Abstract
This randomized placebo-controlled trial evaluates the impact of photobiomodulation (PBMT) on the salivary flow and biochemistry of patients with chronic kidney disease (CKD) on hemodialysis. Forty-four patients on hemodialysis self-responded two questionnaires for oral health and salivary gland function perception. The subjects were evaluated for function of salivary glands and randomly allocated to two groups: PBMT group (three irradiations at 808 nm, 100 mW, 142 J/cm2, and 4 J per site); and placebo group. Patients were submitted to non-stimulated and stimulated sialometry and after the treatment at baseline and 14 days. Salivary volume and biochemical of the saliva were analyzed. At baseline, most subjects had self-perception of poor oral health (52.6%) and salivary dysfunction (63.1%). Clinical exam revealed that 47.3% of subjects presented dry mucosa. PBMT promoted increase of the non-stimulated (p = 0.027) and stimulated saliva (p = 0.014) and decrease of urea levels in both non-stimulated (p = 0.0001) and stimulated saliva (p = 0.0001). No alteration was detected in total proteins and calcium analysis. Patients with kidney disease can present alteration in flow, concentrations, and composition of saliva, affecting oral health, but our findings suggest that PBMT is effective to improve hyposalivation and urea levels in saliva of patients with CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is characterized by the progressive and permanent reductions of renal function due to a reduction in the glomerular filtrate rate. It is a worldwide public health problem and its incidence has been increasing worldwide, based on aging of the population and the higher prevalence of diabetes and hypertension. In Brazil, it is estimated that there are 10 to 15 million people with some degree of CKD, considering an estimated prevalence of 50 cases/100,000 inhabitants. Depending on the level of renal impairment, it can be classified into the five progressive stages according to the Kidney Disease Outcomes Quality Initiative (K/DOQI). The last one is called end-stage renal disease and these patients need hemodialysis or kidney transplant. In the world, there are around 1.8 million patients in this condition [1,2,3,4,5].
Renal function is essential for the preservation of life, since the kidneys are essential to homeostasis. They eliminate undesirable products of metabolism, as well as maintain the extracellular concentration of potassium and the plasma levels of the other electrolytes constant. Besides, renal function plays a key role in regulating blood pressure by the renin-angiotens in system and endocrine functions, such as the production of erythropoietin, as well as in mineral and bone disorders (MBD) by the synthesis of the active form of vitamin [6]. In general, patients with end-stage renal disease, especially those on hemodialysis, show a wide range of clinical symptoms and signs, including biochemical changes such as hyperkalemia, hyperphosphatemia, and hypocalcaemia and hormonal disturbances like secondary hyperparathyroidism and low activity of 1,25(OH) vitamin D [2]. In addition, CKD can directly or indirectly affect flow, concentration, and composition of saliva. Hemodialysis can effectively minimize most of these complications to some extent [6]. Based on the prevalence and impact of CKD on patients’ quality of life and public health care, management and support, an increasing health concern about management and support to patients has arisen [7].
Oral health can also be affected by CKD and dialysis. Some studies indicate that up to 90% of renal patients have some oral alterations that include uremic odor, uremic stomatitis, gingivitis, decreased taste sensitivity, dry mouth, pale oral mucosa, decreased salivary flow, dental erosion, premature tooth loss, enamel hypoplasia, bone alterations as a consequence of secondary hyperparathyroidism, low incidence of caries, and a greater predisposition to the formation of dental calculi [8,9,10,11,12,13,14,15,16]. Alterations related to saliva like xerostomia (subjective feeling of dry mouth), hyposalivation (objective reduction of salivary flow), and biochemical composition are highly prevalent in patients with CKD [17, 18]. Among biochemical modifications, elevated creatinine and urea levels have been detected with significant positive relationship between salivary and serum analysis [19,20,21,22].
The treatment for salivary changes remains unknown, especially those related to chronic systemic diseases such as CKD [23,24,25]. However, some protocols for salivary flow stimulation have been proposed, such as mechanical, drug-based therapy and photobiomodulation therapy (PBMT) in patients with Sjögren’s syndrome[26,27,28,29] and submitted to head and neck radiotherapy [23, 28,29,30,31].
PBMT is described as a form of light therapy that uses non-ionizing light sources, including lasers, LEDs, and broadband light, in the visible and near-infrared spectrum. It is a nonthermal process that acts mainly on mitochondrial membranes and in the increase of ATP production, stimulating cellular metabolism. PBMT relies on light being absorbed by the Cytochorme C Oxidase (Cox), which is an enzyme from the electron transfer chain that mediates the transfer of the electron to the molecular oxygen. As a direct result of the PBMT, when the radiant energy is absorbed by the Cox, there is an increased availability of electrons for the reduction of molecular oxygen in the catalytic center of Cox, which results in increased mitochondrial membrane potential and ultimately increasing the production of adenosine triphosphate, cyclic adenosine monophosphate, and reactive oxygen species [32]. Indirectly, several beneficial therapeutic outcomes such as alleviation of pain or inflammation, immunomodulation, and promotion of wound healing and tissue regeneration have been described [33].
The incidence and prevalence of CKD have increased in the world in the last decades, mainly due to the global burden of diabetes and hypertension [7]. Since this disease is often progressive and irreversible, many patients reach the end-stage renal disease with accumulation of metabolic waste products and multiorgan involvement requiring hemodialysis or kidney transplantation. In addition to the systemic manifestations that appear due to CKD and its treatment modalities, these patients can present complications in the oral cavity [8,9,10,11,12,13,14,15, 34]. Several authors have reported that these patients present altered salivary flow rates and composition when compared to people without this disease. It influences oral health, impairs oral functions and patient’s quality of life [11, 15, 16, 34, 35]. PBMT is a promising approach that is being increasingly used to stimulate salivary glands function [27, 28, 30, 33, 36]. PBMT is also capable of stimulating the salivary glands of rats and increasing salivary flow [36,37,38,39]. However, there are few studies that investigate the effects of PBMT in salivary alterations associated to chronic systemic diseases [26, 27]. Therefore, this study aimed to investigate the effects of PBMT in salivary analysis (quantitative and qualitative) of chronic renal failure patients undergoing hemodialysis.
Methods
Calculation of sample size
The primary outcome (sialometry) presents a normal distribution. For the non-stimulated sialometry, the effect size (magnitude of the difference between the groups normalized by its variability) was 1.0666 and in the stimulated sialometry, 0.9763. The analysis of power indicates that from 11 patients the power of the test will be 1-β > 0.80. With 21 patients, the power of the test for the stimulated collection was 1-β = 0.9890, and for the non-stimulated collection, it was 1-β = 0.9963, demonstrating a high probability of correctly rejecting the null hypothesis. The software GPower 3.1 was used for this calculation.
Participants and initial evaluation
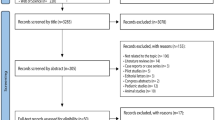
One hundred and fifty adult patients with CKD on hemodialysis at Hospital das Clínicas, University of São Paulo Medical School, São Paulo, Brazil, were enrolled in the study between July 2016 and September 2017. Inclusion criteria were as follows: stable cardiopulmonary and neurological conditions; Hb > 10.9 g/L and hematocrit > 33%; absence of acute systemic infectious processes; systolic blood pressure (SBP) < 140 mmHg and diastolic BP (DBP) < 90 mmHg in at least two measurements in two subsequent dialysis; no hypervolemia; patients over 18 years-old; signed a statement of informed consent. Exclusion criteria were as follows: patients in intensive care unit; hemodynamic instability, signs and symptoms of uremic syndrome related to the cardiovascular and neurological systems; presence of acute systemic infectious processes; presence of acute cardiovascular disease, SBP > 141 mmHg and/or DBP > 91 mmHg; significant anemia (Hb < 11 g/L and Hto < 33%); photosensitivity; pregnancy. From all patients, 44 were included. Figure 1 displays the study flowchart according to CONSORT (Consolidated Standards of Reporting Trials) model.
Initially, all patients’ self-responded two questionnaires. The first was adapted from Geriatric Oral Health Assessment Index (GOHAI) for patients on hemodialysis [40]. The other involved the perception of symptoms related to salivary gland function according to II international criteria for Sjögren syndrome [39, 40]. After that, patients were clinically evaluated using the clinical criterion for the diagnosis of hypofunction of salivary glands (Navazesh, Christensen and Brightman 1992). The presence of dehydration and fissures at the commissures and/or vermilion of the lips was graduated in 4 scores (0, normal; 1, dehydration; 2, dehydrated and/or cracked tissue; 3, angular cheilitis, erythema, or cleft at the commissure with lesions of non-traumatic origin). The alterations in oral mucosa were graduated in 4 scores (0, normal; 1, apparently dry, but the tissues do not adhere to the wooden spatula; 2, apparently dry, but the tissues adhere to the wooden spatula; 3, apparently dry, but the tissues adhere in the wooden spatula, with disappearance of the parotid papilla). After that, all patients were submitted to baseline sialometry test (non-stimulated and stimulated) and randomized.
Randomization and blinding
Forty-four patients were randomized by blocks into two groups:
PBMT group (n = 24): patients were submitted to three sessions of PBMT (baseline (before any intervention), 7 and 14 days). PBMT was administered by a single professional using a continuous wave AsGaAldiode laser (Photon Lase III – DMC, São Paulo, Brazil) with a wavelength of 808 nm (near-infrared). Irradiation was performed in punctual contact mode following the parameters described on Table 1. A total of 20 points were irradiated in each session/day, three extraoral points in the parotid region (right and left n = 6), three points in buccal mucosa (right and left, n = 6), two extraoral (right and left, n = 4), and two intraoral (right and left, n = 4) points in the submandibular and sublingual regions (Fig. 2). The output power of the equipment was measured. Figure 1 shows the irradiation sites.
Placebo group (n = 20): patients were submitted to same protocol but the laser was turned off.
The patients were first submitted to sialometry and after to PBMT or placebo intervention at baseline, 7 and 14 days. Only one researcher knew in which group the patients were allocated and performed all the treatments. A blinded researcher performed all the evaluations after the procedures. The patient was also blinded to the treatment.
Table 1 shows the radiometric parameters used for this work.
Figure 2 shows the flowchart of the experiment.
Salivary collection
Non-stimulated and stimulated saliva were collected from each patient for analysis of salivary volume, total protein, and urea and calcium concentrations. The sialometries were always performed at the same time of the day and before hemodialysis sessions started. Patients were instructed to not eat, drink, or perform oral hygiene at least 1 h before the exams. First, non-stimulated salivary collection was obtained with patient seated with heads slightly forward and a graduated collector tube positioned under the lower lip for 5 min to collect saliva. To obtain the stimulated saliva, patients stayed in the same position and chewed a piece of silicone of a standard size for 5 min. All the saliva produced was dispensed in another graduated tube. The specimens were stored in an icebox and transported to laboratory and then frozen in a freezer at − 80 ° C for further measurement of the amount and biochemistry analysis. To keep the patient’s discomfort to a minimum, the salivary collection was limited to two periods: immediately before any intervention (baseline) and after the three series of PBM at 14 days.
Analysis of saliva
Analysis of salivary total proteins and urea and calcium levels was quantified in triplicates using colorimetric analysis with commercially available kits (Bioclin, Belo Horizonte, Minas Gerais, Brazil) and a spectrophotometer(Anthos 2020, Asys, Austria). The absorbance for each marker was measured using the wavelength indicated by manufacture.
Statistical analysis
The Student t test was used to compare initial and final analysis of salivary volume, total proteins, and urea and calcium levels in each group (PBMT and Placebo). Significance level was considered α = 0.05. Statistical analysis was carried out using GraphPad Prism 5 (GraphPad Software, San Diego, California).
Results
The data regarding the general characteristic of the 38 patients that completed the study are described on Table 2. At baseline, (before any intervention) both groups presented similar general characteristics.
The results of the GOHAI questionnaire adapted for patients with CKD on hemodialysis revealed that most subjects consider their oral health poor and that chronic renal failure is related to this condition (Table 3). The self-perception of symptoms related to salivary gland function questionnaire showed that 24 (63.1%) subjects reported at least one positive answer, indicating that they have oral symptoms associated with salivary gland dysfunction (Table 4).
Clinical evaluation of patient’s commissures/lip showed that 28 (68.4%) exhibited dehydration (Table 5). The buccal mucosa examination showed that 18 (47.3%) subjects presented apparently dry mucosa with variable adherence of wooden spatula (Table 6).
The salivary volumes of non-stimulated and stimulated saliva were significantly higher in subjects that received three sessions of PBMT (p = 0.0270; p = 0.014, Table 7). Other important results were the urea levels measured in both non-stimulated and stimulated saliva that showed a significant decrease after PBMT (p = 0.0001; p = 0.0001). However, these results were not observed in the placebo group. Other parameters, such as total proteins and calcium levels, did not show any significant differences in both types of saliva and groups (Table 7).
Discussion
At the initial examination, most patients declared poor oral health. Few studies reported the analysis of oral health in patients with CKD. However, some important aspects are described in this group of patients such as less frequent visits to dentist associated to economic difficulties, lack of motivation and stress [41]. In addition, our patients also reported the self-perception of salivary dysfunction and signals of dryness of the lip and buccal mucosa were observed. All these findings are in agreement with previous literature [10, 13, 16, 35, 40], and can be justified by the fact that our patients were on hemodialysis for an average of 10.5 years. It is well known that saliva is a complex biologic fluid secreted by major and minor salivary glands with a variety of physicochemical properties [36]. It performs a number of important functions that are essential for the maintenance of oral health and interacts directly with oral mucosa and teeth. Salivary secretion is dependent on high metabolic rate, local blood flow and occurs against an osmotic and pressure gradient. Based on that, various metabolic processes can indirectly influence the rate of salivary secretion [33, 42]. In the case of patients with CKD, and especially in hemodialysis, a decreased mean of stimulated and non-stimulated salivary flow rates compared to control group, associated with modification in saliva composition, has been noticed [15]. Other factors that can justify the dry mouth in these patients are the combination of direct uremic involvement of the salivary glands, dehydration due to the restriction of fluid intake and side effects of drugs (fundamentally antihypertensive agents) [9, 34].
Several strategies have been advocated for dry mouth, using mechanical and gustatory stimulation of salivary glands or by saliva substitutes as palliative care [35, 41]. Pharmacological stimulation using systemic sialogogues, such as pilocarpine and cevimeline hydrochloride, can present good results in dry mouth. As a side effect, they have been associated with cardiovascular and pulmonary disorders and thus are contraindicated in some patients. Conflicting data about the efficacy of all these approaches have been presented [41, 43].
In addition, few studies were reported analyzing the management of dry mouth in patients with CKD [23, 24, 43] and evaluated the effect of gum or a saliva substitute on xerostomia, thirst and interdialytic weight gain [23, 41]. After 2 weeks, the authors detected that gum chewing reduced both thirst and xerostomia, but the saliva substitute had no effect on xerostomia. To date, some studies have demonstrated that PBMT is a new beneficial tool for the reduction of xerostomia, especially in Sjogren syndrome [44]; head and neck irradiated patients [45,46,47,48].Then, we hypothesized that PBMT would be an effective treatment for dry mouth, improving the salivary flow and modifying the composition of saliva in patients with CKD on hemodialysis. Our hypothesis was confirmed in the present randomized, placebo-controlled trial. Non-stimulated and stimulated saliva flows of patients were improved, associated to a decrease of salivary urea levels after three sessions with 808 nm (near-infrared) using 142 J/cm2 of radiant exposure and 4 J per point of radiant energy in major salivary glands. On the other hand, total protein and calcium levels were not affected by the therapy.
PBMT is a non-ablative and non-thermal irradiation, well tolerated by the tissues, with no mutagenic effects that can promote a number of biological effects. The primary effect occurs when light is absorbed in mitochondrial cytochromes, increasing ATP production, reducing oxidative stress and initiating secondary cell-signaling pathways. The overall results of PBMT therapy are the increase of energy metabolism, improvement of cell viability and angiogenesis, modulation of inflammatory process, and stimulation of wound healing [32, 49,50,51]. Patients on hemodialysis have a high concentration of urea in serum and saliva [19, 21]. Urea accumulation in tissues promotes oxidative stress by generation of highly reactive, intermediary, oxygen metabolites known as reactive oxygen species (ROS). ROS imbalance has been considered a major mediator of various complications of CKD including dry mouth, uremic breath, and other oral complications [11]. Oxidative stress results in the activation of the redox-sensitive transcription factor nuclear factor k B (NFKb), which leads to the generation of proinflammatory cytokines. Although the mechanisms involved in salivary gland damage caused by CKD are not well known, our results showed that PBM therapy could improve some deleterious effects promoted by this condition. It could reverse the mitochondrial inhibition of respiration and the generation of ROS.
Decrease of xerostomia and an increase in salivary flow rates have been associated to PBM therapy in other clinical situations in which salivary glands are affected by immunological/inflammatory process such as Sjogren syndrome [26, 27, 44] or radiotherapy damage [24, 28, 29, 45]. In experimental animal models, PBM therapy showed an increase in the number of duct epithelial cell mitoses, stimulated protein synthesis in submandibular glands of rats, increased salivary flow rate, and altered salivary protein concentration [38, 52]. These effects probably occur because of the activation of redox-sensitive transcription factors leading to the expression of an array of gene products that prevent apoptosis and cell death, stimulate cell proliferation and modulation of the inflammatory and antioxidant response, and increase blood microcirculation in the salivary glands [33, 38, 39].
The calcium level in our patients’ saliva was increased in relation to the normal value described in the literature, which is around 2.0 mg/dL. Controversial results about calcium levels in saliva have been described. Some authors claim that lack of differences is observed in calcium concentration between healthy patients [34, 35]. No previous results described the effect of PBM therapy regarding calcium level in patients with xerostomia related to other conditions.
To the best of our knowledge, PBM therapy has never been used for the treatment of dry mouth in patients with CKD. The strength of our study was the study design with a placebo-controlled clinical trial. Also, our patients of both PBM therapy and placebo groups presented similar clinical and salivary biochemical profile at the baseline. We reported that PBMT led to a higher amount of saliva production with improvement of urea levels. Also, non-irradiated subjects maintained the same salivary rate flow and salivary composition. These findings might have a significant clinical impact for these patients since it is easy to perform, and does not increase morbidity or presents side effects.
One limitation is that we do not perform any questionnaire and oral mucosa analysis during and after the treatment. In addition, we do not examine or collect samples of the patients after a long period to determine if this effect was transient. However, recent studies performed in patients submitted to radiotherapy suggest that the effect of laser stimulation on salivary glands is positive when the gland has residual function and it is not transient [24, 53]. Other important point that should be discussed is the difficulty in selecting the PBMT protocol to be used in this clinical trial. The lack of an effective and recognized protocol is clear. In the literature, the power, power density, wavelength, and all other parameters differ considerably in the studies that used PBMT for xerostomia [54, 55]. We decided for using the near-infrared wavelength because of the depth of the glandular parenchyma to be irradiated. The sessions took place once a week for 3 weeks, with the objective of maintaining the patients’ adherence to therapy.
Conclusion
In conclusion, this clinical trial shows that PBMT is effective to improve hyposalivation and urea levels in the saliva of patients with CKD on hemodialysis. Based on these findings, PBMT could be considered a promising therapeutic strategy for xerostomia/hyposalivation in these patients. Further studies need to be performed to investigate the mechanisms involved in the underlying effects of PBMT on salivary gland dysfunction associated to CKD.
References
Grassmann A, Gioberge S, Moeller S, Brown G (2005) ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. 20:2587–2593
Long B, Koyfman A, Lee CM (2017) Emergency medicine evaluation and management of the end stage renal disease patient. 35:1946–1955
Cherchiglia ML, Machado EL, Szuster DAC, Andrade ELG, Acúrcio FDA, Caiaffa WT et al (2010) Epidemiological profi le of patients on renal replacement therapy in Brazil, 2000-2004. 44(4):1–10
Sampaio RMM, Coelho MO, Pinto FJM, Osteme EPR (2011) Epidemiological profile of patients with nephropathy and the difficulties in access to treatment. 26(1):95–101
Silva AT, Soares LB, Magajewski FRL (2016) Epidemiologic and economic aspects related to hemodialysis and kidney transplantation in Santa Catarina in the period of 2012-2013. 48:2284–2288
Khanum N, Mysore-Shivalingu M, Basappa S, Patil A, Kanwar S (2017) Evaluation of changes in salivary composition in renal failure patients before and after hemodialysis. 9(11):1340–1345
Inker LA, Astor BC, Fox CH, Tamara I, Lash JP et al (2014) KDOQI US Commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. 63(5):713–735
Kho H-S, Lee S-W, Chung S-C, Kim Y-K (1999) Oral manifestations and salivary flow rate, pH, and buffer capacity in patients with end-stage renal disease undergoing hemodialysis. 88(3):316–319
García ER, Padilla AM, Camacho MEI, Ramírez MAB (2005) Lesiones bucales en un grupo de pacientes con trasplante renal Oral lesions in a group of kidney transplant patients. 10(3):196–204
García ER, Padilla AM, Romo SA, Ramírez MAB (2006) Oral mucosa symptoms, signs and lesions, in end stage renal disease and non-end stage renal disease diabetic patients. 11(6):467–473
Martins C, Siqueira WL, Oliveira E, Nicolau J, Primo LG (2012) Dental calculus formation in children and adolescents undergoing hemodialysis. 27:1961–1966
Andrade MRTC, Salazar SLA, de Sá LFR, Portela M, Pereira AF, Soares RMA et al (2015) Role of saliva in the caries experience and calculus formation of young patients undergoing hemodialysis. 19(8):1973–1980
Anuradha BR, Katta S, Kode VS, Praveena C, Sathe N, Sandeep N et al (2015) Oral and salivary changes in patients with chronic kidney disease: a clinical and biochemical study. 19(3):297–301
Oyetola EO, Owotade FJ, Agbelusi GA, Fatusi OA, Sanusi AA (2015) Oral findings in chronic kidney disease: implications for management in developing countries. 15(24):2–8
Dioguardi M, Caloro GA, Troiano G, Giannatempo G, Laino L, Petruzzi M et al (2015) Oral manifestations in chronic uremia patients. 38(1):1–6
Honarmand M, Farhad-Mollashahi L, Nakhaee A, Sargolzaie F (2017) Oral manifestation and salivary changes in renal patients undergoing hemodialysis. 9(2):207–210
Venkatapathy R, Govindarajan V, Oza N, Parameswaran S, Dhanasekaran BP, Prashad KV (2014) Salivary creatinine estimation as an alternative to serum creatinine in chronic kidney disease patients. 2014:1–6
Seethalakshmi C, KoteeSwaran D, ChiranjeeVi V (2014) Correlation of serum and salivary biochemical parameters in end stage renal disease patients undergoing hemodialysis in pre and post-dialysis state. 8(12):12–14
Pandya D, Nagrajappa AK, Ravi KS (2016) Assessment and correlation of urea and creatinine levels in saliva and serum of patients with chronic kidney disease, diabetes and hypertension– a research Study. 10(10):58–62
Raimann JG, Calice-Silva V, Thijssen S, Nerbass FB, Vieira MA, Dabel P et al (2016) Saliva urea nitrogen continuously reflects blood urea nitrogen after acute kidney injury diagnosis and management: longitudinal observational data from a collaborative, international, prospective, multicenter study. 42:64–72
Lasisi TJ, Raji YR, Salako BL (2016) Salivary creatinine and urea analysis in patients with chronic kidney disease: a case control study. 17(10):2–6
Renda R (2017) Can salivary creatinine and urea levels be used to diagnose chronic kidney disease in children as accurately as serum creatinine and urea levels? A case–control study. 39(1):452–457
Bots CP, Brand HS, Veerman ECI, Korevaar JC, Valentijn-Benz M, Bezemer PD et al (2005b) Chewing gum and a saliva substitute alleviate thirst and xerostomia in patients on haemodialysis. 20(3):578–584
Bots CP, Brand HS, Veerman ECI, Valentijn-Benz M, Amerongen BMV, Amerongen AVN et al (2005a) The management of xerostomia in patients on haemodialysis: comparison of artificial saliva and chewing gum. 19:202–207
Palma LF, Gonnelli FAS, Marcucci M, Dias RS, Giordani AJ, Segreto RA et al (2017) Impact of low-level laser therapy on hyposalivation, salivary pH, and quality of life in head and neck cancer patients post-radiotherapy. 32(4):827–832
Cafaro A, Arduíno PG, Gambino A, Romagnoli E, Broccoletti R (2014) Effect of laser acupuncture on salivary flow rate in patients with Sjögren’s syndrome. 30(6):1805–1809
Fidelix T, Czapkowski A, Azjen S, Andriolo A, Neto PH, Trevisani V (2017) Low-level laser therapy for xerostomia in primary Sjögren’s syndrome: a randomized trial. 37(3):729–736
Mercadante V, Hamad AA, Lodi G, Porter S, Fedele S (2017) Interventions for the managemente of radiotherapy-induced xerostomia and hyposalivation: a systematic review and meta-analysis. 66:64–74
Loncar B, Stipetic MM, Baricevic M, Risovic D (2011) The effect of low-level laser therapy on salivary glands in patients with xerostomia. 29(3):171–175
Yu C, Tsai Y-F, Fang J-T, Yeh M-M, Fang J-Y, Liu C-Y (2016) Effects of mouthwash interventions on xerostomia and unstimulated whole saliva flow rate among hemodialysis patients: a randomized controlled study. 63:9–17
Duruk N, Eser Í (2016) The null effect of chewing gum during hemodialysis on dry mouth. 12-13
Freitas FL, Hambling MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. 22(3):7000417
Anders JJ, Lanzafame RJ, Arany PR (2015) Low-level light/laser therapy versus photobiomodulation therapy. 33(4):183–184
Epstein SR, Mandel I, Scoppi IW (1980) Salivary composition and calculus formation in patients undergoing hemodialysis. 51(6):336–338
Kaushik A, Reddy SS, Umesh L, Devi BKY, Santana N, Rakesh N (2013) Oral and salivary changes among renal patients undergoing hemodialysis: a cross-sectional study. 23(2):125–129
Simões A, Nicolau J, de Souza DN, Ferreira LS, de Paula Eduardo C, Apel C et al (2007) Effect of defocused infrared diode laser on salivary flow rate and some salivary parameters of rats. 12:25–30
de Jesus VC, Beanes G, Paraguassú GM, Ramalho LMP, Pinheiro ALB, Ramalho MJP et al (2015) Influence of laser photobiomodulation (GaAlAs) on salivary flow rate and histomorphometry of the submandibular glands of hypothyroid rats. 30(4):1275–1280
Gonnelli FAS, Palma LF, Giordani AJ, Deboni ALS, Dias RS, Segreto RA et al (2016) Low-level laser for mitigation of low salivary flow rate in head and neck cancer patients undergoing radiochemotherapy: a prospective longitudinal study. Photomed Laser Surg:326–330
Carvalho C, Manso AC, Escoval A, Salvado F, Nunes C (2013) Geriatric Oral Health Assessment Index (GOHAI). 31(2):153–159
Martins C, Siqueira WL, Primo LSSG (2008) Oral and salivary flow characteristics of a group of Brazilian children and adolescents with chronic renal failure. 23(4):619–624
Villa A, Wolff A, Narayana N, Dawes C, Aframian DJ, Pedersen AML et al (2016) World workshop on oral medicine VI: a systematic review of medication-induced salivary gland dysfunction. 22:365–382
Proctor GB (2016) The physiology of salivary secretion. 2000 70:11-25
De-Nour K, Czaczkes JW (1980) A saliva substitute as a tool in decreasing over drinking in dialysis patients. 16(1):43–44
Simões A, Platero MD, Campos L, Aranha AC, de Paula Eduardo C, Nicolau J (2009) Laser as a therapy for dry mouth symptoms in a patient with Sjögren's syndrome: a case report. 29(3):134–137
Cowen D, Tardieu C, Schubert M, Peterson D, Resbeut M, Faucher C et al (1997) Low energy helium-neon laser in the prevention of oral mucositis in patients undergoing bone marrow transplant: results of a double blind randomized trial. 38(4):697–703
de Oliveira Lopes C, Mas JRI, Zângaro RA (2006) Low level laser therapy in the prevention of radiotherapy-induced xerostomia and oral mucositis. 39(2):131–136
Oton-Leite AF, Elias LSA, Morais MO, Pinezi JCD, Leles CR, Silva MAGS et al (2013) Effect of low level laser therapy in the reduction of oral complications in patients with cancer of the head and neck submitted to radiotherapy. 294-300
Carramolino-Cuéllar E, Lauritano D, Silvestre F-J, Carinci F, Lucchese A, Silvestre-Rangil J (2018) Salivary flow and xerostomia in patients with type 2 diabetes. 47(5):526–530
Pavlic V (2012) The effects of low-level laser therapy on xerostomia (mouth dryness). 5(6):247–250
Hamblin MR (2017) Mechanisms and mitochondrial redox signaling in photobiomodulation. 94:199–212
Hamblin MR (2017) Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. 4(3):337–361
Migliario M, Sabbatini M, Mortellaro C, Renò F (2018) Near infrared low level laser therapy and cell proliferation: the emerging role of redox sensitive signal transduction pathways
Saleh J, Figueiredo MAZ, Cherubini K, Braga-Filho A, Salum FG (2014) Effect of low-level laser therapy on radiotherapy induced hyposalivation and xerostomia: a pilot study. 32(10):546–552
Spanemberg JC, Figueiredo MA, Cherubini K, Salum FG (2016) Low-level laser therapy: a review of its applications in the management of oral mucosal disorders. 22(6):24–31
Pandeshwar P, Roa MD, Das R, Shastry SP, Kaul R, Srinivasreddy MB (2015) Photobiomodulation in oral medicine: a review. 1-12
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The present single-center, randomized, double-blind, placebo-controlled study received approval from the Nove de Julho University Ethics Committee (CAAE: 32437614830010068 and protocol 1.328.881). All participants signed a statement of informed consent prior to any clinical procedure. The protocol was also registered in ClinicalTrial.gov, under number NCT03647813.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pavesi, V.C.S., Martins, M.D., Coracin, F.L. et al. Effects of photobiomodulation in salivary glands of chronic kidney disease patients on hemodialysis. Lasers Med Sci 36, 1209–1217 (2021). https://doi.org/10.1007/s10103-020-03158-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03158-0