations in concentrations were noticed, all compounds showed antibacterial activity against S. mutans and L. casei. Both bur and Er:YAG laser have effectively removed soft dentin and reduced S. mutans counts. Nanoformulations did not promote any additional antibacterial effect in the remaining dentin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental caries is a biofilm-dependent oral disease. The frequent intake of fermentable dietary carbohydrates that are metabolized and converted into acid-end products disrupts the homeostasis in the oral microenvironment leading to initiation and development of dental caries [1]. Oral biofilm is a three-dimensional structure of complex polymicrobial communities [2]. Streptococcus spp. is the initial colonizer of dental biofilm and Streptococcus mutans is considered a common etiologic agent participating in cariology [3]. Besides S. mutans, other species with acidogenic phenotypes play essential roles in prolonged periods of low pH in biofilms, including the Bifidobacterium spp., Lactobacillus spp., and, the new caries-related, Scardovia wiggsiae [4].
The prevalence of primary and permanent dental caries worldwide is still considered high [5]. The management of dental caries requires caries excavation followed by cavity restoration. But, in cases of deep caries lesions, the selective caries removal to soft dentin is an indication to avoid pulp exposure, preserving dentin structure [6].
The erbium-doped:yttrium-aluminum-garnet laser (Er:YAG laser) ablates carious tissue through microexplosions due to its high rate of absorption in water, resulting in minimal thermal side effects [7]. The Er:YAG wavelength (2.94 μm) is in the middle of the infrared region of the electromagnetic spectrum, which coincides with the absorption peak of water present in the dentin. The hydroxyapatite shows an IR absorption band in the same region due to its intrinsic water content [7, 8]. During Er:YAG laser irradiation, the energy absorbed by water molecules of dentin organic content leads to successive microexplosions which causes an ejection of organic and inorganic substrates, thus, removing soft carious dentin [9]. However, after the selective caries removal, independent of the caries removal method (erbium lasers or burs), remaining intratubular cocci, rod, and filamentous bacteria in coronal dentin may be found [10].
Natural herbal plants, as Camellia sinensis (Green tea), show potential antibiofilm effects and are low-cost and show reduced side-effect risk [11]. Green tea is composed of four significant catechins epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG), and epigallocatechin gallate (EGCG). Green tea contains about 40% of EGCG [12]. EGCG targets glucosyltransferase enzymes responsible for converting sucrose inhibiting biofilm development and progression [13, 14].
Chitosan, a natural biopolymer, is obtained by an alkaline deacetylation reaction from chitin, mainly obtained from the exoskeletons of crustaceans [15]. Chitosan is highly reactive due to its amino groups, giving it a positive (cationic) charge toward anionic particles, such as bacteria and biofilms [16]. This biopolymer has an excellent antimicrobial activity against isolated species of S. mutans, Actinomyces naeslundii, and Enterococcus faecalis [17, 18].
Thus, this study aimed to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of green tea, EGCG, chitosan, and chitosan + green tea against S. mutans and Lactobacillus casei isolates. And then, to test the antibacterial effect of a nanoformulation containing chitosan + green tea on dentin after the selective removal of carious lesion induced by S. mutans.
Materials and methods
Preparation of nanoformulations
Five milligrams of EGCG (#E4143, Sigma-Aldrich, Saint Louis, MO, USA) was diluted in 1 mL of PBS. The pH was adjusted to 5 using 0.1 N sodium hydroxide (Sigma-Aldrich, Saint Louis, MO, USA). The diluted solution was protected from UV-visible light with aluminum foil.
The Camelia sinensis (Green Tea 400 mg, NOW Supplements, USA) was diluted in MilliQ water in a microtube and sonicated for approximately 30 min at 85 °C. Intending to provide better solubilization of the green tea, in sequence, it was centrifuged at 25 °C for another 30 min at 10,000 rpm to separate the impurities that did not dissolve. Then, the supernatant was used as a stock solution [19, 20].
The nanoformulations containing chitosan nanoparticles were prepared according to an ionic cross-linking method using tripolyphosphate (TPP) [19]. Low molecular weight chitosan (#448869, 75–85% deacetylation) commercially available (Sigma-Aldrich, Saint Louis, MO, USA) was dissolved in 0.33% (vol/vol) glacial acetic acid for a stock solution of 2 mg/mL. The pH was adjusted to 5 using 0.1 N sodium hydroxide. Under mild stirring, TPP solution (1 mg/mL) was slowly added, drop by drop, to the chitosan solution. The proportion of chitosan to TPP was 4.6:1 w/w. The Camelia sinensis (Green Tea 400 mg, NOW Supplements, USA), 0.26% (w/v), was incorporated into chitosan nanoparticles using the ionic gelation method. Nanoformulations were monitored over 90 days after preparation. All data regarding the preparation of chitosan nanoformulations, extraction of the green tea mass, preparation of green tea chitosan nanoformulation, and the characterization of chitosan-based nanoformulations are presented in a previous study [21].
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of green tea (Gt), epigallocatechin gallate (EGCG), green tea–loaded chitosan nanoparticles (Gt+Nchi), and chitosan nanoparticles (Nchi) were determined. MIC is the lowest concentration of a compound, which prevents visible growth of bacteria, and MBC is the lowest concentration of an antibacterial agent required to kill a specific bacterium. Before the test, microorganisms were reactivated on its specific solid culture media (Table 1) for 48 h. Then, one bacterial colony was transferred into the broth medium and incubated for 24 h at 37°C. Bacterial cultures were centrifuged at 4200 g for 5 min. The resulting pellets were washed twice with phosphate-buffered saline (PBS). The cell concentration (108 colony forming units per milliliter - CFU/mL) was determined by spectrophotometry at 625 nm, obtaining absorbance values of 0.09 for S. mutans and 0.160 for L. casei. Then, the suspensions were diluted into a 107 CFU/mL in PBS. The MIC and MBC values of solutions were determined using 96-well plates in duplicate. First, 100 μL of the medium was added to each well. One hundred microliters of each experimental solution was added in the first well, and then, 10 serial dilutions were obtained in the following wells. Five microliters of each microbial suspension was inoculated bringing a final concentration of approximately 105 CFU/mL. Positive and negative controls were used to confirm the microorganism’s growth and test asepsis, respectively. In the positive control, 100 μL of the culture medium and 5 μL of inoculum were added. In the negative control, 50 μL of the culture medium and 50 μL of its respective experimental solution were added.
Table 1 shows the microorganism and the culture medium used in the experimental tests.
Then, the plates were incubated under microaerophilic conditions at 37°C for 24 h in a microbiological incubator. The MIC values were defined as an absence of visible growth of bacteria in the wells. After the MIC determination, aliquots of 20 μL from all the wells, which showed no visible bacterial growth, were dropped onto brain heart infusion (BHI) agar, and incubated for 24 h at 37 °C. When the bacterial inoculum was killed at the lowest concentration, it was termed the MBC endpoint.
Tooth preparation
This study was approved by the Research Ethics Committee of the University of Sao Paulo (Institutional Review Board protocol CAAE 69600217.4.0000.5419). To test the antibacterial effect of nanoformulations directly on carious dentin, tooth samples were prepared. Human third molars stored in distilled water at 4°C were cleaned and analyzed under a stereomicroscope (Leica S6 D Stereo Zoom, Leica Microsystems AG, Switzerland) to select teeth with no structural defects. Thirty-three sound molars were selected. Roots were sectioned in the cementum enamel junction with a double-faced diamond disk mounted in a cutting machine (Isomet 1000, Buehler, Lake Bluff, IL, USA). Specimens had the enamel removed from the occlusal and lateral surfaces, and two specimens of 3.0 × 3.0 × 2.5 mm dimensions were obtained from each tooth. The dentin surface was polished up to 1200-grade Al2O3 paper (DP-9U2, Struers A/S, Copenhagen, Denmark). Specimens were immersed in deionized water and sonicated for 10 min to remove polishing residues.
Artificial caries induction
As S. mutans is considered the major pathogen of dental caries, a primary culture of microorganism (ATCC25175) was selected to induce artificial caries lesions in the present study. The lateral surface of each tooth was painted with two layers of nail varnish (Colorama Maybelline Ltda, Sao Paulo, Brazil) except for the occlusal surface. On one surface, a nylon line was attached, and the specimens were subsequently sterilized using the following gas mixture: 30% EtO and 70% carbon dioxide at 50–55 °C for 4 h. An oral biofilm model was developed to reproduce the oral environment. Aseptically, the nylon line was fixed to a screen anchored on a beaker opening to keep the specimens suspended. Subsequently, 25 mL of artificial caries solution was added to each dentin specimen. The artificial caries solution contained 100 mL of distilled water, 3.7 g of BHI broth, 0.5 g of yeast (Kasvi), 1.0 g of glucose (Sigma), 2 g of sucrose (Sigma), and 100 μL of S. mutans at a concentration of 108 CFU/mL. Specimens were incubated at 37 °C in a microaerophilic jar (BBL GasPak system, Becton-Dickinson, Franklin Lakes, EUA). At every 48 h, specimens were transferred to a fresh solution [22]. At 14 days, the biofilm was carefully removed by gentle fractioning a sterile gauze. Superficial dentin scrapes were harvested from each tooth and transferred to 1 mL of PBS. Then, caries removal of soft dentin was conducted.
Caries removal of soft dentin
As previously reported, the selective removal of soft dentin was standardized using an automatic custom-designed device (MPC ElQuip, Sao Carlos, Sao Paulo, Brazil) [23, 24]. Briefly, a non-contact 90°-angled Er:YAG handpiece (R02) with incorporated air/water spray nozzle (Er:YAG laser, Fidelis Er III, Fotona, Ljubljana, Slovenia) was applied with a focal distance of 7 mm, pulse energy of 250 mJ, pulse repetition rate of 4 Hz, output beam diameter of 0.9 mm, energy density of 39 J/cm2, and underwater spray (6 mL/min). In the control group, caries removal was performed using a spherical carbide bur (#8, KG Sorensen, Barueri, SP, Brazil) at low speed (1:1 L microseries, Bien-Air Dental, CA, USA). The criteria of removal were based on the dentin consistency, checked by a sharp probe. The tactile softened dentin was removed entirely until the leathery dentin was found. Soft dentin deforms with a latent stickiness when the sharp probe is pressed onto it. In addition, the softened dentin was quickly scooped up with a sharp hand excavator with little force applied. Leathery dentin (remaining) shows more resistance against deformation when an instrument is pressed onto it [25].
Dentin biomodification
The dentin surface was treated with 50 μL chitosan nanoparticles (Nchi) or green tea–loaded chitosan nanoparticles (Gt+Nchi) for 1 min, followed by rinsing with distilled water for 15 s and drying with absorbent paper. Control specimens did not receive biomodification.
Microbiological analysis of the dentin layers affected by caries lesions
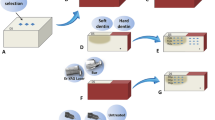
The microbiological analysis of the dentin layers was conducted during the following steps: after caries lesions were formed (baseline), after selective caries removal to leathery dentin, and after dentin biomodification. Dentin scraps (0.3 mg) were collected using aseptic curettes #11, #11½, and #12 (Duflex, SSWhite, Juiz de Fora, MG, Brazil). Dentin scraps were weighed using a high-precision analytical balance (Analytical Plus AP 250D, Ohaus Corp, Florham Park, Nova Jersey, USA), stored in sterile microtubes, and suspended in 1 mL of PBS. Samples were sonicated for 15 s pulses at 20% amplitude using a sonic dismembrator (CL-334 Digital Fisher Scientific Sonicator, Park Lane, Pittsburgh, USA). Tenfold serial dilutions were seeded onto Mitis Salivarius agar (Kasvi) supplemented with 0.2 UI/mL of bacitracin (Sigma) and 20% sucrose (Sigma). Plates were incubated in microarephilia at 37 °C for 48 h. CFU were counted and results are expressed in log10CFU/mL. Figure 1 shows the schematic diagram of the experimental design.
Data analysis
As data presented normal distribution, groups were statistically analyzed by two-way ANOVA, followed by Bonferroni’s post hoc test (α = 0.05), using SPSS version 20.0 (SPSS Inc., v20, Chicago, IL, USA).
Results
All compounds tested showed an antimicrobial effect. The MIC of Gt was found when the stock solution was diluted at 1:32 for S. mutans and 1:16 for L. casei. The isolated green tea catechin, EGCG, completely inhibited the growth of S. mutans and L. casei at 1:4 dilution, and its bactericidal activity for both microorganisms was observed at 1:2 dilution. Independently of the association with Gt, the lowest dilution of Nchi which complete inhibited S. mutans was at 1:4. The dilution noted for L. casei was greater, at 1:32 dilution for Nchi and 1:8 for Nchi+Gt. The MIC and MBC values for the two microorganisms tested are shown in Tables 2 and 3.
The quantitative microbiological analysis of dentin scrapings obtained after selective carious removal showed a reduction in the microbial load of S. mutans compared to baseline (p<0.001). There was no difference between bur and Er:YAG laser (p=0.132). The interaction between the removal method vs time was not significant (p=0.132). Table 4 shows the data obtained after the selective caries removal.
Biomodification of leathery (remaining) dentin with Nchi and Nchi+Gt did not influence the microbial load of S. mutans compared to the non-treatment group (p>0.05), regardless of which method of removal was used (p>0.05). The interaction of the removal method vs dentin biomodification was not significant (p>0.05). Table 5 shows the data obtained after the dentin biomodification.
Discussion
In the present study, the antibacterial activity of green tea nanoformulation (Gt), epigallocatechin gallate (EGCG), chitosan nanoparticles (Nchi), and green tea–loaded chitosan nanoparticles (Gt+Nchi) was evaluated against S. mutans and L. casei by broth microdilution. In parallel, the antibiofilm activity was also tested in the oral biofilm model, using the cariogenic bacteria, S. mutans.
The epigallocatechin-3-gallate (EGCG) is one of the polyphenols present in green tea [11, 12]. Due to the high potential to induce collagen cross-linking via hydrogen bonding, EGCG has been tested to prevent the free access of collagenases to the active sites on the collagen chains, increasing the elastic modulus of dentin [26, 27]. Recently, studies have investigated its antibacterial potential in preventing dental caries [14, 28]. Accordingly, the isolated green tea catechin, EGCG, showed antibacterial activity for both S. mutans and L. casei. This demonstrates that the other catechins included in Camellia sinensis, such as epigallocatechin, epicatechin-3-gallate, and epicatechin, could play an essential role in inhibiting the initiation of biofilm formation. Other evidence indicates that EGCG could inhibit biofilm formation and inhibit glucansucrase, a major virulence factor in S. mutans within the formed biofilms [13].
When associated with Nchi, a lower concentration of green tea completely inhibited S. mutans and L. casei. This exemplifies chitosan nanoformulation has good potential as an antimicrobial compound. Within the desired characteristics of a nanoformulation, the tiny particles that pass through various biological barriers facilitate delivering drugs to target sites [29]. The pH is another critical characteristic since chitosan at high pH is poorly soluble [30]. In the present study, the pH is 5. The adsorption of chitosan on bacterial surfaces is highly regulated by pH. When pH decreases, chitosan adsorption on bacterial surfaces increases [31]. In addition, the outcomings may be favored by an acid microambient promoted either by S. mutans or L. casei, which are acid-producing bacteria.
In the present study, a dentin experimental model previously tested [21] was used to apply the compounds directly on dentin. This model allowed a microbiological analysis of the dentin layers affected by S. mutans. Although other microorganisms are related to caries lesions, S. mutans was selected since it is the major acidogenic-aciduric pathogen associated with dental caries and related to its initial stages [32].
The partial caries removal with bur and Er:YAG laser was performed based on the minimally invasive treatment strategies. Because it reduces the risk of pulp exposure during caries management and lowers the risk of regrowth of the few embedded microbial cells located inside the dentinal tubules [33]. After using bur and Er:YAG laser (energy density of 39 J/cm2), the quantitative microbiological analysis of dentin scrapings was similar, which means both methods promoted reduction in about 100-fold (2 log) in the number of S. mutans colonies compared to baseline. Our findings agree with a previous study reporting that irradiation with a high energy density (25.47 J/cm2) promotes bactericidal effects against S. mutans. In addition, Er:YAG laser irradiation may also clean the dentin promoting open orifices [34].
After partial caries removal, microorganisms are present in the residual dentin [10]. The treatments with Nchi or Nchi+Gt were applied on dentin substrate immediately after the caries removal techniques. However, they did not influence the residual microbial load, which means the tested compounds did not show an antimicrobial additional effect on dentin. As the presence of bacteria after partial caries removal cannot be effectively determined, antibacterial compounds should, preferentially, permeate dentin at some level. In the present study, the nanoformulations reached an average size between 300 and 350 nm, penetrating dentin tubules [21].
The dental biofilms present in the oral cavity are predominant in an acid microenvironment. The nanomaterial benefits from this highly acid microenvironment to act [35]. Under acid conditions, the amine group of chitosan delivers pH-responsive groups that are protonated and provides pH-based activity. Nanomaterials fabricated with pH-responsive block copolymers, as Nchi and Nchi+Gt, could bind to negatively charged tooth surface and deliver green tea. However, a factor that should be considered is drug resistance in biofilms. Compared to planktonic cells, biofilms may tolerate up to 100–1000 times higher concentrations of antibiotics [36]. Other factors that affect chitosan’s antimicrobial properties may include pH, type of microorganism, and neighboring components. In addition, structural conditions such as molecular weight, degree of deacetylation, derivative form, concentration, and source could influence the antimicrobial effect of a compound [31].
This study was limited because the oral environment was not completely reproduced. In the oral cavity, microorganisms exist in multispecies communities, encompassing commensal, symbiotic, and pathogenic microorganisms [37]. These microorganisms interact to modulate biofilm nature. However, only S. mutans were applied to compose the oral biofilm model.
Although the results require additional investigation, based on the above considerations, green tea and chitosan have shown potential as therapeutic agents against cariogenic microorganisms. The concentration evaluated in this study did not show substantial antibiofilm activity, and different approaches should be further explored. Despite the specificity of the dentin substrate, natural product-based nanoformulations may be considered a highly promising tool for biofilm therapy.
Conclusions
-
Independently of variations of Gt, EGCG, Nchi, and Nchi+Gt concentrations, all compounds showed antibacterial activity against S. mutans and L. casei.
-
Bur and Er:YAG laser have effectively removed soft dentin reducing S. mutans colonies in the remaining dentin.
-
Biomodification with Nchi and Nchi+Gt on remaining dentin has not resulted in an additional antibacterial effect.
Data availability
The data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Paes Leme AF, Koo H, Bellato CM, Bedi G, JAC (2006) The role of sucrose in cariogenic dental biofilm formation—new insight. J Dent Res 85:878–887
Bowen WH, Burne RA, Hui W, HK (2018) Oral biofilms: pathogens, matrix and polymicrobial interactions in microenvironments. Trends Microbiol 26:229–242. https://doi.org/10.1016/j.tim.2017.09.008
Ito T, Ichinosawa T, Shimizu T (2017) Streptococcal adhesin SspA/B analogue peptide inhibits adherence and impacts biofilm formation of Streptococcus mutans. PLoS One 12:1–15. https://doi.org/10.1371/journal.pone.0175483
Chen X, Daliri EBM, Tyagi A, Oh DH (2021) Cariogenic biofilm: pathology-related phenotypes and targeted therapy. Microorganisms 9. https://doi.org/10.3390/microorganisms9061311
Kazeminia M, Abdi A, Shohaimi S et al (2020) Dental caries 1995-2019. Head Face Med 1:1–21
Jardim JJ, Mestrinho HD, Koppe B et al (2020) Restorations after selective caries removal: 5-year randomized trial. J Dent 99:103416. https://doi.org/10.1016/j.jdent.2020.103416
Hibst R, Keller U (1989) Experimental studies of the application of the Er:YAG laser on dental hard substances: I. Measurement of the ablation rate. Lasers Surg Med 9:338–344. https://doi.org/10.1002/lsm.1900090405
Keller U, Hibst R (1997) Effects of Er:YAG laser in caries treatment: a clinical pilot study. Lasers Surg Med 20:32–38. https://doi.org/10.1002/(sici)1096-9101(1997)20:1<32::aid-lsm5>3.0.co;2-%23
Valenti C, Pagano S, Bozza S et al (2021) Use of the Er:YAG laser in conservative dentistry: evaluation of the microbial population in carious lesions. Materials (Basel) 14:2387. https://doi.org/10.3390/ma14092387
Nakrathok P, Kijsamanmith K, Vongsavan K et al (2020) The effect of selective carious tissue removal and cavity treatments on the residual intratubular bacteria in coronal dentine. J Dent Sci 15:411–418. https://doi.org/10.1016/j.jds.2020.03.016
Smeriglio A, Barreca D, Bellocco E, Trombetta D (2017) Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br J Pharmacol 174:1244–1262. https://doi.org/10.1111/bph.13630
Reygaert WC (2018) Green tea catechins: their use in treating and preventing infectious diseases. Biomed Res Int 2018. https://doi.org/10.1155/2018/9105261
Hairul Islam MI, Arokiyaraj S, Kuralarasan M et al (2020) Inhibitory potential of EGCG on Streptococcus mutans biofilm: a new approach to prevent cariogenesis. Microb Pathog 143:104129. https://doi.org/10.1016/j.micpath.2020.104129
Han S, Abiko Y, Washio J et al (2021) Green tea-derived epigallocatechin gallate inhibits acid production and promotes the aggregation of Streptococcus mutans and non-mutans streptococci. Caries Res 55:205–214. https://doi.org/10.1159/000515814
Elieh-Ali-Komi D, Michael R (2016) Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int J Adv Res 4:411–427. https://doi.org/10.2307/4145104
Kawakita ERH, Ré ACS, Peixoto MPG et al (2019) Effect of chitosan dispersion and microparticles on older streptococcus mutans biofilms. Molecules 24:1–11. https://doi.org/10.3390/molecules24091808
Gu LS, Cai X, Guo JM et al (2019) Chitosan-based extrafibrillar demineralization for dentin bonding. J Dent Res 98:186–193. https://doi.org/10.1177/0022034518805419
Cavalcante LLR, Tedesco AC, Takahashi LAU et al (2020) Conjugate of chitosan nanoparticles with chloroaluminium phthalocyanine: synthesis, characterization and photoinactivation of Streptococcus mutans biofilm. Photodiagnosis Photodyn Ther 30:101709. https://doi.org/10.1016/j.pdpdt.2020.101709
Cánepa C, Imperiale JC, Berini CA et al (2017) Development of a drug delivery system based on chitosan nanoparticles for oral administration of interferon-α. Biomacromolecules 18:3302–3309. https://doi.org/10.1021/acs.biomac.7b00959
Safer AMA, Hanafy NA, Bharali DJ et al (2015) Effect of green tea extract encapsulated into chitosan nanoparticles on hepatic fibrosis collagen fibers assessed by atomic force microscopy in rat hepatic fibrosis model. J Nanosci Nanotechnol 15:6452–6459. https://doi.org/10.1166/jnn.2015.10608
Curylofo-Zotti FA, Tedesco AC, Lizarelli GTC et al (2021) Effect of green tea-loaded chitosan nanoparticles on leathery dentin microhardness. Odontology 109:860–867. https://doi.org/10.1007/s10266-021-00611-6
Marquezan M, Corrêa FNP, Sanabe ME et al (2009) Artificial methods of dentine caries induction: a hardness and morphological comparative study. Arch Oral Biol 54:1111–1117. https://doi.org/10.1016/j.archoralbio.2009.09.007
Curylofo-Zotti FA, Tanta GS, Zucoloto ML et al (2017) Selective removal of carious lesion with Er:YAG laser followed by dentin biomodification with chitosan. Lasers Med Sci 32. https://doi.org/10.1007/s10103-017-2287-6
Curylofo-Zotti FA, Scheffel DLS, Macedo AP et al (2019) Effect of Er:YAG laser irradiation and chitosan biomodification on the stability of resin/demineralized bovin dentin bond. J Mech Behav Biomed Mater 91:220–228. https://doi.org/10.1016/j.jmbbm.2018.12.022
Banerjee A, Frencken JE, Schwendicke F, Innes NPT (2017) Contemporary operative caries management: consensus recommendations on minimally invasive caries removal. Br Dent J 223:215–222. https://doi.org/10.1038/sj.bdj.2017.672
Aguiar TR, Vidal CMP, Phansalkar RS et al (2014) Dentin biomodification potential depends on polyphenol source. J Dent Res 93:417–422. https://doi.org/10.1177/0022034514523783
Jackson JK, Zhao J, Wong W, Burt HM (2010) The inhibition of collagenase induced degradation of collagen by the galloyl-containing polyphenols tannic acid, epigallocatechin gallate and epicatechin gallate. J Mater Sci Mater Med 21:1435–1443. https://doi.org/10.1007/s10856-010-4019-3
Zayed SM, Aboulwafa MM, Hashem AM, Saleh SE (2021) Biofilm formation by Streptococcus mutans and its inhibition by green tea extracts. AMB Express 11. https://doi.org/10.1186/s13568-021-01232-6
Peng HH, Hong DX, Guan YX, Yao SJ (2019) Preparation of pH-responsive DOX-loaded chitosan nanoparticles using supercritical assisted atomization with an enhanced mixer. Int J Pharm 558:82–90. https://doi.org/10.1016/j.ijpharm.2018.12.077
Qin C, Li H, Xiao Q et al (2006) Water-solubility of chitosan and its antimicrobial activity. Carbohydr Polym 63:367–374. https://doi.org/10.1016/j.carbpol.2005.09.023
Hosseinnejad M, Jafari SM (2016) Evaluation of different factors affecting antimicrobial properties of chitosan. Int J Biol Macromol 85:467–475. https://doi.org/10.1016/j.ijbiomac.2016.01.022
Lemos JA, Palmer SR, Zeng L et al (2019) The biology of Streptococcus mutans. Microbiol Spectr 7:1–26. https://doi.org/10.1128/microbiolspec.gpp3-0051-2018
Conrads G, About I, Conrads G, et al (2022) Pathophysiology of dental caries to cite this version : HAL Id : hal-03547399 Pathophysiology of Dental Caries. 1–10
Elburki MS, Rossa C, Guimarães-Stabili MR et al (2017) A chemically modified curcumin (CMC 2.24) inhibits nuclear factor κB activation and inflammatory bone loss in murine models of LPS-induced experimental periodontitis and diabetes-associated natural periodontitis. Inflammation 40. https://doi.org/10.1007/s10753-017-0587-4
Jessa MV, Makabenta AN, Li C-H, Schmidt-Malan S, Robin Patel VMR (2021) Nanomaterial-based therapeutics of antibiotic-resistant bacterial infections. Nat Rev Microbiol 19:23–36. https://doi.org/10.1038/s41579-020-0420-1.Nanomaterial-based
Macià MD, Rojo-Molinero E, Oliver A (2014) Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin Microbiol Infect 20:981–990. https://doi.org/10.1111/1469-0691.12651
Verma D, Garg PK, Dubey AK (2018) Insights into the human oral microbiome. Arch Microbiol 200:525–540. https://doi.org/10.1007/s00203-018-1505-3
Acknowledgements
The authors would like to thank Ana Paula Macedo for assistance with statistical data analysis.
Funding
This study received financial support from the São Paulo Research Foundation (FAPESP), grant nos. #2017/11582-1, #2017/00720-4, #2018/23862-1, #2019/04807-2, thematic project #2013/50181-1 and FINEP project 01.10.0758.01) and from the National Council for Scientific and Technological Development (CNPq), grant nos. PRONON-SIPAR project #25000.077093/2015-86), CNPq-SCTIE-Decit-DGITIS-CGCIS/CNPq no. 26/2020 – Innovative Platforms in Advanced Therapies, # 441673/2020-1 and CNPq-UN # 404416/2021-7, #2021-3/130399.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Fabiana Almeida Curylofo Zotti, Viviane de Cássia Oliveira, Analu Rodriguez Marquesin, and Hiago Salge Borges. The first draft of the manuscript was written by Fabiana Almeida Curylofo Zotti and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in the study were in accordance with the Research Ethics Committee of the University of São Paulo (Institutional Review Board protocol CAAE 69600217.4.0000.5419 and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants who agree to donate their extracted teeth to be included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Curylofo-Zotti, F.A., Oliveira, V.D.C., Marchesin, A.R. et al. In vitro antibacterial activity of green tea–loaded chitosan nanoparticles on caries-related microorganisms and dentin after Er:YAG laser caries removal. Lasers Med Sci 38, 50 (2023). https://doi.org/10.1007/s10103-023-03707-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-023-03707-3