Abstract
Studies reported the harmful effects of 2,4-D on body tissues, provoking changes in the anatomy and physiology of the kidneys, liver, and testicles. Thus, the objective was to evaluate if there were alterations in the bone quality of the tibia of rats submitted to feed consumption that were exposed to three different 2,4-D doses. Male Wistar rats were divided into four groups: oral control group (C: feed consumption without 2,4-D contamination); low oral concentration group (G3: contaminated feed with low concentration of 2,4-D); medium concentration group (G6: contaminated feed with medium concentration of 2,4-D); and high concentration group (G9: contaminated feed with high concentration of 2,4-D). The results demonstrated alterations of the mechanical properties and Raman ratios of the tibias of the contaminated groups. The maximum load, maximum stress, elastic modulus, and the cortical area were lower in the G6 and G9 compared to C group. The mineral-to-matrix ratio (relative mineral to organic content) was lower in the G6 and G9 groups compared to C group, but carbonate-to-matrix ratio (indicator of bone turnover) was higher in both groups. Thus, it is possible to suggest that the 2,4-D herbicide performed deleterious effects on the bone quality of male Wistar rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dichlorophenoxyacetic acid (2,4-D) is a type of herbicide widely used for weed control and also in cereal crops, forestry control, and especially sugarcane plantations [1]. As this compound is toxic, careful handling and application are required to avoid environmental and worker contamination, since it is rapidly distributed to body tissues and easily absorbed into the gastrointestinal tract [1].
The neurotoxic [2], hepatotoxic [3, 4], and cytotoxic effects [3, 5, 6] of the 2,4-D have been well-documented. This organic compound has also shown harmful effects on reproductive organs by interfering with their reproductive capacities. Joshi et al. [5] studied male rats exposed to 2,4-D and observed degenerative changes on the seminiferous tubules of the testicles, decreased fertility rates and decreased testicular, epididymis, seminal vesicle, and ventral prostate weights. In addition, they observed increased and decreased levels of serum cholesterol and testosterone, respectively. According to the authors, testosterone reduction clearly demonstrates a sign of disturbance in its biosynthesis and also an inhibitory effect on gonadotropic hormones. Thus, it can be suggested that the 2,4-D compound promoted the onset of acquired hypogonadism, which is a situation in which the gonads do not produce adequate amounts of sex hormones [7].
According to Carsote et al. [8], hypogonadism can cause sexual dysfunction, decrease muscle strength, and compromise bone mineralization. There are many articles in the literature that have related this disease to bone health impairment [7, 9, 10]. The association of the results of these studies with those of Joshi et al. [5] suggest that prolonged 2,4-D contamination may indirectly influence bone metabolism.
Other studies have demonstrated that 2,4-D contamination exerts a teratogenic effect, such as fetal skeletal malformation [11, 12]. In the study by Alpöz et al. [11], pregnant rats were fed a diet containing 2,4-D compound during the gestational period and during the breastfeeding period. After 30 days, the pups were sacrificed and it was found that exposure to 2,4-D compromised tooth development (odontogenesis), affected the function of odontoblasts and ameloblasts, and showed a lack of dentin matrix mineralization. In addition, Mazhar et al. [12] also studied offspring of contaminated female by 2,4-D mixed with corn oil and administered by gavage. A significant increase in the number of brood with morphological and skeletal defects was observed in the herbicide-treated group when compared to the control group. Among the defects, the authors observed reduced ossification of vertebrae, absence of long bone in limbs, and absence of ossification of metatarsals and phalanges bones.
There is evidence, therefore, that 2,4-D may compromise bone metabolism. However, there are no studies in the literature that have analyzed the effects of this compound on the bones of animals or even humans that have been exposed to direct or indirect contact with the herbicide. Thus, the aim of this study was to evaluate the bone quality of the tibias of Wistar rats submitted to the consumption of feed contaminated with the herbicide 2,4-D in three different concentrations.
Materials and methods
Animals
Male Wistar rats (n = 40) were used and provided by the Central Animal Facility of the University of Western São Paulo (UNOESTE), Presidente Prudente, SP, Brazil. The average weight was 150–200 g. The animals were kept in plastic cages (30 x 16 x 19 cm) in an experimental room with controlled temperature (22 ± 2 °C) and luminosity (12-12 h light-dark cycle). This study was approved by the Animal Experimentation Ethics Committee under protocol number 5224.
Division of groups and experimental protocol
The feed was nebulized for 15 min with 2,4-D herbicide by Nortox S.A. (Arapongas, Paraná, Brazil), registered with the Ministry of Agriculture, Cattle and Supplying (MAPA) under number 03009, with the following composition: 806 g/l (80.6% m/v) of 2,4-dichlorophenoxyacetic acid dimethylamine salt; 670 g/l (67.0% m/v) of acid equivalent of 2,4-D; 424 g/l (42.4% m/v) of inert ingredients.
The animals were randomly divided into four experimental groups (10 animals per group):
-
1)
Control Group (C): animals daily consumed feed that was previously nebulized with sodium chloride solution (NaCl)
-
2)
Low Concentration Group (G3): animals daily consumed feed that was previously nebulized with the herbicide 2,4-D at a concentration of 3.71 × 10−3 g of active ingredient per hectare
-
3)
Medium Concentration Group (G6): animals daily consumed feed that was previously nebulized with the herbicide 2,4-D at a concentration of 6.19 × 10−3 g of active ingredient per hectare
-
4)
High Concentration Group (G9): animals daily consumed feed that was previously nebulized with the herbicide 2,4-D at a concentration of 9.28 × 10−3 g of active ingredient per hectare
Exposure protocol to 2,4-D herbicide
The handling of the 2,4-D herbicide was carried out using the following personal protective equipment (PPE): rubber gloves, goggles, and gas filter masks. The experimental protocol used two plastic boxes (32x24x32 cm) each one connected to a Pulmosonic Star ultrasonic nebulizer [13]. The feed was exposed for 15 min to 2,4-D nebulization since this is the time required for all solutions to be nebulized. The feed was changed every 2 days and nebulization was performed 1 day before being placed for the animals. The experimental procedure was performed for 6 months and, after this period, the animals were euthanized and their tibias removed. Euthanasia was performed with sodium thiopental at a dose of 100 mg/kg of weight and administered to the peritoneal cavity. Clinical signs of death were the absence of respiratory movements, heartbeat, and loss of reflexes.
Mechanical test and morphometry of the diaphysis
For the mechanical test, tibias (n = 10 per group) were submitted to three-point bending testing until complete fracture at a velocity of 3 mm/min. An Instron (EMIC 23-2S) apparatus with a load cell of 100 kgf was used. The distance between the two lower supports was 17 mm. After testing of the specimens in three-point bending, the failure sites of all bone specimens were photographed, together with a measurement standard, by a high-resolution digital camera at a standardized distance according to Huang et al. [14]. The parameters of cortical thickness and cross-sectional cortical bone area of the diaphysis were measured on the images using the NIS-Elements 3.0 software (Advanced Research, USA).
The load and displacement data were obtained directly from the MTS system and recorded with a computer coupled to the testing machine. These data were used for the acquisition and calculation of the structural properties: maximum load, yield load, and extrinsic stiffness. The extrinsic stiffness was calculated as the slope of the most linear portion of the elastic region of the load–displacement curve [14,15,16,17]. The material properties were obtained from the structural properties [14, 17]. The following material properties were evaluated: maximum stress, maximum strain, and elastic modulus).
Raman spectroscopy
After euthanasia, the tibia was immersed in saline solution and stored at − 20 °C for subsequent Raman analysis. At the time of analysis, intact bones were stripped of the periosteum from the mid-shaft by lightly scraping with a scalpel and were wetted with phosphate-buffered saline. Thus, Raman spectra were then either acquired from the diaphysis of the tibia and were not processed/treated (baseline or offset correction), but the “spikes or cosmic rays” were removed from each one, if necessary. These samples were analyzed in a dry state.
Raman spectroscopy measurements were obtained with a micro-Raman spectrograph (inVia model from Renishaw), equipped with Leica optical microscope. A laser wavelength of 785 nm [18] with a 1200 lines per mm diffraction grating was used. The exposure time adopted was 10 s, with 5 accumulations and spectral range from 200 to 1800 cm−1. Spectra were collected using a 50X objective lens. The analysis was obtained from the cortical bone of the midpoint of the diaphysis of non-decalcified tibias.
In this analysis, we used relative peak intensities for certain pairs of bands (430, 960, 1070, 1272, 1660) from the Raman spectrum (Table 1). Then the following ratios were used: v2PO43−/amide III (430/1272) [19], v1PO43−/amide I (960/1660) [20], and v1CO32−/amide I (1070/1660) [21]. The 430/1272 ratio is associated with calcium content [19], 960/1660 ratio indicates the amount of mineralization, and 1070/1660 ratio may indicate bone turnover and remodeling activity [20, 21].
Statistical analysis
As normality was found in the sample distribution, parametric tests were used to analyze the mechanical parameters of the four independent groups. Thus, one-way analysis of variance (ANOVA) was used, followed by Tukey’s a posteriori test. Results were expressed as mean ± standard deviation. All tests were performed with 5% significance level.
Results
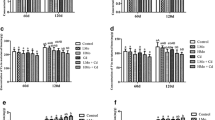
Statistical analysis of structural properties demonstrated that the maximum load was higher in the control group compared to the medium (C vs G6, p < 0.05) and high (C vs G9, p < 0.01) concentration groups but was similar to the low concentration group (G3). For this property, there was no difference between G6 and G9 groups. The maximum displacement showed no difference when comparing the four groups (p = 0.9988). Extrinsic stiffness only differed between the C and G9 groups (p < 0.01) (Fig. 1a, b, c).
In the study of material properties, the maximum stress of the C group was significantly different of the G6 and G9 groups (C vs G6, p < 0.05; C vs G9, p < 0.01), but the maximum strain was similar among all groups (p = 0.3108). The elastic modulus was higher compared to the G6 and G9 groups (C vs G6, p < 0.01; C vs G9, p < 0.01) (Fig. 1d, e, f).
The cross-sectional area (CSA) of the diaphysis of tibia presented higher value compared to the G6 (C vs G6, p < 0.05) and high concentration (GCO vs GACO = p < 0.01) compared to control group. The CSA of the G3 group was higher than the G9 group (p < 0.01) (Fig. 2). However, measurements for thickness showed no difference among all groups (p = 0.8051) (Fig. 3).
The 430/1272 ratio was similar for all groups analyzed (p = 0.1743), but the 960/1660 ratio (mineral-to-matrix) was lower in groups G6 (p < 0.05) and G9 (p < 0.05) compared to the control group. However, the 1070/1660 ratio (carbonate-to-matrix) was higher in groups G6 (p < 0.05) and G9 (p < 0.01) compared to the control group (Table 2). Figure 4 shows a representative Raman spectrum from the diaphysis of tibia of C, G3, G6, and G9 groups.
Discussion
There is a wide use of pesticides in Brazil and particularly Western of state of São Paulo presents a high rate of use of 2,4-D and atrazine [22], allowing contamination of people, animals, and the environment. This contamination can occur through direct (product handling) or indirect contact (respiration and food consumption) [23], and, therefore, many studies have been interested in the effects of 2,4-D under the physiological systems. Studies have reported anatomical and physiological impairment of the kidneys [24], liver [3, 25], and testicles [26, 27], demonstrating that 2,4-D can promote harmful effects on testicular function and, consequently, on testosterone production. Thus, it is interesting to investigate how foods exposed to different doses of 2,4-D could compromise tibias of Wistar rats, since there is no similar study in the literature.
The mechanical properties of bone can be classified into structural and material properties. Structural properties are related to bone geometry (architecture, shape, size, and dimension), while material properties refer to properties related to bone tissue composition, regardless of size and shape, and can be measured in homogeneous bone samples of unknown geometry [28]. Thus, material properties reflect the state of organization and constitution of macromolecules and cells in bone tissue and also its mineral content [17, 28].
In the present study, it was found that the tibias of the C group were more resistant to the load (force) applied during the mechanical test when compared to those of the groups that consumed contaminated feed with medium and high concentration of 2,4-D. In addition, a decrease in cortical area was also observed in all groups exposed to 2,4-D. Thus, there is sufficient evidence to suggest that the 2,4-D herbicide promoted a decrease in bone volume in the tibial shaft and interfered in the development and maturation of the skeleton of growing rats. This fact corroborates the study by Mazhar et al. [12]. These researchers studied the effect of 2,4-D contamination on pregnant rats and found impaired fetal growth and a high incidence of skeletal malformation, which was attributed to 2,4-D-induced oxidative stress. According to the authors, oxidative stress appears to have damaged embryos, causing peroxidation of cell membrane phospholipids and altering cell molecules such as lipids, proteins, and nucleic acids.
The analysis of the material properties demonstrated lower values of maximum tension and elastic modulus for the contaminated groups, indicating impairment to the bone quality of rats that consumed contaminated feed with medium and high doses of 2,4-D, making the bone more susceptible to the fracture. This possibly suggests that the tibias of the contaminated groups presented alterations of cell types, in the composition and organization of bone matrix molecules and in mineral content. Thus, 2,4-D contamination may have promoted osteoblast suppression and increased osteoclast activity per bone tissue area, since there is greater bone volume in the diaphysis of the control group. It is also possible to suggest that the contaminated groups (medium and high concentration) had some impairment in the process of differentiation of mesenchymal osteoprogenitor cells [29]. This hypothesis may be supported by the study by Miyakoshi et al. [30], in which it was verified that material properties are regulated by bone remodeling rate, cellular activity, and oxidative stress.
According to Marouani et al. [27], 2,4-D can cause damage to various body systems. They observed that oral exposure to 2,4-D for 30 days caused a decrease in serum testosterone level, histological changes in the testicles, and a decrease in sperm count. Thus, there is another factor that may have contributed to the impairment of the quality and mechanical resistance of the tibias of the contaminated groups. This other factor would be the level of testosterone found in animals since it is an essential hormone for maintaining bone integrity for both trabecular and cortical bone [31] by preventing osteoblast apoptosis and stimulating osteoclast apoptosis [29]. Another research group from our university found changes related to spermatic quality, such as reduced sperm cell production, reduced progressive motility, and also changes in sperm morphology (Ana Paula Alves Favareto - personal communication). These parameters may have been affected by the change in testosterone levels, but it cannot be stated that its reduction directly caused the changes mentioned.
According to Morris and Mandair [21], mechanical properties are related with mineral-to-matrix and carbonate-to-matrix ratios. The mineral-to-matrix (960/1660) ratio “represents the amount of mineral per amount of collagen per volume analyzed” [20]. The animals that consumed contaminated feed with medium and high concentration of 2,4-D demonstrated lower mineral-to-matrix ratios and higher carbonate-to-matrix ratios. This indicates that the herbicide promoted the decrease of the amount of mineralization of bone. However, these groups also demonstrated higher carbonate-to-matrix ratios. This can be attributed to high levels of bone remodeling since this ratio is an indicator of bone turnover, and it also has been associated with increased risk of fracture [21]. Thus, this might explain the lower values found for maximum stress and elastic modulus.
Considering the facts presented and the limitations of this study, such as number of analyses (cell count and hormonal dosage), it can be concluded that the herbicide 2,4-D had deleterious effects on the mechanical properties of tibias from male Wistar rats in the growing process and biological maturation. It should be considered that the amount of 2,4-D to which the organism is exposed may be a crucial factor for bone tissue strength.
References
Islam F, Wang J, Farooq MA, Khan MSS, Xu L, Zhu J, Zhao M, Muños S, Li QX, Zhou W (2018) Potential impact of the herbicide 2,4-dichlorophenoxyacetic acid on human and ecosystems. Environ Int 111:332–351. https://doi.org/10.1016/j.envint.2017.10.020
Freitas LM, Valadares LA, Camozzi M, de Oliveira PG, Ferreira Machado MR, Lima FC (2019) Animal models in the neurotoxicology of 2,4-D. Hum Exp Toxicol 38:1178–1182. https://doi.org/10.1177/0960327119860172
Tayeb W, Nakbi A, Trabelsi M, Attia N, Miled A, Hammami M (2010) Hepatotoxicity induced by sub-acute exposure of rats to 2,4-Dichlorophenoxyacetic acid based herbicide "Désormone lourd". J Hazard Mater 180:225–233. https://doi.org/10.1016/j.jhazmat.2010.04.018
Kalipci E, Ozdemir C, Oztas H (2013) Assessing eco-toxicological effects of industrial 2,4-D acid iso-octylester herbicide on rat pancreas and liver. Biotech Histochem 88:202–207. https://doi.org/10.3109/10520295.2012.758312
Joshi SC, Tibrewal P, Sharma A, Sharma P (2012) Evaluation of toxic effect of 2,4-D (2,4-dichlorophenoxyacetic acid) on fertility and biochemical parameters of male reproductive system of albino rats. Int J Pharm Pharm Sci 4:338–342
Mello FA, Quinallia G, Marion AC, Jorge FC, Marinelli LM, Salge AKM, Fagiani MAB, Mareco EA, Favareto APA, Silva RCR (2018) Evaluation of the nasal cavity mice submitted to the inhalation exposure to the herbicide 2,4-dichlorophenoxyacetic acid. Medicina (Ribeirão Preto Online) 51:247–253. https://doi.org/10.11606/issn.2176-7262.v51i4p247-253
Irwig MS (2014) Bone health in hypogonadal men. Curr Opin Urol 24:608–613. https://doi.org/10.1097/MOU.0000000000000104
Carsote M, Capatina C, Valea A, Dumitrascu A (2016) Vanishing testes syndrome-related osteoporosis and high cardio-metabolic risk in an adult male with long term untreated hypergonadotropic hypogonadism. Arch Endocrinol Metab 60:79–84. https://doi.org/10.1590/2359-3997000000127
Bolanowski M, Halupczok J, Jawiarczyk-Przybyłowska A (2015) Pituitary disorders and osteoporosis. Int J Endocrinol 2015:206853. https://doi.org/10.1155/2015/206853
Mirza F, Canalis E (2015) Management of endocrine disease: secondary osteoporosis: pathophysiology and management. Eur J Endocrinol 173:R131–R151. https://doi.org/10.1530/EJE-15-0118
Alpöz AR, Tosun N, Eronat C, Delen N, Sen BH (2001) Effects of 2,4-dichlorophenoxy acetic acid dimethyl amine salt on dental hard tissue formation in rats. Environ Int 26(3):137–142. https://doi.org/10.1016/S0160-4120(00)00095-7
Mazhar FM, Moawad KM, El-Dakdoky MH, Amer AS (2014) Fetotoxicity of 2,4-dichlorophenoxyacetic acid in rats and the protective role of vitamin E. Toxicol Ind Health 30:480–488. https://doi.org/10.1177/0748233712459915
Domingues RR, Mesquita GL, Cantarella H, Júnior DM (2011) Suscetibilidade do capim-colonião e de cultivares de milho ao flúor. Bragantia 70:729–736. https://doi.org/10.1590/S0006-87052011000400001
Huang TH, Lin SC, Chang FL, Hsieh SS, Liu SH, Yang RS (2003) Effects of different exercise modes on mineralization, structure, and biomechanical properties of growing bone. J Appl Physiol 95:300–307. https://doi.org/10.1152/japplphysiol.01076.2002
Akther MO, Cullen DM, Gong G, Recker RR (2001) Bone biomechanical properties in prostaglandin EP1 and EP2 knockout mice. Bone 29:121–125. https://doi.org/10.1016/s8756-3282(01)00486-0
Nakagaki WR, Bertran CA, Matsumura CY, Santo-Neto H, Camilli JA (2011) Mechanical, biochemical and morphometric alterations in the femur of mdx mice. Bone 48:372–379. https://doi.org/10.1016/j.bone.2010.09.011
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14:595–608. https://doi.org/10.1016/8756-3282(93)90081-k
Mandair GS, Morris MD (2015) Contributions of Raman spectroscopy to the understanding of bone strength. BoneKEy Rep 7:620–628. https://doi.org/10.1038/bonekey.2014.115
Roschger A, Gamsjaeger S, Hofstetter B, Masic A, Blouin S, Messmer P, Berzlanovich A, Paschalis EP, Roschger P, Klaushofer K, Fratzl P (2014) Relationship between the v2PO4/amide III ratio assessed by Raman spectroscopy and the calcium content measured by quantitative backscattered electron microscopy in healthy human osteonal bone. J Biomed Opt 19:065002. https://doi.org/10.1117/1.JBO.19.6.065002
Ciubuc JD, Manciu M, Maran A, Yaszemski MJ, Sundin EM, Bennet KE, Manciu FS (2018) Raman spectroscopic and microscopic analysis for monitoring renal Osteodystrophy signatures. Biosensors (Basel) 8(2)piiE38. https://doi.org/10.3390/bios8020038
Morris MD, Mandair GS (2011) Raman assessment of bone quality. Clin Orthop Relat Res 469:2160–2169. https://doi.org/10.1007/s11999-010-1692-y
Oliveira TG, Favareto APA, Antunes PA (2013) Agrotóxicos: levantamento dos mais utilizados no Oeste Paulista e seus efeitos como desreguladores endócrinos. Periódico Eletrônico Fórum Ambiental da Alta Paulista 9:375–390. https://doi.org/10.17271/198008279112013684
Souza A, Medeiros AR, Souza AC, Wink M, Siqueira IR, Ferreira MBC, Fenandes L, Hidalgo MPL, Torres ILS (2011) Evaluation of the impact of exposure to pesticides on the health of the rural population. Vale do Taquari, state of Rio Grande do Sul (Brazil). Ciênc. Saúde Coletiva 16:3519–3528. https://doi.org/10.1590/S1413-81232011000900020
Uyanikgil Y, Ateş U, Baka M, Biçer S, Oztaş E, Ergen G (2009) Immunohistochemical and histopathological evaluation of 2,4-dichlorophenoxyacetic acid-induced changes in rat kidney cortex. Bull Environ Contam Toxicol 82:749–755. https://doi.org/10.1007/s00128-009-9689-5
Duchnowicz P, Koter M (2003) Damage to the erythrocyte membrane caused by chlorophenoxyacetic acid herbicides. Cell Mol Biol Lett 8:25–30
Zhang D, Wu Y, Yuan Y, Liu W, Kuang H, Yang J, Yang B, Wu L, Zou W, Xu C (2017) Exposure to 2,4-dichlorophenoxyacetic acid induces oxidative stress and apoptosis in mouse testis. Pestic Biochem Physiol 141:18–22. https://doi.org/10.1016/j.pestbp.2016.10.006
Marouani N, Tebourbi O, Cherif D, Hallegue D, Yacoubi MT, Sakly M, Benkhalifa M, Ben Rhouma K (2017) Effects of oral administration of 2,4-dichlorophenoxyacetic acid (2,4-D) on reproductive parameters in male Wistar rats. Environ Sci Pollut Res Int 24:519–526. https://doi.org/10.1007/s11356-016-7656-3
Cole JH, van der Meulen MC (2011) Whole bone mechanics and bone quality. Clin Orthop Relat Res 469:2139–2149. https://doi.org/10.1007/s11999-011-1784-3
Mohamad NV, Soelaiman IN, Chin KY (2016) A concise review of testosterone and bone health. Clin Interv Aging 11:1317–1324. https://doi.org/10.2147/CIA.S115472
Miyakoshi N, Fujii M, Kasukawa Y, Shimada Y (2019) Impact of vitamin C on teriparatide treatment in the improvement of bone mineral density, strength, and quality in vitamin C-deficient rats. J Bone Miner Metab 37:411–414. https://doi.org/10.1007/s00774-018-0941-0
Saki F, Kasaee SR, Sadeghian F, Talezadeh P, Ranjbar Omrani GH (2018) The effect of testosterone itself and in combination with letrozole on bone mineral density in male rats. J Bone Miner Metab 32:1–8. https://doi.org/10.1007/s00774-018-0972-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rocha, D.R., Nery, J.F., Furini, L.N. et al. Effects of consumption of contaminated feed with 2,4-dichlorophenoxyacetic acid (2,4-D) on the rat tibia: analysis by Raman spectroscopy and mechanical properties. Lasers Med Sci 35, 1703–1709 (2020). https://doi.org/10.1007/s10103-020-02961-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-02961-z