Abstract
The present study aimed to evaluate the new heterologous fibrin biopolymer associated, or not, with photobiomodulation therapy for application in tendon injuries, considered a serious and common orthopedic problem. Thus, 84 Rattus norvegicus had partial transection of the calcaneus tendon (PTCT) and were randomly divided into: control (CG); heterologous fibrin biopolymer (HFB); photobiomodulation (PBM); heterologous fibrin biopolymer + photobiomodulation (HFB + PBM). The animals received HFB immediately after PTCT, while PBM (660 nm, 40 mW, 0.23 J) started 24 h post injury and followed every 24 h for 7, 14, and 21 days. The results of the edema volume showed that after 24 h of PTCT, there was no statistical difference among the groups. After 7, 14, and 21 days, it was observed that the treatment groups were effective in reducing edema when compared to the control. The HFB had the highest edema volume reduction after 21 days of treatment. The treatment groups did not induce tissue necrosis or infections on the histopathological analysis. Tenocyte proliferation, granulation tissue, and collagen formation were observed in the PTCT area in the HFB and HFB + PBM groups, which culminated a better repair process when compared to the CG in the 3 experimental periods. Interestingly, the PBM group revealed, in histological analysis, major tendon injury after 7 days; however, in the periods of 14 and 21 days, the PBM had a better repair process compared to the CG. In the quantification of collagen, there was no statistical difference between the groups in the 3 experimental periods. The findings suggest that the HFB and PBM treatments, isolated or associated, were effective in reducing the volume of the edema, stimulating the repair process. However, the use of HFB alone was more effective in promoting the tendon repair process. Thus, the present study consolidates previous studies of tendon repair with this new HFB. Future clinical trials will be needed to validate this proposal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Musculoskeletal injuries place a significant burden on society and can have a considerable impact on patient morbidity and mortality. Soft tissue injuries, including tendon and ligaments, account for 50% of these injuries [1]. Tendons are elongated structures, formed by dense connective tissue that transmit muscle contraction force to the bone tissue so that it can produce movement or simply maintain its posture. This tissue is formed predominantly of type I collagen, produced by tenocytes and has a triple helix structure that stretches when subjected to tensile strength (resistance to stretching), offering the tendon its mechanical properties [2].
Due to their complex highly organized structure, the tendons are able to withstand heavy loads and stress [3]. This organization allows the calcaneus tendon to carry the tension force of the gastrocnemius and soleus muscle to the calcaneus bone, generating plantar flexion movement. During a physical effort, this tension is multiplied and can cause injury [4, 5]. Thus, the evidence suggests that the tendon is susceptible to several traumas such as micro injuries or overuse (low energy traumas) and macro injuries (high energy traumas) that can cause acute, chronic injuries and/or partial/total rupture. Tendons that have a high load demand, such as the calcaneus tendon and are usually the most affected [6]. The injury occurs mainly in men between 30 and 50 years of age and affects professional athletes, as well as recreational athletes. It is associated with a high incidence (average of 18 out of 100,000 people), appearing among the most common orthopedic problems [7, 8].
The ideal treatment for acute ruptures has been debated for a long time, with surgical and non-surgical options presenting unique risks and benefits [9,10,11]. Some authors report that the recurrence of injury to the calcaneus tendon is lower when surgical treatment is performed. However, operative treatment poses a risk of complications such as infections [2, 12].
In this perspective, several strategies have been studied to obtain a minimally invasive approach and minimize surgical risks, such as the use of a fibrin biopolymer, which is a hemostatic agent that can be used in several types of operations [13]. The heterologous fibrin biopolymer (HFB), a genuinely Brazilian bioproduct, is composed of a cryoprecipitate extracted from buffalo blood (Bubalus bubalis) associated with a thrombin-like enzyme extracted from rattlesnake venom (Crotalus durissus terrificus). It is known that the thrombin-like enzyme, in the presence of calcium, acts on the fibrinogen molecule transforming it into fibrin monomers, forming a stable clot with adhesive, hemostatic, and sealant effects [14, 15], and more recently a scaffold [16,17,18,19] and drug delivery system [20].
As a result, a heterologous fibrin sealant can be used in surgical procedures as an efficient method to replace conventional sutures, making the process less invasive and can still control bleeding, thus reducing recovery time and increasing the success rate [15, 21]. Its effectiveness has been demonstrated in several tissues, for example, in the repair of surgical wounds [22] and cartilage [23], in the reduction of hematoma and complications in facelifts [24], in the treatment of the nervous system after injuries and illnesses [25], and in the treatment of tendon transection [14]. In addition, the heterologous fibrin biopolymer has several advantages such as lack of human viral transmission risk, a fast production process, low cost, potential to act as a scaffold for stem cells [16, 17], and as a new drug delivery system [20].
Still, a non-invasive resource that has been extensively investigated, and has shown effective results in the repair process, is the use of photobiomodulation therapy (PBMT). This therapy can be defined as the use of non-ionizing light sources for therapeutic purposes [26]. The action of PBMT is based on the absorption of light by the tissues, which results in an increased expression of adenosine triphosphate (ATP), molecular oxygen production, and transcription factors [27]. These effects can increase the synthesis of DNA, RNA, and regulatory proteins of the cell cycle, promoting cell proliferation [28]. In addition, PBM modulates the expression of some inflammatory mediators and stimulates angiogenic factors and collagen production, thus playing an essential role in the repair process [29]. Thus, this therapeutic modality has been used to stimulate tendon repair [30].
Despite the encouraging data on the potential effects of PBM and heterologous fibrin biopolymer on tissue repair, the association of these therapies in the treatment of tendon rupture is not yet fully understood. Thus, it is hypothesized that the association of fibrin biopolymer with PBM could stimulate the tendon repair process, which would culminate in the acceleration of this process. Therefore, the present study aimed to evaluate the effects of heterologous fibrin biopolymer and photobiomodulation treatments, associated or not, in the tendon repair process after 7, 14, and 21 days.
Material and methods
For the study, 84 Rattus norvegicus belonging to the Wistar strain, with 60 days of age (body weight 202.8 ± 27.2 g) were used. The animals were kept in a light/dark photoperiod of 12 h, with a temperature of 24 ± 1 °C, with free access to water and food. The present study was approved by the Animal Use Ethics Committee.
The sample calculation was performed based on data published in the literature to determine the number of animals used in the study [31, 32]. Thus, it determined that a total of 84 animals would be needed, randomized into 4 experimental groups:
The groups were subdivided, in a blinded manner, into 3 experimental periods: 7, 14, and 21 days, with 7 animals each.
Preparation of heterologous fibrin biopolymer
The HFB was kindly supplied by the Center for the Study of Venoms and Venomous Animals (CEVAP) from São Paulo State University (UNESP), São Paulo, Brazil. The product was supplied in three microtubes, namely fraction 1 (thrombin-like enzyme, 0.4 mL), fraction 2 (cryoprecipitated, 1 mL), and diluent (0.6 mL), that were stored at − 20 °C. Immediately before use, the components were previously thawed, the diluent was added to fraction 1 (syringe 1), and fraction 2 was added to another syringe (syringe 2), and then both were applied directly to the lesion site. The composition of the product, as well as its use, is described in detail in the patents BR 10 2014 011,432 7 [33] and BR 10 2014 011,436–0 [34], and in the publications by Ferreira Jr. et al. [15] and Buchaim et al. [20].
Experimental procedure
For the surgical procedure, the animals were previously anesthetized with an intraperitoneal injection of ketamine hydrochloride 80 mg/kg (Agener®) and xylazine hydrochloride at 10 mg/kg (Syntec®). Next, trichotomy and asepsis with 70% alcohol were performed on the right lower leg of each animal. Next, a longitudinal incision of approximately 2 cm was made in the animal’s skin to expose the calcaneus tendon. Subsequently, with a surgical microscope, partial transection of the tendon was performed.
Immediately after inducing the tendon lesion, 9 μL heterologous fibrin biopolymer was applied to each lesion in the animals of the HFB and HFB + PBM groups. Next, the skin was sutured with nylon thread (Shalon 5–0) and needle (1.5 cm) (Johnson & Johnson, St. Stevens-Woluwe, Belgium). The animals received analgesia (0.05 mg/kg of dornil) immediately after and within 5 days after surgery. The health status of the rats was monitored daily.
Photobiomodulation therapy protocol
Photobiomodulation, applied to the HFB and HFB + PBM groups, was performed with the laser 660 nm (InGaAlP) MMOpticsTM equipment. The device has been properly calibrated by the supplier. PBM laser applications were performed with the laser pen positioned at 90° and a punctual technique was used with contact, following the parameters in Table 1. It was started 24 h after the surgical procedure, and it was repeated every 24 h, totaling 6, 13, or 20 applications. The samples for histological analysis were collected 24 h after the last PBM application.
Edema analysis
The presence of edema in the injured paw was assessed using an adapted paw plethysmometer. This equipment records the volume of liquid displaced in a system of communicating vessels in response to the introduction of the animals’ paw in one of the system vats. For this, the animals’ right paw was inserted in the plethysmometer up to the knee joint, which caused the displacement of liquids to the other reservoir capable of quantifying the volumetric change in milliliters; thus, the equipment quantified the edema.
Edema analyses were performed at different times: The first assessment—(AV1)—was done immediately before the tendon transection; the second assessment—(AV2)—was done 24 h after the tendon transection; and the third assessment—(AV3)—was performed on the day of the animals’ euthanasia (after 7, 14, and 21 days) [35]. The initial edema value was calculated by AV2 − AV1. The final edema value was calculated by AV3 − AV1.
Sample collection
After each experimental period, 7, 14, and 21 days, the animals were euthanized by an overdose of ketamine anesthesia (240 mg/kg) and xylazine (30 mg/kg) for subsequent dissection of the calcaneus tendon. The tendon samples were washed 3 to 4 times in saline solution and any type of peripheral tissue and other visible artifacts were removed. After collection, the pieces remained in 10% formaldehyde solutions for fixation for 24 h. They were then washed in running water for 24 h. Subsequently, they underwent dehydration in an increasing 70%, 90%, and 100% ethyl alcohol solution. After dehydration baths, the diaphanization of the pieces was started in an alcohol/xylol solution (1: 1) and then in 2 pure xylol baths. Next, the pieces were embedded in paraffin and cut using a Spencer 820 rotating microtome with 5-μm-thick serial cuts. The cuts were made in the longitudinal plane. From the cuts obtained, slides stained with hematoxylin–eosin (HE) were made to perform the histopathological analysis: Masson’s trichrome to quantify collagen fibers.
Histopathological analyses
The histopathological analysis of the partial transection region in the calcaneus tendon was performed using slides stained with HE using a light microscope (Olympus, Optical Co. Ltd, Tokyo, Japan) at an increase of × 100. The slides were blindly classified by two experienced professionals, according to the Bonar score, adapting the recommendations of Fearon et al. [36].
Thus, the evaluated parameters included the arrangement of the collagen fibers, cellular morphology, cellularity, vascularization, and accumulation of the extracellular matrix. Each parameter was classified on a 4-point scale (from 0 to 3), being 0 = normal; 1 = slightly abnormal; 2 = abnormal; and 3 = markedly abnormal. The classification of the tissue alteration of the calcaneus tendon of each animal was considered the sum of the scores of each of the four categories evaluated. Consequently, the highest possible score value is 12 (twelve) for a tendon with maximum pathological changes.
Quantification of blood vessels
The quantification of blood vessels in the tendon transection region was performed using slides stained with HE. Twelve different fields of the lesion were photographed using an optical microscope (Olympus, Optical Co., Ltd, Tokyo, Japan) with a digital camera attached (Sony DSCs75, Tokyo, Japan) in an increase of × 100. The images were analyzed using the ImageJ software, version 1.8 (National Institute of Health in Bethesda), in which all blood vessels were considered. Quantification was performed by two experienced evaluators; then, the results were compared, and the mean of blood vessels was obtained.
Collagen quantification
Collagen quantification was performed using slides stained with Masson’s trichrome. The region of the tendon transection was photographed in 6 different fields using an optical microscope (Olympus, Optical Co., Ltd, Tokyo, Japan). The images were obtained in a × 100 magnification with a digital camera attached to the microscope (Sony DSCs75, Tokyo, Japan).
First, the images were loaded into the MATLAB R2019a software (MathWorks, Inc., Natick, MA, USA) and separated into its three-color channels (red—R, green—G, and blue—B). The ratios between the blue and red (B/R) and green and red (G/R) channels are computed. Two masks were generated from the threshold of 15% of the highest intensity observed in the blue channel. The two masks were then combined using the AND digital logic gate, so the white region represents areas of collagen with black in the background.
Next, the number of black pixels in each image was used to calculate the percentage of the image area that corresponded to the collagen. The number of pixels was determined and expressed as a total percentage of collagen. The analysis was adapted from Quinn et al. [37].
Statistical analysis
The results obtained were subjected to the Kolmogorov–Smirnov test to verify whether they had a normal distribution, then subjected to the two-way analysis of variance test (two-way ANOVA) to indicate possible differences between treatments and experimental periods and the Tukey test HSD to find the differences with a 5% significance level. The statistical analysis was performed with the aid of the SPSS 19 program (SPSS Inc., Chicago, IL, USA).
Results
Volume of edema
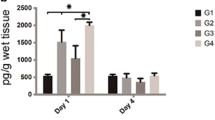
The results of the edema analysis are shown in Figs. 1 and 2. The volume of initial edema observed 24 h after the induction of partial tendon transection was verified in all experimental periods, 7, 14, and 21 days (Fig. 1).
Still, the analysis of the final edema volume, after the experimental periods (7, 14, and 21 days), pointed out that there was an interaction between the treatments and the experimental periods (p = 0.003), as well as a difference between treatments (p < 0.001) and experimental periods (p < 0.001). Thus, it was observed that the control group had greater edema than the other groups in the three periods evaluated. Furthermore, the statistical analysis revealed that the HFB + PBM group exhibited a greater volume of edema compared to the HFB group after 21 days (Fig. 2).
In addition, when we evaluated the different periods, a significant difference was observed in the GC and HFB groups. The volume of edema on the 7th and 14th days were higher when compared to the 21st-day period. For the PBM group, a significant decrease in the edema volume was verified only when comparing the periods 7 to 21 days. In the HFB + PBM group, no statistical differences were observed between different experimental periods (Table 2).
Histopathological analysis
The histological evaluation showed that after 7 days, the control group presented an extracellular matrix (ECM) with the presence of edema, containing few neoformed blood vessels and a moderate inflammatory infiltrate. The fibroblasts were grouped in bundles randomly arranged in different orientations (Fig. 3A). The HFB group also observed ECM swollen with a slight presence of inflammatory cells and with fibroblasts arranged in poorly organized bundles and few newly formed bloods vessels (Fig. 3B). The PBM group presented ECM with edema and many newly formed blood vessels and an intense presence of inflammatory cells and randomly arranged fibroblasts (Fig. 3C). The HFB + PBM group demonstrated ECM with edema and few newly formed blood vessels, inflammatory cells, and fibroblasts arranged in bundles parallel to the large axis of the tendon (Fig. 3D).
After 14 days of partial tendon transection, the control group showed a marked reduction in inflammatory activity; few neoformed blood vessels and fibroblasts were arranged in poorly organized bundles in an ECM that was still loose and swollen (Fig. 3E). The HFB group revealed a denser ECM with newly formed blood vessels, absence of inflammatory cells and intense presence of fibroblasts organized in bundles parallel to the great axis of the tendon (Fig. 3F). Likewise, the PBM group had a denser ECM with numerous neoformed blood vessels and fibroblasts arranged in parallel bundles (Fig. 3G). In addition, the HFB + PBM group demonstrated a dense ECM with newly formed blood vessels and intense proliferation of fibroblasts, arranged in bundles parallel to the large axis of the tendon (Fig. 3H).
After 21 days of treatment, it was observed that the calcaneus tendon did not yet present a full repair process in all experimental groups. In the control group, numerous fibroblasts were still arranged in bundles with different orientations and the ECM showed areas of edema (Fig. 3I). In contrast, the HFB group revealed a dense ECM with fibroblastic proliferation with better organization when compared to the CG (Fig. 3J). Likewise, the PBM group exhibited dense ECM with fibroblasts arranged in parallel and compact to the groups (Fig. 3K). Also, in the HFB + PBM group, the dense ECM had the presence of fibroblasts organized in parallel bundles (Fig. 3L).
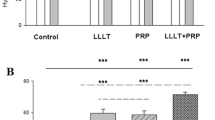
According to the above, when applying the Bonar score, it was observed that there was an interaction between treatments and experimental periods (p < 0.0001). On the 7th day, there was a statistical difference between the control group and all treated groups. It was also possible to observe that the PBM showed a higher score when compared to the HFB and HFB + PBM groups. After 14 days, the CG had a higher score on the Bonar analysis compared to the other groups. However, after 21 days, the CG had a higher score when compared to the HFB, PBM, and HFB + PBM groups (Fig. 4).
In addition, observing the different experimental periods, it was noted that all groups showed statistical differences when comparing the period of 7 and 21 days. Additionally, the control group showed a statistical difference only when comparing the period of 14 and 21 days. Still, the PBM and HFB + PBM groups showed a decrease in the Bonar score, that is, a higher score on the 7th and 14th days compared to the 14th and 21st day, respectively (Table 3).
Quantification of blood vessels
In the analysis of blood vessels, it can be noted that there was an interaction between treatments and experimental periods (p = 0.002). Likewise, the effect of treatments (p < 0.001) and experimental periods (p = 0.043) was evidenced. Thus, it was detected that the control group had a lower number of vessels when compared to the PBM and HFB + PBM groups in the period of 7 days. Still, in the same experimental period, it was shown that the PBM group had a higher number of blood vessels compared to the HFB group. After 14 and 21 days, the statistical analysis showed that the PBM group had statistically higher numbers of blood vessels than the other groups (Fig. 5).
For the comparison of the experimental periods, the HFB and PBM groups showed statistical differences between the 7 and 14 days, that is, with the largest experimental period, the number of blood vessels increased. Still, the HFB + PBM group had a higher number of blood vessels on the 7th day when compared to the 21st day.
Collagen quantification
In the collagen quantification, it was not possible to observe the interaction between treatments and experimental periods (p = 0.956), as well as no difference between treatments (p = 0.254). Only differences between the experimental periods were observed (p < 0.001), as can be seen in Fig. 6. It is possible to observe that the increased collagen over time occurred independently of the treatment group (p < 0.001) (Tables 4 and 5).
Discussion
Calcaneus tendon rupture is a frequent injury with increasing incidence. The ideal treatment strategy for acute tendon rupture remains controversial since surgical and non-surgical methods have distinct advantages and disadvantages, which has culminated doubts about what treatment is the most appropriate or efficacious [9, 38, 39]. Thus, the present study investigated the application of HFB associated with photobiomodulation, or not, in partial transection of the calcaneus tendon. After 24 h of the partial transection of the tendon, the edema analysis was performed. It is known that after an injury or surgical procedures, an inflammatory response is expected, so this process is performed in an attempt to perform local phagocytosis and repair damaged tissues. This inflammatory process is most strongly manifested during the first 4–6 h after trauma and inflammatory mediators such as prostaglandins, leukotrienes, bradykinin, and others are released and, consequently, there is an increase in dilation and vascular permeability, resulting in edema [40, 41]. Thereby, the results of the edema volume evaluation showed that there was no statistical difference between the experimental groups 24 h after the injury induced. These findings evidence that partial transection of the tendon used promoted a similar inflammatory process in all experimental groups.
Therefore, when the effect of treatments on edema reduction was investigated, it was observed that the HFB and PBM groups used alone or in combination were effective in reducing edema when compared to the control group, regardless of the experimental period. In addition, the HFB group showed a greater reduction in edema volume compared to the group that associated HFB + PBM after 21 days.
Many studies have suggested the advantages of the biomodulatory effects of PBM on the inflammatory process [42,43,44]. Albertini et al. [45] have demonstrated that PBM with InGaAlP (650 nm) can reduce edema caused by carrageenan inflammation in rat paw. Similarly, Marcos et al. [46] found that PBM administered with an energy dose of 3 J significantly reduced edema formation compared to no treatment. The effects of photobiomodulation in reducing edema may be related to its ability to activate cells such as lymphocytes and stimulate lymphatic flow, as well as reduced COX-2 gene expression and COX-2-derived PGE2 production, and vascular permeability which could modulate the inflammatory process [44, 47, 48]. Similarly, some studies have demonstrated fibrin biopolymer on the inflammatory process [49,50,51]. Giodano et al. [24] detected a significant decrease in edema in patients treated with fibrin biopolymer. These results could be related to the fact that fibrin biopolymer is a hemostatic agent composed of fibrinogen and thrombin and its interaction mimics the coagulation cascade, forming a stable and adhesive clot [17, 52]. The hemostatic, adhesive, and sealing properties of fibrin biopolymer can reduce postoperative bleeding and ecchymosis by sealing capillaries and reducing postoperative swelling [53]. Furthermore, the fibrin biopolymer forms a complex fibrin network that can serve as a framework, as it has already been shown to promote cell migration over its highly reticulated structure, in addition to allowing cell proliferation without structural deformation to occur [54].
In this context, several studies have shown that the fibrin biopolymer had a positive effect on the tendon repair process [55,56,57]. Using a new heterologous fibrin biopolymer, Frauz et al. [14] demonstrated that this therapy is a good treatment option due to its effectiveness in promoting tendon repair. Still, Ferraro et al. [55, 58] developed two studies in 2005 which evaluated the effect of heterologous fibrin biopolymer on the tendon of dogs. The first study assessed the strength of the repaired tendon tissue, and the second study verified its ability to promote repair. In both studies, the animals were divided into three groups evaluated after 7, 15, and 30 days. Regarding the strength of the newly formed tendon tissue, a progressive increase in resistance to obtain maximum strength and permanent traction and deformations was allowed. In the second study, it was found that the use of biopolymer modulates inflammation after 7 days, facilitating the maturation stages of the tendon repair process.
These findings are in agreement with the results of the present study, in which the histological analysis revealed that the HFB and HFB + PBM groups had a lower score when compared to the control group in the three experimental periods, demonstrating a better tendon repair process. However, when the number of blood vessels was evaluated, the HFB group did not show a statistical difference compared to the control group in any evaluated period. Such findings are supported by the literature; unlike other highly vascularized tissues, such as skin or bone, neovascularization after injury is not necessarily a hallmark of functional tissue repair. Instead, it is associated with degeneration as healthy tendons are generally poorly vascularized with relatively few cells embedded in an abundant collagen matrix [59]. Thus, it is possible to suggest that the heterologous fibrin biopolymer is capable of stimulating tendon repair.
Interestingly, the PBM group presented histological findings compatible with a greater tendon injury when compared to the other groups after 7 days of tendon transection. However, after 14 and 21 days, the PBM group showed less tendon injury when compared to the control group. Likewise, the PBM group had a higher number of blood vessels when compared to the other groups in the 3 periods evaluated. In view of these results, it is possible to suggest that the parameters used for photobiomodulation were not efficient to promote tendon repair after 7 days. These results are supported by Arndt Schulz’s law where a very low dose of light has no effect, and a slightly higher dose has a positive effect until a plateau is reached. However, if the light dose is increased beyond that point, the benefit will progressively decrease until the baseline is reached (no effect), and further increases will begin to have inhibitory effects on the tissue. This behavior of light in tissues is also known as the dose-dependent effect [60, 61]. Still, for Sommer et al. [62], the stimulating effect of photobiomodulation on biological tissues is dependent on the wavelength, energy, irradiation area, total irradiation time, and the energy density. Thus, it is possible to suggest that, in the parameters used in the present study, photobiomodulation promoted an effect dependent on the dose and frequency of treatment, that is, the stimulatory effect of photobiomodulation was only observed after 14 and 21 days of treatment. This effect may be related to the low energy used in the present study, thus requiring several treatment sessions to achieve a stimulatory effect. Similarly, Martignango et al. [63] demonstrated that the effects of photobiomodulation were dependent on the number of days the animals received the treatment, with 5 consecutive applications of PBM not being efficient in promoting tissue viability, while 8 consecutive applications of PBM promoted greater tissue viability.
Interestingly, the group that associated the treatments, HFB + PBM, promoted a better repair process only when compared to the control group. When the HFB + PBM group was compared to the use of therapies alone (HFB and PBM), there was no statistical difference between the groups. Thus, the present study demonstrated that HFB (associated or not with PBM) was able to stimulate tendon repair. Interestingly, the addition of PBM to the use of HFB in the treatment of tendon injuries did not optimize the repair process. On the other hand, the heterologous fibrin biopolymer has already been associated with photobiomodulation to stimulate the morphofunctional repair of the facial nerve [64] and during the reconstruction of lesions in long bones [65, 66]. The authors demonstrated the great potential of the association of heterologous fibrin biopolymer and photobiomodulation to assist the repair process of the peripheral nerve as well as in bone reconstruction.
Still, it is well-known that the response to tendon injury can be divided into three overlapping stages. In the inflammatory phase, a fibrin clot is formed to provide temporary support where inflammatory cells phagocytize the lesion site and tenocytes are recruited to the injured area. In the second stage, known as the proliferative or repair stage, tenocytes deposit a mechanically inferior temporary matrix composed mainly of collagen III. Then, in the third and last stage, known as the remodeling phase, collagen I synthesis begins to dominate, and the extracellular matrix (ECM) becomes more aligned. This phase starts 1–2 months after the injury and can last for more than a year [67]. Given the above, it is possible to suggest that the present study investigated the inflammatory and proliferative phases of the repair process which may be directly related to the amount of collagen presented in the experimental groups.
Taken together, the results showed that HFB is a unique fibrin biopolymer produced using heterologous blood derivatives, and it is a promising treatment for acute tendon rupture since it is a product that does not present a risk of viral transmission, possesses a fast production process, is low cost, and allows anatomical reconstruction with less soft tissue involvement. PBM also has a potential therapeutic resource in the treatment of tendon injuries. However, the combination of both treatments did not promote an extra significant effect on the tendon. Since the present study was limited to investigating the inflammatory and proliferative phases, information on the influence of therapies on the remodeling phase has yet to be provided. In addition, other photobiomodulation parameters should be investigated.
Conclusion
The findings suggest that the treatments of heterologous fibrin biopolymer and photobiomodulation, isolated or associated, were effective in reducing the volume of the edema, preventing degenerative morphological alterations of the tendon observed in the reduction of the Bonar score, providing positive contributions to the regenerative process. However, the use of HFB alone was most effective in promoting the tendon repair process. These data consolidate previous tendon repair studies with this new HFB and highlight the potential of this therapy to be used as a promising effective therapeutic strategy for the treatment of partial injury of the calcaneus tendon. HFB was recently evaluated and approved in a phase I/II clinical safety study [68]. Still, future clinical trials will be necessary to validate this proposal.
References
James R, Kesturu G, Balian G, Chhabra AB (2008) Tendon biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am 33:102–112. https://doi.org/10.1016/j.jhsa.2007.09.007
Andarawis-Puri N, Flatow EL, Soslowsky LJ (2015) Tendon basic science: development, repair, regeneration, and healing. J Orthop Res 33:780–784. https://doi.org/10.1002/jor.22869
Thorpe CT, Peffers MJ, Simpson D et al (2016) Anatomical heterogeneity of tendon fascicular and interfascicular tendon compartments have distinct proteomic composition. Sci Rep 6:1–12. https://doi.org/10.1038/srep20455
Bogaerts S, Desmet H, Slagmolen P, Peers K (2016) Strain mapping in the Achilles tendon-a systematic review. J Biomech 49:1411–1419. https://doi.org/10.1016/j.jbiomech.2016.02.057
Egger AC, Berkowitz MJ (2017) Achilles tendon injuries. Curr Rev Musculoskelet Med 10:72–80. https://doi.org/10.1007/s12178-017-9386-7
Karaaslan F, Mermerkaya MU, Çıraklı A et al (2016) Surgical versus conservative treatment following acute rupture of the Achilles tendon: is there a pedobarographic difference? Ther Clin Risk Manag 12:1311–1315. https://doi.org/10.2147/TCRM.S116385
Zhao J-G, Meng X-H, Liu L et al (2017) Early functional rehabilitation versus traditional immobilization for surgical Achilles tendon repair after acute rupture: a systematic review of overlapping meta-analyses. Sci Rep 7:39871. https://doi.org/10.1038/srep39871
Lawrence JE, Nasr P, Fountain DM et al (2017) Functional outcomes of conservatively managed acute ruptures of the Achilles tendon. Bone Joint J 99:87–93. https://doi.org/10.1302/0301-620X.99B1.BJJ-2016-0452.R1
Deng S, Sun Z, Zhang C et al (2017) Surgical treatment versus conservative management for acute Achilles tendon rupture: a systematic review and meta-analysis of randomized controlled trials. J Foot Ankle Surg 56:1236–1243. https://doi.org/10.1053/j.jfas.2017.05.036
Meulenkamp B, Stacey D, Fergusson D et al (2018) Protocol for treatment of Achilles tendon ruptures a systematic review with network meta-analysis. Syst Rev 7:1–7. https://doi.org/10.1186/s13643-018-0912-5
Stavenuiter XJR, Lubberts B, Prince RM et al (2019) Postoperative complications following repair of acute Achilles tendon rupture. Foot Ankle Int 40:679–686. https://doi.org/10.1177/1071100719831371
Ochen Y, Beks RB, Van Heijl M et al (2019) Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ 7(364):k5120. https://doi.org/10.1136/bmj.k5120
Kryukova AE, Shpichka AI, Konarev PV et al (2018) Shape determination of bovine fibrinogen in solution using small-angle scattering data. Crystallogr Reports 63:871–873. https://doi.org/10.1134/S1063774518060202
Frauz K, Teodoro L, Carneiro G et al (2019) Transected tendon treated with a new fibrin sealant alone or associated with adipose-derived stem Cells. Cells 8:56. https://doi.org/10.3390/cells8010056
Ferreira RS, de Barros LC, Abbade LPF et al (2017) Heterologous fibrin sealant derived from snake venom: from bench to bedside-an overview. J Venom Anim Toxins Incl Trop Dis 23:1–12. https://doi.org/10.1186/s40409-017-0109-8
Gasparotto VPO, Landim-Alvarenga FC, Oliveira ALR et al (2014) A new fibrin sealant as a three-dimensional scaffold candidate for mesenchymal stem cells. Stem Cell Res Ther 5(3):78. https://doi.org/10.1186/scrt467
Orsi PR, Landim-Alvarenga FC, Justulin LA et al (2017) A unique heterologous fibrin sealant (HFS) as a candidate biological scaffold for mesenchymal stem cells in osteoporotic rats. Stem Cell Res Ther 8:1–14. https://doi.org/10.1186/s13287-017-0654-7
Cassaro CV, Justulin Jr. LA, Lima PR de, et al (2019) Fibrin biopolymer as scaffold candidate to treat bone defects in rats. J Venom Anim Toxins Incl Trop Dis 25:. https://doi.org/10.1590/1678-9199-jvatitd-2019-0027
Creste CFZ, Orsi PR, Landim-Alvarenga FC et al (2020) Highly effective fibrin biopolymer scaffold for stem cells upgrading bone regeneration. Materials (Basel) 13:2747. https://doi.org/10.3390/ma13122747
Buchaim DV, Cassaro CV, Shindo JVTC, et al (2019) Unique heterologous fibrin biopolymer with hemostatic, adhesive, sealant, scaffold and drug delivery properties: a systematic review. J Venom Anim Toxins Incl Trop Dis 25 https://doi.org/10.1590/1678-9199-jvatitd-2019-0038
He M, Gan AWT, Lim AYT et al (2013) The effect of fibrin glue on tendon healing and adhesion formation in a rabbit model of flexor tendon injury and repair. J Plast Surg Hand Surg 47:509–512. https://doi.org/10.3109/2000656X.2013.789037
Rahal SC, Amaral MSP, Pai VD, et al (2004) Effect of fibrin glue derived from snake venom on the viability of autogenous split-thickness skin graft. J Venom Anim Toxins Incl Trop Dis 10.https://doi.org/10.1590/S1678-91992004000200006
de Barros CN, Miluzzi Yamada AL, Junior RSF et al (2016) A new heterologous fibrin sealant as a scaffold to cartilage repair—experimental study and preliminary results. Exp Biol Med 241:1410–1415. https://doi.org/10.1177/1535370215597192
Giordano S, Koskivuo I, Suominen E, Veräjänkorva E (2017) Tissue sealants may reduce haematoma and complications in face-lifts: a meta-analysis of comparative studies. J Plast Reconstr Aesthetic Surg 70:297–306. https://doi.org/10.1016/j.bjps.2016.11.028
Biscola NP, Cartarozzi LP, Ulian-Benitez S et al (2017) Multiple uses of fibrin sealant for nervous system treatment following injury and disease. J Venom Anim Toxins Incl Trop Dis 23:1–11. https://doi.org/10.1186/s40409-017-0103-1
Vaghardoost R, Momeni M, Kazemikhoo N et al (2018) Effect of low-level laser therapy on the healing process of donor site in patients with grade 3 burn ulcer after skin graft surgery (a randomized clinical trial). Lasers Med Sci 33:603–607. https://doi.org/10.1007/s10103-017-2430-4
De Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22:1–37. https://doi.org/10.1109/JSTQE.2016.2561201
Hamblin MR (2018) Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol 94:199–212. https://doi.org/10.1111/php.12864
Tim CR, Bossini PS, Kido HW et al (2016) Low-level laser therapy induces an upregulation of collagen gene expression during the initial process of bone healing: a microarray analysis. J Biomed Opt 21:088001. https://doi.org/10.1117/1.jbo.21.8.088001
Naterstad IF, Rossi RP, Marcos RL et al (2018) Comparison of photobiomodulation and anti-inflammatory drugs on tissue repair on collagenase-induced Achilles tendon inflammation in rats. Photomed Laser Surg 36:137–145. https://doi.org/10.1089/pho.2017.4364
Ferreira R, Silva R, Folha RA et al (2015) Achilles tendon vascularization of proximal, medial, and distal portion before and after partial lesion in rats treated with phototherapy. Photomed Laser Surg 33:579–584. https://doi.org/10.1089/pho.2015.3974
Guerra FDR, Vieira CP, dos Santos de Almeida M, et al (2014) Pulsed LLLT improves tendon healing in rats: a biochemical, organizational, and functional evaluation. Lasers Med Sci 29:805–811. https://doi.org/10.1007/s10103-013-1406-2
Ferreira Junior R, Barraviera B (2014) Arcabouço tridimensional para células tronco, processo de obtenção do mesmo e seu uso
Ferreira Junior R, Barraviera B, Barraviera S (2014) Selante de fibrina para uso tópico, método de formação do mesmo e seu uso
Karvat J, Antunes JS, Bernardino GR et al (2014) Effect of low-level LASER and neural mobilization on nociceptive threshold in experimental sciatica. Rev Dor 15:207–210. https://doi.org/10.5935/1806-0013.20140045
Fearon A, Dahlstrom JE, Twin J et al (2014) The Bonar score revisited: region of evaluation significantly influences the standardized assessment of tendon degeneration. J Sci Med Sport 17:346–350. https://doi.org/10.1016/j.jsams.2013.07.008
Quinn KP, Golberg A, Broelsch GF et al (2015) An automated image processing method to quantify collagen fibre organization within cutaneous scar tissue. Exp Dermatol 24:78–80. https://doi.org/10.1111/exd.12553
Khan RJ, Carey Smith RL (2010) Surgical interventions for treating acute Achilles tendon ruptures. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd003674.pub4
Wilkins R, Bisson LJ (2012) Operative versus nonoperative management of acute Achilles tendon ruptures: a quantitative systematic review of randomized controlled trials. Am J Sports Med 40:2154–2160. https://doi.org/10.1177/0363546512453293
Aller MA, Arias JL, Sánchez-Patán F, Arias J (2006) The inflammatory response: an efficient way of life. Med Sci Monit 12:225–234
Arias JI, Aller MA, Arias J (2009) Surgical inflammation: a pathophysiological rainbow. J Transl Med 7:1–15. https://doi.org/10.1186/1479-5876-7-19
Tim CR, Bossini PS, Kido HW et al (2016) Effects of low level laser therapy on inflammatory and angiogenic gene expression during the process of bone healing: a microarray analysis. J Photochem Photobiol B Biol 154:8–15. https://doi.org/10.1016/j.jphotobiol.2015.10.028
Pallotta RC, Bjordal JM, Frigo L et al (2012) Infrared (810-nm) low-level laser therapy on rat experimental knee inflammation. Lasers Med Sci 27:71–78
Boschi ES, Leite CE, Saciura VC et al (2008) Anti-inflammatory effects of low-level laser therapy (660 nm) in the early phase in carrageenan-induced pleurisy in rat. Lasers Surg Med 40:500–508. https://doi.org/10.1002/lsm.20658
Albertini R, Aimbire FS, Correa F et al (2004) Effects of different protocol doses of low power gallium–aluminum–arsenate (Ga–Al–As) laser radiation (650 nm) on carrageenan induced rat paw ooedema. J Photochem Photobiol B Biol 74:101–107. https://doi.org/10.1016/j.jphotobiol.2004.03.002
Marcos RL, Leal Junior ECP, de Moura MF et al (2011) Infrared (810 nm) low-level laser therapy in rat Achilles tendinitis: a consistent alternative to drugs. Photochem Photobiol 87:1447–1452. https://doi.org/10.1111/j.1751-1097.2011.00999.x
Aimbire F, Albertine R, De Magalhães RG et al (2005) Effect of LLLT Ga-Al-As (685 nm) on LPS-induced inflammation of the airway and lung in the rat. Lasers Med Sci 20:11–20. https://doi.org/10.1007/s10103-005-0339-9
Albertini R, Aimbire FSC, Correa FI et al (2004) Effects of different protocol doses of low power gallium-aluminum-arsenate (Ga-Al-As) laser radiation (650 nm) on carrageenan induced rat paw ooedema. J Photochem Photobiol B Biol 74:101–107. https://doi.org/10.1016/j.jphotobiol.2004.03.002
Mooney E, Loh C, Pu LLQ (2009) The use of fibrin glue in plastic surgery. Plast Reconstr Surg 124:989–992. https://doi.org/10.1097/PRS.0b013e3181b039a3
Marchac D, Sa’ndor GKB, (1994) Face lifts and sprayed fibrin glue: an outcome analysis of 200 patients. Br J Plast Surg 47:306–309. https://doi.org/10.1016/0007-1226(94)90087-6
Fredricks S (2001) Comment on zones of adherence: role in minimizing and preventing contour deformities in liposuction. Plast Reconstr Surg 108:2100. https://doi.org/10.1097/00006534-200112000-00044
Lee KC, Park SK, Lee KS (1991) Neurosurgical application of fibrin adhesive. Yonsei Med J 32:53–57
Yu MS, Jung MS, Kim BH et al (2018) Aerosolized fibrin sealant is effective for postoperative edema and ecchymosis in open rhinoplasty without osteotomy. J Oral Maxillofac Surg 76:2000.e1-2000.e8. https://doi.org/10.1016/j.joms.2018.05.019
Yamada Y, Boo JS, Ozawa R et al (2003) Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J Cranio-Maxillofacial Surg 31:27–33. https://doi.org/10.1016/S1010-5182(02)00143-9
Ferraro GC, Moraes JR, Shimano AC et al (2005) Effect of snake venom derived fibrin glue on the tendon healing in dogs: clinical and biomechanical study. J Venom Anim Toxins Incl Trop Dis 11:261–274. https://doi.org/10.1590/s1678-91992005000300005
Solakoǧlu C, Mahiroǧullari M, Çakmak S et al (2010) Fibrin sealant in the treatment of acute ruptures of the Achilles tendon: long-term results. Eklem Hast ve Cerrahisi 21:124–129
Ferguson REH, Rinker B (2006) The use of a hydrogel sealant on flexor tendon repairs to prevent adhesion formation. Ann Plast Surg 56:54–58. https://doi.org/10.1097/01.sap.0000181666.00492.0e
Ferraro GC, Moraes JRE, Pereira GT, et al (2005) Clinical and morphological evaluation of snake venom derived fibrin glue on the tendon healing in dogs. J Venom Anim Toxins Incl Trop Dis 11:.https://doi.org/10.1590/S1678-91992005000400005
Tempfer H, Traweger A (2015) Tendon vasculature in health and disease. Front Physiol 18(6):330. https://doi.org/10.3389/fphys.2015.00330
Hamblin MR, Huang YY, Sharma SK, Carroll J (2011) Biphasic dose response in low level light therapy-an update. Dose-Response 9:602–618. https://doi.org/10.2203/dose-response.11-009.Hamblin
Zein R, Selting W, Hamblin MR (2018) Review of light parameters and photobiomodulation efficacy: dive into complexity. J Biomed Opt 23:1. https://doi.org/10.1117/1.jbo.23.12.120901
Sommer AP, Pinheiro ALB, Mester AR et al (2001) Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA’s light-emitting diode array system. J Clin Laser Med Surg 19:29–33. https://doi.org/10.1089/104454701750066910
Martignago CCS, Tim CR, Assis L et al (2019) Comparison of two different laser photobiomodulation protocols on the viability of random skin flap in rats. Lasers Med Sci 34:1041–1047. https://doi.org/10.1007/s10103-018-2694-3
de Rosso MP, O, Rosa Júnior GM, Buchaim DV, et al (2017) Stimulation of morphofunctional repair of the facial nerve with photobiomodulation, using the end-to-side technique or a new heterologous fibrin sealant. J Photochem Photobiol B Biol 175:20–28. https://doi.org/10.1016/j.jphotobiol.2017.08.023
de Oliveira Rosso MP, Oyadomari AT, Pomini KT et al (2020) Photobiomodulation therapy associated with heterologous fibrin biopolymer and bovine bone matrix helps to reconstruct long bones. Biomolecules 10:1–17. https://doi.org/10.3390/biom10030383
de Oliveira Gonçalves JB, Buchaim DV, de Souza Bueno CR et al (2016) Effects of low-level laser therapy on autogenous bone graft stabilized with a new heterologous fibrin sealant. J Photochem Photobiol B Biol 162:663–668. https://doi.org/10.1016/j.jphotobiol.2016.07.023
Voleti PB, Buckley MR, Soslowsky LJ (2012) Tendon healing: repair and regeneration. Annu Rev Biomed Eng 14:47–71. https://doi.org/10.1146/annurev-bioeng-071811-150122
Abbade LPF, Barraviera SRCS, Silvares MRC, Lima ABB de CO, Haddad GR, Gatti MAN, Medolago NB, Carneiro MTR, dos Santos LD, Ferreira RS, Barraviera B (2021) Treatment of chronic venous ulcers with heterologous fibrin sealant: A phase I/II clinical trial. Frontiers in Immunology 12
Acknowledgements
Special thanks are due to Aristides Pavan of Fazenda Céu Azul, Pereiras, SP, Brazil; the Center for the Study of Venoms and Venomous Animals (CEVAP) of São Paulo State University (UNESP), Guilherme Shin Iwamoto Haga, for enabling the publication of this paper.
Funding
The present study was supported by the National Council for Scientific and Technological Development, CNPq, Proc. No. 563582/2010–3 (BB) and CNPq Proc. No. 401170/2013–6 (BB); and the Coordination for the Improvement of Higher Education Personnel, CAPES, through Toxinology CAPES Call No. 063/2010, Proc. No. 23038.006285/2011–21, AUXPE Toxinology 1219 (BB). RSF Jr. is a CNPq PQ1C fellow researcher No. 303224/2018–5, FAPESP grant nº 2017/21851-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The present study was approved by the Ethics Committee on the Use of Animal under number 0326/2019 and conducted according to the international norms of ethics on animal experimentation (National Research Council, 1996).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Freitas Dutra Júnior, E., Hidd, S.M.C.M., Amaral, M.M. et al. Treatment of partial injury of the calcaneus tendon with heterologous fibrin biopolymer and/or photobiomodulation in rats. Lasers Med Sci 37, 971–981 (2022). https://doi.org/10.1007/s10103-021-03341-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03341-x