Abstract
Radiotherapy is an extensively used treatment for most tumor types. However, ionizing radiation does not discriminate between cancerous and normal cells surrounding the tumor, which can be considered as a dose-limiting factor. This can lead to the reduction of the effectiveness of tumor cell eradication with this treatment. A potential solution to this problem is loading the tumor with high-Z materials prior to radiotherapy as this can induce higher toxicity in tumor cells compared to normal ones. New advances in nanotechnology have introduced the promising use of heavy metal nanoparticles to enhance tumor treatment. The primary studies showed that gold nanoparticles (GNPs) have unique characteristics as biocompatible radiosensitizers for tumor cells. This study aimed to quantify the dose enhancement effect and its radial dose distribution by Monte Carlo simulations utilizing the EGSnrc code for the water–gold phantom loaded with seven different concentrations of Au: 0, 7, 18, 30, 50, 75, and 100 mg-Au/g-water. The phantom was irradiated with two different radionuclide sources, Ir-192 and Cs-137, which are commonly used in brachytherapy, for all concentrations. The results exhibited that gold nanoparticle-aided radiotherapy (GNRT) increases the efficacy of radiotherapy with low-energy photon sources accompanied with high Au concentration loads of up to 30 mg-Au/g-water. Our finding conducts also to the detection of dose enhancement effects in a short average range of 650 μm outside the region loaded with Au. This can indicate that the location determination is highly important in this treatment method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite all the current technological advances, cancer is still a leading cause of death for people under the age of 85 years worldwide [1]. According to statistical data, most cancer patients die during the first 5 years after diagnosis. Due to the concern caused by such statistical facts, cancer research has become one of the most active research areas nowadays. Because of the intensive scientific research in this field, we now have a wide variety of cancer diagnosis and therapeutic methods at our disposal. The most common ones are surgery, chemotherapy, and radiotherapy, whether separately or in combination with each other. Unfortunately, all these treatment methods have side effects; for example, surgical solutions are invasive and usually performed for only a limited number of cases. However, chemotherapy commonly causes undesirable side effects like anemia, diarrhea, hair loss, bleeding and digestive problems, nausea, mouth ulcers, fatigue, and many other side effects like secondary “metastatic” malignant tumors, mainly due to the damage to healthy cells [1, 2].
Radiotherapy uses ionizing radiation, including gamma rays, X-rays, and charged particles to kill cancer cells. This treatment method is employed extensively for the treatment of almost all types of solid tumors. Unlike chemotherapy, radiotherapy is more controllable as it does not affect distant, healthy organs through blood circulation. In addition, many patients have said that radiotherapy was much less painful than chemotherapy. Nevertheless, radiotherapy has some common side effects including general fatigue, loss of appetite, changes in blood, and sensitive skin [2]. However, the most challenging problem in radiotherapy is that ionizing radiation cannot discriminate between cancerous and normal cells that surround the tumor or lie in the field of radiation during treatment. This problem can reduce the effectiveness of tumor cell eradication with this method of treatment. This can be considered as a limiting factor for a given radiation dose and thereby to avoid damaging healthy tissue. A suggested solution to this problem in radiotherapy is loading high-Z materials into the tumor region, which can induce higher toxicity to tumor cells and lower toxicity for normal ones [3,4,5].
The use of metallic nanoparticles for the treatment of cancer is a promising development in this field. Among various nanoparticles, preclinical studies have shown a radiosensitization effect for gold nanoparticles (GNPs) in combination with different photon beams. In addition, gold is a favored material because it is a noble, chemically inert metal, nontoxic, very biocompatible, and stable for human use. Furthermore, the synthesis of gold nanoparticles having different forms, such as spheres, shells, cages, or wires, could be simple and cost-effective comparative to bulk quantity synthesis. Gold nanotechnology relies on the ability of the tiny gold nanoparticles to pass through the leaky blood vessels that surround the tumor and accumulate inside of it. The combination of radiotherapy with GNPs increases the local radiation dose by inducing a higher absorption of photons and more photoelectric interactions in the gold-loaded tumor site. This can produce an enhanced radiobiological damage to cancer cells and is considered to be a noninvasive radiotherapy method [3,4,5,6,7,8].
The overall aim of this study was to collect valid and reliable data about the effect of gold nanoparticles on radiation dose enhancement and its distribution with the radial dose employing Monte Carlo simulations. Within this broad theme, this study had a number of specific objectives. First of all, to understand the impact of two factors: photon beam energy and gold (Au) concentration on both of the dose enhancement effect and its variation with the radial distance measured from the source, in the presence of Au particles loaded in the tumor tissue. Thereafter, we compute the dose enhancement factor (DEF) at various concentrations of Au loaded in the tumor: 0, 7, 18, 30, 50, 75, and 100 mg-Au/g-water, for Ir-192 and Cs-137, two different radionuclide sources that are commonly used in brachytherapy (a type of internal radiation therapy). Importantly, we will quantify the maximum distance range measured from the source at which the DEF value drops to 1 (no enhancement) (R max) for the same Au concentrations and for both radionuclide sources. For this enhanced treatment method, we will finally assess the optimized combination of both photon energy and Au concentration in order to provide the optimum DEF values.
Methods and materials
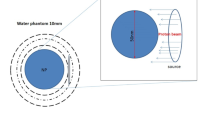
Monte Carlo (MC) methods are statistical simulation methods that provide approximate solutions to a wide variety of physical and mathematical problems by performing statistical sampling processes, which utilize a sequence of random numbers and probability to obtain an approximation to the problem under simulation [9]. EGSnrc is a MC code that considers the transport of photons and electrons for an arbitrary geometry [10]. In this study, we have carried out MC simulations for the phantom configurations and for 10 billion histories (i.e., incident photons), as illustrated in the Fig. 1a, b. The calculations are conducted using the DOSXYZnrc program along with BEAMnrc and both are implemented in the EGSnrc package. Two cases were simulated: the first one is the pure water phantom (as an equivalent for human soft tissue) where the concentration of Au is 0 mg-Au/g-water (as a reference case for comparison), and the second one is the gold-tissue phantom with six different concentrations of Au into it: 7, 18, 30, 50, 75, and 100 mg-Au/g-water. All phantom configurations were irradiated using two different sources: Ir-192 and Cs-137, which are commonly used in the brachytherapy method of treatment.
For mimicking a typical brachytherapy treatment, the spherical phantoms are determined to be 10 cm in radius. The tumor region loaded with Au is defined as a sphere in the center of the described water phantom with a radius of 1 cm, while both sources that are used in this study are located at the center of the spherical phantom. To simulate the various concentrations of Au within the region of interest, a PEGS (preprocessor for EGS) file is created for each one of the considered cases to obtain the DEF values and their variation with the radial distance (r) measured from the source. In order to take into account all electrons with considerable kinetic energies (higher than the rest mass of the electron) during the dose deposition calculations, the global cutoff energy for electron transport (ECUT) was set to 0.521 MeV. In addition, the chosen boundary-crossing algorithm was EXACT to produce a more accurate calculation of the dose inside the voxel. However, the histories for the electrons that carry energies below this value are terminated and their energies are deposited in the present voxel they existed in.
In general, the MC simulations are considered a benchmark for radiation dose calculations and dose distribution prediction in heterogeneous media such as different tissue structures of living bodies [11, 12]. Nevertheless, the results of the MC method are only approximations of the true values and always imply statistical uncertainties for each calculated parameter due to the statistical nature of the method. There are two main methods to lower such uncertainties: the first one is to reduce the statistical noise or the variance and the other one is to increase the number of histories for each calculation. Increasing number of histories can achieve more accuracy and limit the statistical error, which is challenging and very time consuming. This is a big issue in the MC simulation method. One solution to the time consumption issue is to determine a cutoff energy for both photons and electrons, in which the particles of energies less than that threshold will deposit their energies locally without being transported (stopped). This solution may generate problem of its own as it simplifies the physics. This might not reflect how the real interactions occur, as both types of particles actually travel for a short distance before their energies are totally absorbed.
Additionally, techniques of the variance reduction are routinely performed to increase the accuracy of MC calculations and lower the uncertainty. This is usually achieved by selecting certain parts of the actual geometry (e.g., limited volume or certain angles of concern) and probing them more than others. This will actually reduce the variance into that selected geometry. On the other hand, this may neglect relevant contributors of dose deposition in reality, such as the scattered photons from parts of the geometry that were neglected (e.g., head of the accelerator and shielding walls) in order to reduce the variance. Such methods and techniques, if not chosen carefully, can provide misleading results, as large dose depositions will not be calculated, while the error will stay small as these parts of geometry were not chosen initially. Finally, these two simplification methods may have a great influence on the results and may generate a huge disagreement with experimental results that involve interactions with biological and chemical systems as well as the physical aspect. For this reason, we restricted ourselves to perform MC simulation as described in Refs. [9, 10].
Results and discussion
In the current study, the impact of two factors are investigated on the dose enhancement factor (DEF) value as well as its variation with the radial distance measured from the source at which the DEF value drops to 1 (no enhancement) (R max). The first factor is the photon beam energy, which is investigated by choosing two different radionuclide sources that are commonly used in brachytherapy: Ir-192 (with an average photon energy of 380 keV) and Cs-137 (with an average photon energy of 662 keV). The second factor is the concentration of Au loaded in the human tissue (represented by the formerly described water–gold phantom). The results of the simulations can be divided into two parts: the first one is dedicated to the discussion of the DEF value calculation for all different concentrations and both radionuclide sources. The second one is dedicated to the investigation of the variation of the DEF values along the radial distance measured from the source (r) and, finally, the determination of R max value for each case.
In radiotherapy, a depth dose profile (DDP) is defined as the absorbed dose deposited by a radiation beam into a medium as it varies with depth (distance) along the axis of the beam, or the radial distance in the case of a point source at the isocenter of the phantom. In order to quantify the dose enhancement effect, we can define the DEF as the deposited dose in tissue with the presence of Au divided by the deposited dose in tissue without the presence of Au (i.e., the case of pure water or zero concentration of Au). Hence, we can write
Since we deal with a mixture of three elements in the phantom (hydrogen, oxygen, and gold), it is necessary to calculate the effective atomic number Z eff values for the different concentrations instead of the atomic number Z using the following equation:
where f n is the fraction of the total number of electrons associated with each element and Z n is the atomic number of each element [13].
Dose enhancement factor for different concentrations of Au
Calculation of dose enhancement factor for different concentrations of Au for the Ir-192 source
Table 1 gathers the calculated DEF values obtained by Monte Carlo simulations for the seven different concentrations of Au (0, 7, 18, 30, 50, 75, and 100 mg-Au/g-water), in which the phantom was irradiated by the Ir-192 source. As can be seen in Table 1, the calculated DEF value increased as the Au concentration went higher and reached its maximum value of 1.32 at a concentration of 30 mg-Au/g-water.
This general behavior is expected, as there is a strong direct relationship between the probability of all photon interactions in the mixture (i.e., tumor phantom) and the number of Au atoms in it, which is directly proportional to the concentration of gold and the atomic number (Z) of the medium. Specifically, this behavior can indicate that the photoelectric interaction, which is proportional to (Z 3) of the medium of interaction (represented by Z eff in this study), is the major contributor to the dose enhancement in the gold nanoparticle-aided radiotherapy (GNRT) method. This is due to the energy deposition of various energetic yields for this type of interaction, such as photoelectrons and Auger-electron cascades. However, the DEF values decreased slightly at a concentration of 50 mg-Au/g-water and remained almost the same for the higher concentrations of 75 and 100 mg-Au/g-water. A possible explanation for this small reduction is that the accumulation of Au atoms at high concentrations in a small limited volume can affect the ability of photons to penetrate more voxels in the region of interest and experience backscatter interactions instead of ionization for Au atoms. In fact, this can be due to the relatively high Z eff values, which are all illustrated in Table 1.
Calculation of dose enhancement factor for different concentrations of Au for the Cs-137 source
Table 2 displays the same generally proportional relationship between DEF values and Au concentration for the Cs-137 source as with the previous source. The DEF values exhibited an increase for Au concentrations from 0 to 30 mg-Au/g-water, which has an equivalent Z eff of 7.42 to 17.81, respectively. The DEF value reached its maximum value of 1.31 for the Cs-137 source at the Au concentration of 30 mg-Au/g-water; beyond this concentration, the DEF showed a reduced value. From Table 2, the same general behavior is noticed with the Cs-137 source as it was previously found with the Ir-192 source. This can be explained in the same manner as in the previous section.
In order to understand the overall behavior of the dose enhancement effect according to both factors: photon beam energy and Au concentration in the tumor (proportional to the effective atomic number of the medium), a comparison of the DEF values for all concentrations should be made between both sources. Figure 2 displays a comparison between the Ir-192 and Cs-137 sources for the DEF values versus the different Au concentrations. The same general behavior is noticed for both sources. The DEF value increases with the Au concentration until it reached a maximum at an Au concentration of 30 mg-Au/g-water. The DEF decreased for higher Au concentrations for both sources. For all concentrations, the calculated DEF values obtained from the Ir-192 source (avg. energy of 380 keV) are higher than those of the Cs-137 source (avg. energy of 662 keV). Photoelectric interactions are known to be inversely proportional to the energy of the incident photons (∝ E−3) [7, 13] and because these interactions make the major contribution to the dose enhancement effect in the GNRT method as previously stated [7], the DEF is also inversely proportional to the incident photon energy. The inverse proportionality of DEF values with photon beam energy and direct proportionality with Au concentration are consistent with the findings of previous studies [3,4,5,6,7,8, 11,12,13,14,15,16].
Dose enhancement factor variation with the radial distance (r) for different concentrations of Au
Calculation of R max values for different concentrations of Au for the Ir-192 source
Although the variations are relatively slight, Table 3 spotlights an inverse relationship between the concentration of Au in the mixture and R max value. Note that according to the definition of R max (mentioned previously), it is meaningless to include the 0 mg-Au/g-water concentration (pure water case) in this part, as there is no need for comparison to the reference case.
In radiation physics, it is known that there is a direct relationship between the photoelectric interaction probability (i.e., interaction cross-section) and the attenuation coefficient μ; the larger the value of the attenuation coefficient μ for a given photon energy, the more likely it is that photon interactions will occur in a given thickness of material. However, both the photoelectric interaction probability and the attenuation coefficient μ are directly related to the quantity (Z 3/E 3). On top of that, the range of photoelectric interaction products (proportional to R max) is inversely proportional to μ and increasing Au concentration will increase the effective atomic number of the mixture [13, 14]. By adding all these facts together, one can conclude that the value of R max is directly proportional to (E 3/Z 3). In this study, Z is represented by Z eff of the medium. Hence, the inverse relationship between Au concentration and R max value can be understood. This inverse relationship between R max and Z eff (Au concentration accordingly) might not be seen explicitly in Fig. 3, as these differences are negligible on the used scale as they are in the order of magnitude of microns. This is consistent with earlier results [14, 15]. In fact, this is due to the short range of the photoelectric interaction products (e.g., photoelectrons, characteristic X-rays, and Auger electrons).
Calculation of R max values for different concentrations of Au for the Cs-137 source
In both Table 4 and Fig. 4, one can see that R max values are changing slightly and inversely with the increase of Au concentration, as it has been previously noticed for the Ir-192 source and can be explained in the same manner. Figure 4 illustrates the DEF variation with the radial distance (r) for the Cs-137 and as we have seen for the Ir-192 source, the changes were relatively small due to the short range of the photoelectric products, which are in the order of magnitude of microns.
Finally, a comparison of the R max values between the two sources for each specific concentration is illustrated in Fig. 5. It can be seen that R max values for Ir-192 are generally less than those for the CS-137 source, although the differences are relatively negligible due to the short range of the electrons produced by photon interactions with Au atoms. This finding supports the previous studies’ results [14, 15], while the slight insignificant direct relationship between photon energy and R max value can be explained by the inverse relationship between the photoelectric interaction probability and the energy of incident photons. Thus, as the Cs-137 emits photons of higher average energy than the Ir-192 source photons, the penetration ability and the range of interaction for the photons emitted by the former source are higher. The photons emitted by the Cs-137 travel with a quite great distance irrespective to the Ir-192 photons and they can deposit their energies at slightly further distances. From the results of both sources, we can conclude that the maximum range of a total deposition for photon energy was between 400 and 900 μm (avg. 650 μm) for all concentrations. These findings lie in the range of values shown by a previous study on the range of photoelectrons created from a GNP, spanning approximately from 3 up to 1000 μm (1 mm) [14].
Although the results of this study have shown that the dose enhancement would increase proportionally with the concentration of heavy element particles loaded in a tumor in general, there is a biological concern about injecting tiny heavy metals with relatively high concentration into the human body. Regarding this issue, a previous study conducted by Zhang et al. [6] has shown that the high concentrations of GNPs could cause a sharp drop off in cell viability, body weight, red blood cells, and hematocrit, while low concentrations did not. Furthermore, other studies have demonstrated similar side effects for high concentrations of GNPs and showed that delivering high concentrations of nanoparticles into the tumor region would be clinically infeasible regardless of the particles’ size and shape, and this has been seen during various experiments [17,18,19]. These findings along with the current results (which showed a reduction in the DEF value for high concentrations of Au) conclude that the use of high concentration of GNPs in medical treatments is not recommended.
One great challenge is that some MC simulations for the GNRT models are not able to be consistent with the experimental data for some preclinical models and cell line studies as well [12]. This is due to some additional factors related to real interactions of radiation within biological tissues. A clear example of that is the experimental study conducted previously [20] that involved 50-nm GNPs with kilovoltage photon beams of 35 and 660 keV and it was found that the dose enhancement factors (DEF) are about 1.66 and 1.18, respectively. These results conflict with the calculated DEFs calculated by another MC simulation [6] for the same GNP size and same photon beam energies, when the beam energy goes down from 660 to 35 keV, the dose enhancement ratio must be 483 instead of increasing by a factor of 1.4. Such deviations in results between MC calculations and experimental data can be explained based on the additional nonphysical (biological and chemical) damages to the DNA after interaction with electrons of low energy (Auger electrons) emitted from the GNPs after interactions [21]. Moreover, despite the results of many MC calculations which have showed that the combination of GNPs and kilovoltage photon beams can enhance the dose significantly, the experimental results illustrated that the photon beams with energies in the kilovoltage range are greatly attenuated by the soft tissue of human body. This may suggest that the GNRT method can only be used to treat superficial tumors in order to give sufficient treatment for real patients [21].
Summary
In summary, the EGSnrc code was utilized to carry out multiple Monte Carlo (MC) simulations to perform a comparative study between the absence and presence of heavy metals in the tumor. A water–gold phantom was used as an equivalent for the human soft tissue and was irradiated with Ir-192 and Cs-137, two radionuclide sources commonly used in the brachytherapy technique. Different concentrations of Au were introduced into the water phantom: 0, 7, 18, 30, 50, 75, and 100 mg-Au/g-water. The DEF and its variation with the radial distance measured from the source were all computed and analyzed for all considered cases. In addition, the maximum distance range, measured from the source at which the DEF value drops to 1 (no enhancement) (R max), was quantified for each considered case as well. The results of the study can be summarized in the following points:
-
The calculated DEF value increases directly with Au and reaches its maximum value at a certain limit of concentration, which was found to be 30 mg-Au/g-water for all sources and configurations used in this study. Beyond this limit, the DEF value decreases and remains relatively unchanged for higher concentrations for both used sources.
-
The calculated DEF values for the Ir-192 source were higher than those for the Cs-137 source for all concentrations. This suggests that the dose enhancement effect is inversely related to the photon beam energy.
-
The calculated R max value changes slightly and inversely with the increase of Au concentration (and with Z eff, accordingly).
-
The calculated R max values for Ir-192 were generally lower than those for the Cs-137 source. Although the differences are relatively negligible, we may conclude that there is a direct, but insignificant, relationship between photon beam energy and R max values.
More investigations should be carried out to solve some challenges in the promising GNRT method of treatment. One of these challenges is synthesizing the appropriate nanoparticles with specific characteristics, which are optimized for a treatment method. Moreover, cancer targeting needs special biocompatible stable nanoparticles because this type of treatment is highly dependent on surface chemical interactions between the nanoparticles and the targeted cells. In addition, there is a growing concern about the long-term toxicity of the utilized nanoparticles to the human healthy tissue. Hence, there is an obvious necessity to direct efforts towards the study of side effects and toxicity of the GNPs on the human body in the long term, as well as the assessment of the risks and investigating the impacts on the environmental resources.
References
E. Ferlie, K. Montgomerey, and A. R. Perdersen 2016 The Oxford handbook of health care management, https://doi.org/10.1093/oxfordhb/9780198705109.001.0001
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics. CA Cancer J Clin 67:7
Xiang-Yu S, Liu P-D, Wu H, Gu N (2014) Enhancement of radiosensitization by metal-based nanoparticles in cancer radiation therapy. Cancer Biol & Med 11:2095
Haume K et al (2016) Gold nanoparticles for cancer radiotherapy: a review. Cancer Nanotechnology 7(8):12645
McQuaid HN (2016) Imaging and radiation effects of gold nanoparticles in tumour cells. Sci Rep 6:19442
Zhang SX et al (2009) Quantifying tumor-selective radiation dose enhancements using gold nanoparticles: a monte carlo simulation study. Biomed Microdevices 11(4):925–933
Ma N et al (2017) Shape-dependent radiosensitization effect of gold nanostructures in cancer radiotherapy: comparison of gold nanoparticles, nanospikes, and nanorods. ACS Appl Mater Interfaces 9:13037
S. Jelveh, D.B. Chithrani (2011). Gold nanostructures as a platform for combinational therapy in future cancer therapeutics, Cancers, 3
Kalos, Malvin H., and Paula A. Whitlock 2009 Monte Carlo methods. John Wiley & Sons
http://www.nrc-cnrc.gc.ca, National Research Council (NRC), Canada, accessed: Apr. 2013
Cho SH, Bernard LJ, Sunil K (2009) The dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy using low-energy gamma-/x-ray sources. Phys Med Biol 54(16):4889
Mesbahi A, Jamali F (2013) Effect of photon beam energy, gold nanoparticle size and concentration on the dose enhancement in radiation therapy. BioImpacts: BI 3(1):29
J. T. Bushberg, J. A. Seibert, E. M. Leidholdt Jr., J. M. Boone (2012) The essential physics of medical imaging, Third Edition 3rd Edition
Leung MK et al (2011) Irradiation of gold nanoparticles by x-rays: Monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Med Phys 38:624
Cho S et al (2010) Monte Carlo simulation study on dose enhancement by gold nanoparticles in brachytherapy. J Korean Phys Soc ss(6):1754–1758
Gual, Maritza Rodríguez, et al. 2009 Use of nanoparticles in brachytherapy—an alternative for enhancing doses in cancer treatment. World congress on medical physics and biomedical engineering, September 7–12, Munich. Springer 2009
Rambanapasi C et al (2016) Bioaccumulation and subchronic toxicity of 14 nm gold nanoparticles in rats. Molecules 21:763
Zhao J, Zhou M, Li C (2016) Synthetic nanoparticles for delivery of radioisotopes and radiosensitizers in cancer therapy. Cancer Nanotechnology 7(9):12645
J. P. Pignol, E. Lechtman (2012) Reply to Comment on ‘Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location’, Physics in Medicine and Biology, 57
Monte Carlo treatment planning—an introduction (report 16 of the Netherlands) 2017 Commission on Radiation Dosimetry (Retrieved from http://radiationdosimetry.org, May 4)
Alexandru Mihai Grumezescu, Anton Ficai 2017 Nanostructures for cancer therapy—micro and nano technologies. Elsevier, ISBN: 0323461506, 9780323461504
Acknowledgments
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Author information
Authors and Affiliations
Contributions
All authors contributed equally for the computation and writing of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
No informed consent process was necessary.
Rights and permissions
About this article
Cite this article
Al-Musywel, H.A., Laref, A. Effect of gold nanoparticles on radiation doses in tumor treatment: a Monte Carlo study. Lasers Med Sci 32, 2073–2080 (2017). https://doi.org/10.1007/s10103-017-2329-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2329-0