Abstract
The aim of this study was to evaluate the bactericidal effect of ultraviolet (UV) light at 265-nm (UVC) and 310-nm (UVB) wavelengths from a newly developed UV light-emitting diode (LED) device against cariogenic bacteria in vitro. Suspensions of Streptococcus mutans (S. mutans) and Streptococcus sobrinus were directly irradiated by UVB or UVC for 2.5 min or 5 min. Numbers of colonies were counted and calculated as colony forming units (CFU) per milliliter. Fluorescence microscopy (FM) and optical density measurements at 490 nm (OD490) were also taken after irradiation. In addition, the bactericidal effects of irradiation against S. mutans under 0.5 mm-thick dentin were compared using culture tests and OD490 measurements. Direct UV-LED irradiation with both UVB and UVC showed strong bactericidal effects. UVB showed superior bactericidal effect through 0.5-mm-thick dentin than did UVC, especially after demineralization. These results suggest that UVB irradiation could be utilized for the prevention and management of dental caries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental caries is the most prevalent chronic disease and involves infection by cariogenic bacteria and destruction of tooth structure. It is directly caused by acid metabolites of bacterial fermentation of dietary carbohydrates in oral biofilm. Oral biofilm is an aggregate of microbes found on tooth surfaces and embedded in a matrix of polymers of bacterial and salivary origin. It is an etiological factor for dental caries and other oral diseases [1]. Although several acidogenic (acid-producing) and aciduric (acid-tolerating) bacteria can lower pH to the level at which enamel is demineralized, oral biofilm-forming Streptococcus mutans is considered the main pathogenic contributor to dental caries [2]. Despite control of oral biofilm by mechanical tooth cleaning, which is key to prevention and control of dental caries and other oral diseases, maintaining microbial organisms in the correct balance is nearly impossible when traditional methods fail to remove biofilm effectively [1]. The inability of older people to maintain a clean oral environment can increase the risk of caries. Moreover, reduced saliva and loss of periodontal attachment in aged people are risk factors for the development of root caries [3].
New technologies based on phototherapy have been suggested to suppress subgingival bacteria species with the pervasive resistance to antibacterial therapy [4]. Previous studies have demonstrated that photodynamic therapy employing photosensitizers has a potent antibacterial effect. Ultraviolet (UV) irradiation has bactericidal effects that result from photochemical damage to nucleic acids. UV light wavelengths are classified as UVC (100–280 nm), UVB (280–315 nm), and UVA (315–400 nm). UV light irradiation can alter the union between the double chains to inhibit DNA replication and transcription by forming thymine-thymine dimers and thymine-cytosine dimers [5]. Of the UV light classes, UVC has the strongest bactericidal effect, while narrowband (NB)-UVB at 310 nm has fewer side effects in humans and is effective for treatment of various skin diseases that show strong immune responses [6]. Recently, NB-UVB irradiation was reported to have a direct bactericidal effect on oral bacteria with little cytotoxicity to oral epithelial cells [7]. Moreover, UVB light irradiation has an indirect bactericidal effect by producing reactive oxygen species (ROS) in oral epithelial cells that may kill Porphyromonuas gingivalis (P. gingivalis)[7, 8].
Recently, photodynamic therapy (PDT) has been used for the treatment of various diseases caused by bacteria, fungal, and viral infection [9,10,11]. Previous studies have shown that growth or inhibition of oral bacteria after irradiation depends on wavelength and irradiation time [12, 13]. The bactericidal effects of blue-light irradiation in general are well evidenced in several hundreds of in vitro studies and clinical case reports [14]. The bactericidal effects of irradiation with a 425 nm LED on P. gingivalis have been reported [12]. However, to obtain enough bactericidal effect, photosensitizers are being used in addition to the blue light to perform photochemical reactions in many cases. Photosensitizers, such as methylene blue, toluidine blue, porphyrin, and others, are being used in the clinics targeting both gram-positive and gram-negative bacteria [9]. Unfortunately, use of those chemicals carries certain disadvantages, including rapid loss of the photochemical reaction due to degradation and adverse effects on the teeth and restorations. On the other hand, UVB are being used widely in the field of dermatology for long under the established biological safety guideline. Therefore, UVB is a potential option for control of intraoral pathogenic bacteria [7].
A pen-type light-emitting diode (LED) device that permits intraoral UV irradiation was newly developed. The purpose of this study was to evaluate the bactericidal effect of UVB and UVC irradiation from the device against cariogenic bacteria in vitro.
Materials and methods
Bacterial strains
Streptococcus mutans MT8148 (S. mutans) and Streptococcus sobrinus 6715 (S. sobrinus) were used in this study. Separate suspensions of S. mutans and S. sobrinus in phosphate-buffered saline (PBS) were prepared using bacteria freshly cultured for 16 h in brain heart infusion broth (BHI; BD Biosciences, Franklin Lakes, NJ, USA) washed three times with PBS and were then stored at 4 °C with gentle stirring. Afterwards, the bacteria were resuspended in PBS to an optical density of 490 nm (OD490) = 0.5 (approximately 3.6 × 108 colony forming unit (CFU)/mL) using a spectrophotometer (Model 680 Microplate Reader; Bio-Rad, Hercules, CA, USA). The suspensions were then used immediately for the tests described below after aliquoting 100 μL into each well of sterile 96-well flat-bottomed culture plates.
UV-LED irradiation device
A UV-LED irradiation device and two handheld emitters (310 nm UVB and 265 nm UVC) were used in this study. The handheld emitters were cylindrical in shape with a 6 mm diameter irradiation aperture for the UV-LED light driven by a DC power unit built inside the UV control system (10–500 mA and 12 V). The current value of the UVB was 90 mA, and that of UVC was 350 mA on the surface area at the aperture point, as set by the manufacturer (NIKKISO, Ishikawa, Japan) to deliver approximate irradiance at the surface of each well. The exposure amount at each radiation time was calculated as E = P × t, where E is the energy density (dose) (mJ/cm2), P is the power intensity (irradiance) (mW/cm2), and t is the time (s).
Direct UV-LED irradiation
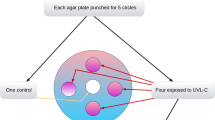
Aliquoted suspensions in a 96-well plate were individually irradiated by UVC for 5 min (UVC5) or 2.5 min (UVC2.5) and by UVB for 5 min (UVB5) or 2.5 min (UVB2.5) as separate groups, and a non-irradiated group was prepared as a control (PBS). For all conditions, three suspension- wells were used. The irradiation dose for each group was as follows; UVC5 = 3.2 J/cm2, UVC2.5 = 1.6 J/cm2, UVB5 = 3.0 J/cm2, and UVB2.5 = 1.5 J/cm2. The irradiation times were determined to keep the irradiation dose (energy density E = P × t) within the safe range as mentioned in The Japanese Dermatological Association guideline and elsewhere [15], targeting to acquire effective results on the cariogenic bacteria.
Bacterial viability test
A LIVE/DEAD BacLight Bacterial Viability Kit (Thermo Fisher Scientific, Waltham, MA, USA) was to evaluate the effect of irradiation on the viability of each organism. In each group, a 100-μL suspension was transferred into a dark microtube and stained with 0.5-μL BacLight stain (a mixture of SYTO 9 and propidium iodide). In this staining system, viable bacterial cells exhibit green fluorescence, whereas nonviable cells exhibit red fluorescence. Selective dye uptake depends upon cell membrane integrity, allowing dead bacteria to be easily distinguished from viable bacteria. The excitation/emission wavelengths of the dyes were approximately 480/530 nm for SYTO 9 (green signals) and 520/580 nm for propidium iodide (red signals). Viability of bacterial cells was evaluated using a fluorescence microscope (FM, CKX41; Olympus, Tokyo, Japan).
Bacterial cell growth after irradiation (CFU/mL count)
Each bacterial cell suspension (10 μL) was serially diluted and plated with a spiral plating instrument (Eddy Jet; IUL Instruments, Barcelona, Spain). All S. mutans and S. sobrinus samples were plated in petri dishes containing Mitis Salivarius (MS) agar medium (BD Biosciences). After a 48-h incubation under anaerobic conditions at 37 °C, the number of CFU per milliliter was counted using a light microscope.
Growth curve of the irradiated bacteria (OD490)
One hundred microliters of autoclaved BHI solution was added to each well containing the bacterial suspensions 3 h after irradiation and incubated at 37 °C, and optical density was measured every 1 h until 15 h using the same spectrophotometer at 490 nm.
Penetration UV-LED irradiation through dentin
Eighteen bovine incisors were used for this experiment (ethics committee approval from the Local Government Authority, Yokohama, Japan was obtained). All teeth were sectioned vertically to the occlusal surface with a low-speed saw (Isomet; Buehler, Lake Bluff, IL, USA) under running water to obtain approximately 1.5 mm-thick mid-coronal dentin slices. The slices were then ground flat with 800-grit silicon carbide paper under running water and polished to 0.5-mm thickness. The dentin slices were then divided into two groups and immersed in two types of demineralizing solutions: (1) ethylenediaminetetraacetic acid (EDTA) and (2) acetic acid-based demineralizing solution (AADS) containing 2.2 mmol/L CaCl2, 2.2 mmol/L KH2PO4, 50 mmol/L acetic acid, and 0.02% NaN3 (10 mL per specimen) at pH 4.5 and 37 °C under constant agitation for 7 days. A total of six dentin slices were used in one experiment: two each for the two above mentioned demineralization conditions and two more as un-demineralized controls. A fresh S. mutans suspension was prepared, and 100 μL was aliquoted into each well of a 96-well culture plate as described above. Afterwards, six dentin slices were placed on the culture plate over the aliquoted bacterial suspensions, and UVB or UVC light was applied thereon for 5 min. All suspensions were then plated on MS agar plates as above for counting CFU/mL. In addition, effects on bacterial proliferation were monitored hourly by measuring OD490 as described above. Group distribution of demineralization and irradiation conditions is shown by two letter abbreviations, e.g., UVC after EDTA (EC), UVB after EDTA (EB), UVC after AADS (AC), UVB after AADS (AB), UVC after no treatment (NC), and UVB after no treatment (NB). The survival rate of S. mutans after UVB irradiation through demineralized dentin was estimated by the formula:
- S:
-
Bacterial survival rate
- PO:
-
Number of bacteria (CFU) before UV irradiation
- P:
-
Number of bacteria (CFU) after UV irradiation.
Statistical analysis
All experiments were performed in triplicate to assure reproducibility. The bactericidal effects of direct UV-LED irradiation on S. mutans and S. sobrinus were statistically analyzed using Dunnett’s T3 test with Bonferroni correction. The photoinactivation effects of UVB and UVC on S. mutans under demineralized dentin were statistically analyzed using two-way analysis of variance (ANOVA) followed by pairwise comparisons using Bonferroni correction. All statistical analyses were performed with 95% level of confidence using Statistical Package for Medical Science (Version 23.0 for Windows; SPSS, Chicago, IL, USA).
Results
Bactericidal effects of UVB and UVC on S. mutans and S. sobrinus
The results of CFU/mL measurements of S. mutans and S. sobrinus after direct exposure to UVB and UVC are shown in Fig. 1 and Table 1. Significant bactericidal effects from UV-LED irradiation were observed for both S. mutans and S. sobrinus compared with control group (p < 0.05). There was no significant difference between UVC5, UVC2.5, and UVB5 exposure (p > 0.05), but UVB2.5 showed significantly lower bactericidal effects than did the other three groups (p < 0.05).
Growth inhibition of S mutans and S. sobrinus after UVB and UVC irradiation
UVB and UVC irradiation both resulted in growth inhibition of both S. mutans and S. sobrinus (Fig. 1). When BHI was added after 3 h, the optical density temporarily increased in all groups. Afterwards, the bacteria in all UV-LED irradiated groups entered a growth inhibitory phase showing substantially lower OD490 than did the control culture. UVC5 showed the strongest growth inhibition ability throughout the observation period.
Fluorescence microscopy photographs
The viability of S. mutans is depicted in Fig. 2. UVC5 irradiation resulted in most S. mutans cell death, i.e., the greatest proportion of red cells was observed by FM. On the other hand, all other UV-LED irradiated groups (UVC 2.5, UVB 5, and UVB 2.5) displayed very weak bactericidal effects, with only a few red cells and many green cells detected.
Bactericidal effects up on penetration through demineralized dentin
The CFU/mL scores measured for S. mutans after UVB and UVC irradiation for 5 min through the dentin slices are presented in Fig. 3 and Table 2. Regardless of the demineralization method, UVB irradiation showed a significantly stronger bactericidal effect than did UVC irradiation. In addition, regardless of wavelength, UV-LED irradiation through EDTA-decalcified dentin showed a significantly stronger bactericidal effect than that through AADS-decalcified dentin (p < 0.05). However, when intact dentin was used, significantly lower bactericidal effects were observed for both UVB and UVC irradiations than for both demineralization conditions (p < 0.05). As the measured bacterial concentrations remained well below the control growth curve, inhibition of S. mutans growth through demineralized dentin slices under all UV-LED irradiation conditions compared to growth in non-irradiated controls in BHI was clear (Fig. 3). In the group using intact dentin, growth inhibition of S. mutans was observed only in the case of NB, while the growth curve in the case of NC was almost the same as that of the control group. The survival rate of S. mutans was minimum in the case of EB and maximum in the case of NC; others were in-between (Table 3).
Discussion
The bactericidal effects of UV light are well evidenced by many studies. Despite UV light showing a photoinactivation effect against microorganisms, its use in the intraoral environment is limited due to the size of emitting devices [11]. The pen-sized devices used in this study were newly developed for an efficient blue LED that enables emitting UV light for intraoral usage. In this study, direct irradiation of S. mutans and S. sobrinus produced significant bactericidal effect after 2.5- and 5-min emission of both UVB and UVC. Moreover, UVB transmitted through demineralized dentin showed a superior ability to kill and inactivate S. mutans than did UVC.
In dermatology, UVB dosage is determined according to the minimal erythema dose (MED). Despite the broad range of MED values for UVB according to skin type, the maximum dose is usually set at 4 MED, which ranges from 2.8 to 4.0 J/cm2 [16]. In this experiment, UVB light was output at 10.1 mW/cm2 for 5 min (300 s). Consequently, the energy density was calculated as 3.03 J/cm2, which is within the range of 4 MED. Moreover, as a directable, monochromatic beam, LED light has an advantage in treating the affected lesions. Furthermore, since the diameter of the UVB and UVC light aperture is only 6 mm, it appears to be convenient enough to irradiate UV-LED comfortably to deliver sufficient doses to the lesion sites leaving the unaffected areas unharmed at the time of treating a carious tooth in the clinics. Therefore, in targeted phototherapy, the risk of exposure to UV radiation at sites other than the irradiation site would be minimum [17].
In this study, the bactericidal effects of UVB and UVC transmitted through 0.5 mm-thick dentin discs were dramatically decreased compared to those of direct irradiation. However, transmitted UVB showed significant bactericidal effect over growth seen in the control. Meanwhile, UVC irradiation through intact dentin exhibited no bactericidal effect compared with growth seen in the control; thus, it is probable that attenuation of UV light transmitted through dentin differs according to UV wavelength. Several studies have shown that as wavelength increases, the translucency of teeth increases [18, 19]. Whether light propagating through dentin follows the Beer-Lambert law is not well-known. Linearity between absorbance and material concentration is limited if the media is highly scattering.
Dentin contains 45% mineral hydroxyapatite, 33% organic material, and 22% water, consists of microscopic tubular structures, and shows absorption of light with a low light transmission ratio. Upon light irradiation of dentin, the tubules behave as a cylindrical Mie scatterers, thereby showing greater light scattering than that shown by enamel and high anisotropy [20]. Moreover, hydroxyapatite crystals in the peritubular and intertubular dentin likely refract and reflect the beam at the surface [21]. In this study, once the dentin discs were demineralized using EDTA or AADS, the bactericidal effect of transmitted UV-LED dramatically increased. Although the amount of mineral loss after demineralization was not evaluated in this experiment, we speculated that demineralization decreased light scattering from the mineral crystals, resulting in the increased bactericidal effect of transmitted UVB and UVC.
UV-LED irradiation after EDTA demineralization showed significant bactericidal effects, and UVB showed a stronger effect than did UVC. Since the bactericidal effect of the UV-LED through demineralized dentin was also influenced by wavelength, longer wavelengths appears advantageous effect for killing bacteria within demineralized dentin. In this experiment, the survival rate of S. mutans after UVB irradiation through demineralized dentin was 16% for EDTA group and 32% for AADS group.
Although carious dentin removal is considered essential to the prognosis of restorations, whether or not to leave infected dentin beneath the restoration is controversial. In general, complete carious dentin removal is difficult to achieve clinically. Microorganisms have been detected after removal of all soft demineralized dentin [22]. Motta et al. [23] reported that the survival rate of total bacteria after caries removal by traditional low-speed bur was 56%. In recent years, it has been reported that what determines the prognosis of dental caries treatment is not complete removal of dental caries, but sealing, and sealed carious dentin did not affect the short-term prognosis of restoration [24, 25]. However, it is difficult to make a complete seal in clinical situations, considering the clinical environment and patient diversity. For that reason, the importance of reducing the number of bacteria as much as possible is clear. In addition, there have been reports that some minimally invasive (MI) methods of removing carious dentin showed favorable results compared to conventional methods using low-speed burs [26,27,28]. Therefore, the 16% and 32% survival rates of bacteria obtained from transmitted UVB are superior to the conventional partial removal method.
There is reportedly a direct relationship between permeability of dentin and its transmittance of light [29]. Although dentinal tubules are considered the most important scatterers in dentin [30, 31], some studies have reported that collagen fibers play an important role in light transmission [29, 32]. It was also reported that treatment with EDTA causes less damage to the collagen matrix compared to demineralization with acetic acid [33]. Demineralization with acetic acid exhibits faster kinetics in dentin lesion production and abundant residual minerals [34]. The refraction and reflection of light at the surface of hydroxyapatite crystals in peritubular and intertubular dentin complicate transmission [21]. We suggest that these facts account for the difference in bacterial viability after UV-LED irradiation through demineralized dentin in this study.
The bactericidal effect of UV-LED irradiation can be explained by two mechanisms same as for UV that were reported previously; one is DNA damage, and the other is bacterial cell membrane damage [35]. Limei et al. [36] reported that irreversible damage to cytoplasmic membranes and that cellular ATP was negligible at low UV doses. However, the UV-LED irradiation was applied on S. mutans and S. sobrinus in this study, and both are gram-positive bacteria and have got thick cell wall around their cell membrane. The UV-LED irradiated group showed a 3 log or more reduction in bacteria in the culture test and only a slight increase in OD490 compared to that of PBS demonstrating that UV-LED irradiation has a significant bactericidal effect on cariogenic bacteria. Moreover, in all groups subjected to UV-LED irradiation, considerable numbers of PI stained red cells were observed on inspection of the FM images unlike the control group displaying the evidence of the bactericidal effect. Although the effect of UV-LED irradiation is obvious, in microscopic images, we observed a good number of green bacterial cells with no membrane damage. Also, dead cells stained PI did not have bright red color indicating less penetration of the stain through their cell wall and membrane. Previously, it was reported that ROS produced by UV irradiation damages bacterial DNA [37]. These facts suggest that the effect of UV-LED irradiation in this study is mainly due to DNA damage rather than due to direct structural damage of the bacteria [38]. As these bacteria are part of human oral microbiota, they tend to find an opportunity better for their survival. They are considered to always re-colonize the surfaces of teeth where nutrition exists. This was observed in our study, and OD490 was equivalently increased in all groups when BHI was added. BHI medium is a favorable environment for bacterial growth, closely mimicking oral conditions after intake of sweets, even though UV-LED irradiation was not able to eliminate the cariogens completely but displayed that potent enough to be applied for prevention of caries or secondary caries, especially treating cases with high caries risks.
There are several limitations of present study. When bacterial biofilm is formed on the tooth surface, it is produced by interactions between bacteria, rather than by a single bacterium. In addition, it is well-known that the composition of bacteria in dental plaque changes over time. Since this experiment was conducted only with S. mutans and S. sobrinus which are involved in the initial pathogenesis of dental caries, it may not precisely model the effect of UV-LED irradiation after bacterial colonization [39]. Further, for UV light use in the oral cavity, high safety standards will be required to obtain the desired effects in a short time. Before clinical application, further studies will be required to determine the optimum wavelength and application method of UV-LED for human teeth.
As clinical significance, this study report would play an important role in the following clinical conditions such as after caries removal both from the crown structures and root surfaces when MI is considered leaving the base of the carious tissue. As obvious, some cariogenic bacteria remain there, and those may regenerate spontaneously and develop secondary caries. Even, in an ideally prepared cavity after complete removal of caries bacteria, additional disinfection would be useful. In those conditions, UV-LED irradiation at a safe low dose might be useful as a preventive therapy, as same can be useful after plaque removal or treating the undercut areas from where caries removal is difficult. Moreover, progression of shallow pit and fissure caries can be slowed down by irradiating with this UV-LED. As commonly known, UV irradiation can cause DNA damage including fragmentation or mutation of human cells resulting in developing carcinoma. The device tested in this study is built with the principal of controlled delivery of low-dosed UV-LED to irradiate a certain target area. For caries control treatment, UV-LED irradiation can be limited to the carious part area only, not involving risk-prone tissues—use of rubber dam would be safer. Studies on the cytotoxicity of this UV-LED irradiation would be useful.
Conclusion
Controlled UVB irradiation displayed strong bactericidal effects against cariogenic bacteria. In addition, it was shown to have great effects against S. mutans under demineralized dentin. These findings suggest that adding UVB irradiation to conventional dental treatment may efficiently prevent and treat dental caries.
References
Gomez GF, Huang R, MacPherson M, Ferreira Zandona AG, Gregory RL (2016) Photo inactivation of Streptcoccus mutans biofilm by violet-blue light. Curr Microbiol 73(3):426–433
Rosier BT, De Jager M, Zaura E, Krom BP (2014) Historical and contemporary hypotheses on the development of oral diseases: are we there yet? Front Cell Infect Microbiol 4(92). https://doi.org/10.3389/fcimb.2014.00092
Murray CG (2015) Advanced restorative dentistry—a problem for the elderly? An ethical dilemma. Aust Dent J 60(suppl 1):106–113
ten Cate JM, Zaura E (2012) The numerous microbial species in oral biofilms: how could antibacterial therapy be effective? Adv Dent Res 24(2):108–111
Blázquez E, Rodríguez C, Ródenas J, de Rozas AP, Segalés J, Pujols J, Polo J (2017) Ultraviolet (UV-C) inactivation of enterococcus faecium, Salmonella choleraesuis and Salmonella typhimurium in porcine plasma. PLoS One 12(4):e0175289. https://doi.org/10.1371/journal.pone.0175289
Patrizi A, Raone B, Ravaioli GM (2015) Management of atopic dermatitis: safety and efficacy of phototherapy. Clin Cosmet Investig Dermatol 8:511–520
Takada A, Matsushita K, Horioka S, Furuichi Y, Sumi Y (2017) Bactericidal effects of 310 nm ultraviolet light-emitting diode irradiation on oral bacteria. BMC Oral Health 17(1):96. https://doi.org/10.1186/s12903-017-0382-5
Leke N, Grenier D, Goldner M, Mayrand D (1999) Effects of hydrogen peroxide on growth and selected properties of Porphyromonas gingivalis. FEMS Microbiol Lett 174(2):347–353
Maisch T, Hackbarth S, Regensburger J, Felgentrager A, Baumler W, Landthaler M, Roder B (2011) Photodynamic inactivation of multi-resistant bacteria (PIB)—a new approach to treat superficial infections in the 21st century. J Dtsch Dermatol Ges 9(5):360–366
Wainwright M (1998) Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother 42(1):13–28
Morimoto K, Ozawa T, Awazu K, Ito N, Honda N, Matsumoto S, Tsuruta D (2014) Photodynamic therapy using systemic administration of 5-aminolevulinic acid and a 410-nm wavelength light-emitting diode for methicillin-resistant Staphylococcus aureus-infected ulcers in mice. PLoS One 9(8):e105173. https://doi.org/10.1371/journal.pone.0105173
Kim S, Kim J, Lim W, Jeon S, Kim O, Koh JT, Kim CS, Choi H, Kim O (2013) In vitro bactericidal effects of 625, 525, and 425 nm wavelength (red, green, and blue) light-emitting diode irradiation. Photomed Laser Surg 31(11):554–562
Cieplik F, Spath A, Leibl C, Gollmer A, Regensburger J, Tabenski L, Hiller KA, Maisch T, Schmalz G (2014) Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clin Oral Investig 18(7):1763–1769
Yin R, Dai T, Avci P, Jorge AE, de Melo WC, Vecchio D, Huang YY, Gupta A, Hamblin MR (2013) Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr Opin Pharmacol 13(5):731–762
Furuhashi T, Saito C, Torii K, Nishida E, Yamazaki S, Morita A (2013) Photo (chemo) therapy reduces circulating Th17 cells and restores circulating regulatory T cells in psoriasis. PLoS One 8(1):e54895. https://doi.org/10.1371/journal.pone.0054895
Morita M, Etoh T, Torii H, Ito T, Nemoto O, Kamide R, Kawada A (2016) Phototherapy guideline for psoriasis. Jpn J Dermatol 126(7):1239–1262
Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, Gottlieb A, Koo JY, Lebwohl M, Lim HW, Van Voorhees AS, Beutner KR, Bhushan R (2010) Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol 62(1):114–135
Arikawa H, Kanie T, Fujii K, Ban S, Takahashi H (2004) Light-attenuating effect of dentin on the polymerization of light-activated restorative resins. Dent Mater J 23(4):467–473
Uusitalo E, Varrela J, Lassila L, Vallittu PK (2016) Transmission of curing light through moist, air-dried, and EDTA treated dentine and enamel. Biomed Res Int 2016:5713962. https://doi.org/10.1155/2016/5713962
Chan AC, Darling CL, Chan KH, Fried D (2014) Attenuation of near-IR light through dentin at wavelengths from 1300-1650-nm. Proc SPIE Int Soc Opt Eng 8929:89290M. https://doi.org/10.1117/12.2045629
Burns T, Wilson M, Pearson GJ (1995) Effect of dentine and collagen on the lethal hotosensitization of Streptococcus mutans. Caries Res 29(3):192–197
Maltz M, Henz SL, de Oliveira EF, Jardim JJ (2012) Conventional caries removal and sealed caries in permanent teeth: a microbiological evaluation. J Dent 40(9):776–782
Motta LJ, Bussadori SK, Campanelli AP, Silva AL, Alfaya TA, Godoy CH, Navarro MF (2014) Efficacy of Papacarie(®) in reduction of residual bacteria in deciduous teeth: a randomized, controlled clinical trial. Clinics (Sao Paulo) 69(5):319–322
Bjørndal L, Reit C, Bruun G, Markvart M, Kjaeldgaard M, Näsman P, Thordrup M, Dige I, Nyvad B, Fransson H, Lager A, Ericson D, Petersson K, Olsson J, Santimano EM, Wennström A, Winkel P, Gluud C (2010) Treatment of deep caries lesions in adults: randomized clinical trials comparing stepwise vs. direct complete excavation, and direct pulp capping vs. partial pulpotomy. Eur J Oral Sci 118:290–297
Orhan AI, Oz FT, Ozcelik B, Orhan K (2008) A clinical and microbiological comparative study of deep carious lesion treatment in deciduous and young permanent molars. Clin Oral Investig 12:369–378
Banerjee A, Kellow S, Mannocci F, Cook RJ, Watson TF (2010) An in vitro evaluation of microtensile bond strengths of two adhesive bonding agents to residual dentine after caries removal using three excavation techniques. J Dent 38(6):480–489
Neves Ade A, Coutinho E, De Munck J, Van Meerbeek B (2011) Caries-removal effectiveness and minimal-invasiveness potential of caries-excavation techniques: a micro-CT investigation. J Dent 39(2):154–162
Li H, Wang WM, Yu SL, Wen Q (2011) Morphological and microtensile bond strength evaluation of three adhesive systems to caries-affected human dentine with chemomechanical caries removal. J Dent 39(4):332–339
Turrioni AP, de Oliveira CF, Basso FG, Moriyama LT, Kurachi C, Hebling J, Bagnato VS, Costa CA (2012) Correlation between light transmission and permeability of human dentin. Lasers Med Sci 27(1):191–196
Pop-Ciutrila IS, Ghinea R, Perez Gomez MDM, Colosi HA, Dudea D, Badea M (2015) Dentine scattering, absorption, transmittance and light reflectivity in human incisors, canines and molars. J Dent 43(9):1116–1124
Vaarkamp J, ten Bosch JJ, Verdonschot EH (1995) Propagation of light through human dental enamel and dentine. Caries Res 29:8–13
Kienle A, Michels R, Hibst R (2006) Magnification—a new look at a long-known optical property of dentin. J Dent Res 85:955–959
Ghisi AC, Kopper PM, Baldasso FE, Stürmer CP, Rossi-Fedele G, Steier L, de Figueiredo JA, Morgental RD, Vier-Pelisser FV (2015) Effect of superoxidized water and sodium hypochlorite, associated or not with EDTA, on organic and inorganic components of bovine root dentin. J Endod 41(6):925–930
Chien YC, Burwell AK, Saeki K, Fernandez-Martinez A, Pugach MK, Nonomura G, Habelitz S, Ho SP, Rapozo-Hilo M, Featherstone JD, Marshall SJ, Marshall GW (2016) Distinct decalcification process of dentin by different cariogenic organic acids: kinetics, ultrastructure and mechanical properties. Arch Oral Biol 63:93–105
Azizi A, Shademan S, Rezai M, Rahimi A, Lawaf S (2016) Effect of photodynamic therapy with two photosensitizers on Streptococcus mutants: in vitro study. Photodiagn Photodyn Ther 16:66–71
Xu L, Zhang C, Xu P, Wang XC (2018) Mechanisms of ultraviolet disinfection and chlorination of Escherichia coli: culturability, membrane permeability, metabolism, and genetic damage. J Environ Sci (China) 65:356–366
Yao L, Rong Q, Zaat SAJ, Eefjan B, Michal H (2015) Antibacterial photodynamic therapy: overview of a promising approach to fight antibiotic-resistant bacterial infections. J Clin Translat Res 1(3):140–167
Kim S, Ghafoor K, Lee J, Feng M, Hong J, Lee DU, Park J (2013) Bacterial inactivation in water, DNA strand breaking, and membrane damage induced by ultraviolet-assisted titanium dioxide photocatalysis. Water Res 47(13):4403–4411
Kim S, Song M, Roh BD, Park SH, Park JW (2013) Inhibition of Streptococcus mutans biofilm formation on composite resins containing ursolic acid. Resror Dent Endod 38(2):65–72
Funding
This research project was supported by the Research Grant for Longevity Science (29-3) from the Japanese Ministry of Health, Labor and Welfare, and by a Grant-in-Aid for Scientific Research (16 K11544) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Uchinuma, S., Shimada, Y., Matin, K. et al. Effects of UVB and UVC irradiation on cariogenic bacteria in vitro. Lasers Med Sci 34, 981–989 (2019). https://doi.org/10.1007/s10103-018-2685-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2685-4