Abstract
The aim of the current study was to evaluate the proliferative effect of low-level laser therapy on long-term cryopreserved dental pulp stem cells (DPSCS) and stem cells from human exfoliated deciduous teeth (SHEDS). The DPSCS and SHEDS were divided into 2 main groups according to gallium aluminum arsenide (GaAIAs) diode laser irradiation densities as 5 J/cm2 and 7 J/cm2. Each main group was further divided into 4 groups according to laser irradiation periods as 0, 24, 48, 72 h groups. During the incubation periods, cells received laser irradiation in every 24 h according to their groups and were put into incubator after irradiation. Cell groups that were not subjected to laser irradiation were served as control groups. Viabilities of cells were determined via MTT assay at the end of all incubation periods, and data were statistically analyzed. Laser irradiation demonstrated significant effects on proliferation rate of DPSCs and SHEDs in comparison with control. Intragroup comparison data of DPSCS revealed that repetitive laser irradiation for long term (72 h) increased the cellular viability significantly in comparison with all other treatment groups; however, no significant differences were found when energy densities were compared within each time interval, except for 48 h group at which irradiation with 7 J/cm2 provided significantly higher cell viability rates of SHEDS. DPSCs showed significantly higher cellular viability than SHEDs only for the 7 J/cm2 energy density in 72 h. Longer term (72 h) repetitive laser irradiation with energy densities of 5 and 7 J/cm2 (wavelength of 980 nm) may be recommended to induce the proliferative effect on long-term cryopreserved DPSCS and SHEDS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue engineering relies on three necessities for tissue regeneration. The components of this triad are scaffold formation, stem cells, and growth signals [1]. In this context, factors providing the proliferative effect on dental mesenchymal stem cells (DMSCS) are largely important in the field of dentistry [2]. DMSCS are derived from dentoalveolar tissues and exhibit a high proliferation capacity with multiple differentiation potentials such as osteoblasts, adipocytes, odontoblasts, and chondrocytes [3]. Generally, DMSCS are derived from adult pulp tissue (DPSCS), human exfoliated deciduous teeth (SHEDS), periodontal ligaments (PDLSCS), and apical papilla cells of immature teeth (SCAP) [4,5,6].
Several studies have reported that undifferentiated stem cells derived from dental pulp (DPSCS and SHEDS) have a great differentiation capacity and can be used for the treatment of necrotic immature permanent teeth [7, 8]. For this purpose, isolation and cryopreservation of these dental stem cells were suggested to be very important for individual stem cell therapies. However, their efficiency in in vitro culture was reported to decrease after thawing processes [9]. Thus, biostimulation of the in vitro proliferation capacity of stem cells are crucial, and current studies focused on the methods for increasing cell expansion. Treatment with an insulin-like growth factor (IGF-1) [10], an enamel matrix derivative [11], an epiregulin from the epidermal growth factor family, Trichostatin A from histone deacetlyase inhibitors [12], and low-level laser therapy (LLLT) are the aforementioned methods [13].
LLLT is preferred in various clinical conditions to speed up the regenerative processes of tissues [13]. LLLT shows its efficacy via the photons provided by laser irradiation which are absorbed by chromophores of the cells resulting in a change in their biological activities [13]. In addition, biostimulative effects of LLLT on tissue responses and cell growth have been shown. LLLT was also reported to promote bone formation by increasing the growth factor that is related with the differentiation of bone cells which in turn stimulates cell proliferation for dentin-pulp complexes [13]. It has been also indicated that LLLT had a promotive effect on differentiating various stem cell types such as adipose stem cells, bone marrow stem cells, or human dental pulp stem cells [13]. Mechanisms of the photostimulatory effect of LLLT can be explained by two main reactions. Primary reactions occur after light absorption via photoreceptors into the cell membrane and mitochondria. These reactions lead to the regulation of redox state in the mitochondria to create greater oxidation and stimulate cellular signaling by secondary reactions [14].
There is little known about the effect LLLT on the proliferation capacity of the DPSCS and SHEDS which exhibit slow proliferation rates after long-term cryopreservation especially when the cells intended to be used in stem cell therapies. The objective of the current study was to evaluate the cellular proliferative capacity of LLLT on long-term cryopreserved DPSCS and SHEDS for future tissue engineering therapies.
Materials and methods
This study was approved by the Ethical Board of Near East University (Number: 2015/34-243).
Thawing and cultivation of DPSCS and SHEDS
DPSCS were isolated and fully characterized according to the criteria proposed by Dominici et al. [15]. For this purpose, isolated cells were characterized according to the surface antigens (CD90, CD73, CD105, CD44, CD34, CD45, CD11b, CD14, CD19, CD79, HLA-DR) (BD, Biosciences, USA). After characterization studies, cells were differentiated into different tissues, and their stem cell characteristics were verified by investigating differentiated tissues under microscope. Isolated and fully characterized DPSCS were cryopreserved according to liquid nitrogen cryopreservation (controlled rate method) protocol. Cells were first trypsinized and then centrifuged at 1000 rpm for 5 min. Cells were then put into medium consisting of 90% fetal bovine serum (FBS) (Gibco, Visp, Switzerland) and 10% dimethylsulphoxide (DMSO). Cells were put into − 20 °C for 1 h and − 80 °C for 24 h and put into liquid nitrogen tank (− 196 °C) until being used. Cryopreserved DPSCs (passage 2) stored in liquid nitrogen for 24 months were used in the present study.
Previously isolated and characterized SHEDs were cryopreserved according to a rapid freezing protocol [16]. Cells isolated and characterized were firstly trypsinized and centrifuged at 1000 rpm for 5 min. Centrifuged cells were transferred into freezing media consisting of 90% FBS and 10% DMSO. Cells were later transferred directly into − 80 °C and kept there until being used (rapid freezing method). SHEDs used in this study were incubated in − 80 °C for 24 months. Firstly, cryopreserved DPSCS and SHEDS were thawed at 37 °C, and cell suspensions were centrifuged for 5 min at 1000 rpm. Following the centrifugation, thawed cells were transferred into 25 cm3 flasks and cultured in DMEM/F12 (Gibco, Switzerland) medium, supplemented with 10% FBS, 2 mM glutamine, 100 U/mL of penicillin, and 100 μg/mL of streptomycin (Gibco, Visp, Switzerland) at 37 °C in a humidified atmosphere of 5% CO2.The cells were subcultured every 2 days. After reaching 80% of confluence, cells were trypsinized by Trypsin-EDTA (Capricorn, Germany) at 37 °C for 10 min and extended to passage 3. The cells from passage 3 were used in the study for both of cell types.
Experimental design
A schematic illustration of the study design was presented in Fig. 1. The effect of laser irradiation was evaluated by using two different energy densities of diode laser irradiation at four different time intervals (0 h, 24 h, 48 h, 72 h). The DPSCS and SHEDS were divided into 2 main groups according to laser irradiation densities as 5 J/cm2 and 7 J/cm2. Each main group was further divided into 4 subgroups according to laser irradiation periods as 0, 24, 48, 72 h groups. Cells in 0 h group were subjected to single laser irradiation and incubated for 24 h. For the other groups, laser irradiation was repeated at the end of each 24 h, and then cells were put into incubator again. In this manner, the cells in 0 h, 24 h, 48 h, and 72 h groups were subjected to irradiation for 1, 2, 3, and 4 times, respectively. Control groups were composed for both cell groups in which cells were not subjected to laser irradiation. The test groups and control groups are presented in Table 1.
Prior to diode laser irradiation, cells grown to 80% confluence levels were trypsinized and counted. A density of 104 cells/mL was transferred to 96-well plates and maintained in an incubator at 37 °C with 5% CO2 overnight. Next day, diode laser irradiation was applied on cells to evaluate the cell proliferative capacity and viability in all groups. Study was set as triplicates.
Laser irradiation
Laser irradiation was applied to isolated DPSCS and SHEDS and was performed by gallium aluminum arsenide (GaAIAs) diode laser (Medency, Italy). To achieve the intended energy density levels of 5 and 7 J/cm2, laser was used with 980-nm wavelength with an average power of 0.1 W in continuous action mode for 20 and 28 s, respectively. The laser probe with a diameter of 0.4 cm2 was fixed perpendicularly and applied to the cells at a distance of 0.5 cm to each plate in the class II cell culture cabinet. Laser irradiation was repeated according to the test groups of cells, and after application of each laser irradiation, cells were put back into the incubator (37 °C).
Cell proliferation assay
The rates of cellular proliferation were analyzed by performing a MTT assay (Acros Organics, NJ, USA) in order to investigate the activity of mitochondrial enzymes of viable cells in the experimental and control (non-irradiated) groups (Fig. 1). Viable cells successfully cleaved MTT and formed formazan crystals with the help of cellular succinate dehydrogenase enzyme. Formazan crystals formed were later dissolved by DMSO. For this purpose, both DPSCs and SHEDs were cultured overnight in 96-well plates with an initial concentration of 1 × 104 cells/mL with 5% CO2, at 37°C. For each group, MTT assay was performed after 24 h of incubation following the laser irradiation, and cell viabilities were measured to detect the effect of phototherapy on proliferation rate of irradiated cells. Control (non-irradiated cells) group was set according to each different time intervals separately in order to surpass the cells’ normal proliferation rate (Table 1). MTT was added to the cells (1 mg/mL) and incubated for 4 h. Following incubation, crystals formed by viable cells were dissolved by DMSO. UV-visible spectrophotometer was used to measure the optical density of the dissolved material (wavelength λ = 570) (Thermo Multiskan Spectrum). The cellular proliferation rate (%) was determined by the following formula:
Statistical analysis
The data were measured with mean standard errors, the statistical significance of differences was examined by using one-way ANOVA, Tukey’s test was performed for multiple intragroup comparisons, and t test was performed for intergroup comparisons by using GraphPad Prism 5.0 and SPSS for Windows. P values of less than 0.05* were stated as statistically significant.
Results
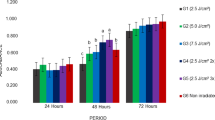
Diode laser irradiation with energy densities of 5 or 7 J/cm2 was applied to treat DPSCs at each time interval. Among the control group and laser-irradiated test groups, the only significant difference was detected between the control and 72 h groups for both energy densities (5 J/cm2: P < 0.05, 7 J/cm2: P < 0.001), no significant differences were found among other time groups (Fig. 2). Intragroup comparisons of DPSCs irradiated with 5 J/cm2 energy density revealed that there were no significant differences among the cellular proliferation of 24, 48, and 72 h groups, while the value obtained in 72 h group was significantly higher than 0 h group (Fig. 2). When the effects of energy densities on cell proliferation of DPSCs were compared for different time periods, 72 h group of DPSCs irradiated with 7 J/cm2 energy density showed higher proliferation rates than the other time periods among which no significant differences were observed (Fig. 2). The differences in proliferation rates of DPSCs subjected to 5 J/cm2 and 7 J/cm2 energy density groups were reported to be nonsignificant within each time interval (P > 0.05) (Fig. 2).
Cellular viabilities of SHEDs in both energy density groups for all time intervals were found statistically higher than the control group. Repeated laser application in different time intervals did not have any significant effect on the cellular proliferation rates of SHEDs irradiated with both energy densities (P > 0.05) (Fig. 3). No significant differences were found when energy densities were compared within each time interval (P > 0.05), except for 48 h group at which irradiation with 7 J/cm2 provided significantly higher cell viability rates of SHEDs (P < 0.05) (Fig. 3).
The different stem cell types were also compared within each energy density and time intervals. DPSCs only showed significantly higher cellular viability than SHEDs for the 7 J/cm2 energy density at 72 h group (P < 0.05). No significant differences were observed between other time points and energy density groups (P > 0.05) (Fig. 4).
Discussion
Currently, recent improvements in biomedical technology encouraged the development of new devices and methods. Low-level laser irradiation is one of these with its numerous clinical applications [13]. Although the effect of laser irradiation on proliferation of cells has been investigated in various cell types including fibroblasts, endothelial cells, and osteoblasts, a lot remained to be unknown about the comparative effect of LLLT on the proliferation of different dental mesenchymal stem cells that have been cryopreserved for long period of time [13].
DPSCS and SHEDS are regarded as good candidates for stem cell therapies and dental pulp tissue engineering studies due to their multi-lineage differentiation capacities [17]. However, a great number of stem cells are required for regenerative approaches. Therefore, LLLT could be a good candidate for the stimulation of proliferation, viability, migration, and differentiation activities of stem cells [13]. Helium-neon (HeNe) or diode lasers such as GaAlAs and indium-gallium-aluminum-phosphide (InGaAlP) are usually preferred for this purpose [18]. In the present study, 980-nm GaAlAs diode laser irradiation with a setting of 0.1 W of power output was applied using two different energy densities of 5 and 7 J/cm2, and the effects of repeated application at 0, 24, 48, and 72 h were investigated. When the cell proliferation data were evaluated, DPSCS demonstrated similar biological responses in agreement with a previous study reporting that single irradiation with 2.5 J/cm2 of energy density did not show any efficacy in comparison with higher densities [19]. In the present study, long-term (72 h) and repeated LLLT with an energy density of 7 J/cm2 showed significantly higher proliferation rate compared with short-term LLLT with energy density of 5 J/cm2. These findings may be attributed to multiple factors including the differences between energy densities, repetitive application of LLLT with longer time period, or the type of stem cells used in the current study. The effect of different energy densities on biostimulation is explained by the biphasic dose response phenomenon, where higher energy densities may lead to a decrease in the biostimulative response of LLLT at a certain energy density and wavelength [20]. On the other hand, if an inadequate energy density was applied, the biostimulation threshold could not be reached and biological response would not be observed on targeted cells [20]. Therefore, selection of correct density is crucial for the efficacy of LLLT. Besides, the energy density emission modes (continuous wave/pulse mode), stem cell types, wavelengths, and power output are important parameters which can change the irradiation protocol and cellular responses [20]. Furthermore, laser therapies exhibit a dose-dependent activity on cellular responses, and repetitive irradiation probably had a cumulative effect when each new dose was applied [19, 21]. Huang et al. [21] and Zaccara et al. [22] demonstrated this cumulative effect of laser therapy supporting to the findings of the present study in which higher proliferation rates were detected in repetitive long-term (72 h) irradiation on both DPSCS and SHEDS in comparison with short-term (0–24 h).
Borzabadi Faharani [13] demonstrated in vitro proliferative effect of laser irradiation on human DMSCS including DPSCs, SCAP, PDLSCs, and SHEDS. It was concluded that various types of diode laser, wavelengths between 660 and 980 nm, and energy densities between 0.1 and 30 J/cm2 had proliferative effect on these cells. The findings of the present study suggested that repetitive long-term (72 h) diode laser application with energy densities of 5 and 7 J/cm2 (wavelength of 980 nm) had proliferative effect on DPSCS and SHEDS.
Even though the exact mechanism of LLLT on cell proliferation was not completely understood, experimental data suggested that, following the laser irradiation, an increased secretion of growth factors, ATP, DNA, RNA, nitric oxide, and reactive oxygen species was detected [23]. According to the study of Ginani et al. [24] on mesenchymal stem cell proliferation, laser irradiation was found to induce in vitro stem cell proliferation. The same proliferation trend of in vitro stem cells was also observed in the present study. The MTT assay data suggested that repetitive laser irradiation with 7 J/cm2 energy density on DPSCs significantly induced proliferation rate in comparison with SHEDs at 72 h. The percentage proliferation rate of DPSCs increased more than twofold upon repetitive irradiation with 7 J/cm2 energy density when compared with the non-irradiated, control group at 72 h. The outcomes of the present study suggested that the most effective proliferative time on both cells was 72 h of repetitive irradiation regardless of energy densities. Zaccara et al. [22] and Ginani et al. [24] detected higher proliferation rates upon long-term (72 h) irradiation compared with short-term (0 h and 24 h) for both DPSCS and SHEDS supporting the findings of the present study [22, 24].
Studies related with DPSCS and SHEDS revealed significant differences in the biology of these stem cells. SHEDS were known by their higher proliferation rates and cell division numbers in comparison with DPSCS [25]. Contrary in the current study, significantly higher proliferation rates were observed on DPSCS in comparison with SHEDs subjected to repetitive long-term (72 h) irradiation with energy density of 7 J/cm2. This finding could be a result of different cryopreservation protocol applications on cells. Cryopreservation protocols should also be taken into consideration since they could influence the viability and proliferation capacity of cells [26]. Liquid nitrogen cryopreservation (controlled rate method), providing storage of cells at a temperature of − 196 °C, is a recommended protocol for mesenchymal stem cells [27]. However, higher cost and limited accessibility of such storage conditions were reported as disadvantages of this protocol [27]. Hence, the rapid cryopreservation protocol with a storage temperature of − 80 °C is preferred due to its lower cost and higher accessibility. SHEDS used in the present study were long-term cryopreserved by following the uncontrolled/rapid freezing protocol. The study of Nhat Hyunh et al. [28] revealed that cells cryopreserved with the rapid freezing method did not exhibit the similar proliferation rates when compared with the controlled rate method. In agreement with this study, application of uncontrolled/rapid freezing technique for cryopreservation and long storage period (exceeding 180 days) of SHEDS may explain the lower cellular proliferation rate in comparison with liquid nitrogen cryopreservation (controlled rate method) DPSCS.
In conclusion, it is possible to state that repetitive irradiation with energy densities of 5 and 7 J/cm2 (wavelength of 980 nm) at 72 h may be recommended to induce a proliferative effect on long-term cryopreserved DPSCS and SHEDS without causing cell cytotoxicity. This irradiation protocol on dental mesenchymal stem cells may represent a clinical therapeutic importance in the field of stem cell-based therapies and tissue engineering. Future in vitro investigations of laser irradiation effect on proliferation mechanisms via PCR, apoptosis, or cell cycle assays are needed to be performed together with in vivo studies in order to determine the optimal conditions for dental stem cell biostimulation.
References
Ripamonti U (2004) Soluble, insoluble and geometric signals sculpt the architecture of mineralized tissues. J Cell Mol Med 8:169–180
Jin H, Park JY, Choi H, Choung PH (2013) Inhibitor trichostatin a promotes proliferation and odontoblast differentiation of human dental pulp stem cells. Tissue Eng Part A 19(5–6):613–624
Huang GT, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 8:792–806
Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang J, Xu GT, Liang A, Liu S (2015) Concise reviews: characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 33:627–638
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GTJ (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study, J. Endod 34(2):166–171
(2014) Apexification, apexogenesis and regenerative endodontic procedures: a review of the literature. Minerva Stomatol 63(11–12):375–389
Marti DE, De Almeida JF, Henry MA, Khaing ZZ, Schmidt CE, Teixeira FB, Diogenes A (2014) Concentration dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod 40(1):51–55
Pilbauerová N, Suchánek J (2018) Cryopreservation of dental stem cells. Acta Med (Hradec Kralove) 61(1):1–7
Feng X, Huang D, Lu X, Feng G, Xing J, Lu J, Xu K, Xia W, Meng Y, Tao T, Li L, Gu Z (2014) Insulin-like growth factor 1 can promote proliferation and osteogenic differentiation of human dental pulp stem cells via mTOR pathway. Develop Growth Differ 56:615–624
Wu SM, Chiu HC, Chin YT, Lin HY, Chiang CY, Tu HP, Fu MM, Fu E (2014) Effects of enamel matrix derivative on the prolifertaion and osteogenic differentiation of human gingival mesenchymal stem cells. Stem Cell Res Ther 5(2):52. https://doi.org/10.1186/scrt441
Jin H, Park JY, Choi H, Choung PH (2013) HDAC inhibitor trichostatin a promotes proliferation and odontoblast differentaition of human dental pulp stem cells. Tissue Eng Part A 19(5–6):613–624
Borzabadi Farahani A (2016) Effect of low-level laser irradiation on proliferation of human dental mesenchymal stem cells; a systematic review. J Photochem Photobiol B Biol 162:577–582
Karu TI (2012) Low-power laser therapy. CRC Press, Boca Raton
Dominici M, Le Blanc K, Muelleri I, Slaper-Corternbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Hortwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317
Islam A, Mammadov E, Kendirci R, Aytaç A, Vatansever HS (2017) In vitro cultivation, characterization and osteogenic differentiation of stem cells from human exfoliated deciduous teeth on 3D printed polylactic acid scaffolds. Iran Red Crescent Med J 19(8):e55593
Martens W, Bronckaers A, Politis C, Jacobs R, Lambrichts I (2013) Dental stem cells and their promising role in neuralregeneration: an update. Clin Oral Investig 17(9):1969–1983
Kushibiki T, Hirasawa T, Okawa S, Ishihara M (2015, 2015) Low reactive level laser therapy for mesenchymal stromal cells therapies. Stem Cells Int. https://doi.org/10.1155/2015/974864
Almedia-Junior LA, Marques NCT, Prado MTO, Oliveira TM, Sakai VT (2019) Effect of single and multiple doses of low-level laser therapy on viability and proliferation of stem cells from human exfoliated deciduous teeth (SHED). Lasers Med Sci 34(9):1917–1924
Peplow PV, Chung TY, Baxter GD (2010) Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies. Photomed Laser Surg 28:3–40
Huang YY, Chen ACH, Carroll JD, Hamblin MR (2009) Biphasic dose response in low level light therapy. Dose-Response 7:358–383
Zaccara IM, Ginani F, Mota-Filho HG, Henriques ACG, Barboza CAG (2015) Effect of low-level laser irradiation on proliferation of human dental pulp stem cells. Lasers Med Sci 30(9):2259–2264
Gao X, Xing D (2009) Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci 16(4). https://doi.org/10.1186/1423-0127-16-4
Ginani F, Soares DM, de Oliveira Rocha HA, de Souza LB, Barboza CAG (2018) Low- level laser irradiation induces in vitro proliferation of stem cells from human exfoliated deciduous teeth. Lasers Med Sci 33(1):95–102
Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M (2009) Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod 35:1536–1542
Ginani F, Soares DM, Rabelo LM, Rocha HAO, de Souza LB, Barboza CAG (2016) Effect of a cryopreservation protocol on the proliferation of stem cells from human exfoliated deciduous teeth. Acta Odontol Scand 74(8):598–604
Gonda K, Shigeura T, Sato T, Matsumoto TD, Suga H, Inoue K, Aoi N, Kato H, Sato K, Murase I, Yoshimura K (2008) Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long term cryopreservation. Plast Reconstr Surg 121:401–410
Nhat Huynh NC, Le SH, Doan VN, Ngo LTQ, Tran HLB (2017) Simplified conditions for storing and cryopreservation of dental pulp stem cells. Arch Oral Biol 84:74–81
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved with 2015/34-243 ethical number.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
İslam, A., Özverel, C.S. & Yilmaz, H.G. Comparative evaluation of low-level laser therapy on proliferation of long-term cryopreserved human dental pulp cells isolated from deciduous and permanent teeth. Lasers Med Sci 36, 421–427 (2021). https://doi.org/10.1007/s10103-020-03090-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03090-3