Abstract
A diode-pump Nd:YAG high-power laser (wavelength 1320 nm, power 100 W) is routinely used to surgically remove lung metastases. Even pulmonary lesions in central locations are resectable via this method, yet it also carries a potential risk of damaging the larger bronchi and vessels in the vicinity. Studies investigating the safety of using high-power lasers are lacking. We therefore aimed to examine the direct effects of a 100-watt laser on the bronchi and pulmonary artery at a standard working velocity. From freshly slaughtered pigs, we isolated cylindrical specimens of the trachea, the main and lobar bronchi, and the central pulmonary artery from the both lungs. These specimens were fixed consecutively in rows behind each other on a Styrofoam surface in the laboratory. The laser’s handle was clamped into a hydraulic feed unit so that the laser was focused at constant distance perpendicular to the tissue and would move at 10 mm/s over the specimens. The Nd:YAG Laser LIMAX® 120 functioned at a consistent power of 100 W during all the experiments. The lasered specimens were examined macroscopically and histologically for tissue damage. None of the trachea or bronchial walls were perforated. Compared to the pulmonary parenchyma, we observed no vaporization effects—only minor superficial coagulation (with a mean depth of 2.1 ± 0.8 mm). This finding was histologically confirmed in each specimen, which revealed mild superficial coagulation and no damage to the cartilage. In the presence of a residual peribronchial fatty tissue, the laser effect was even attenuated. The pulmonary arteries presented no lumen openings whatsoever, merely a discrete trace of coagulation. The vessel wall revealed increased vacuolization without alteration of the remaining vessel wall. In conclusion, laser resection at 100 W of the central lung areas is safe with respect to airways and blood vessels and the laser output does not need to be reduced when treating these areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thirty percent of cancer patients develop lung metastases [1]. Computer tomographic imaging of the thorax often reveals multiple bilateral tumor clusters. These metastases may be located in the periphery or in the center closely to the larger vessels and bronchi. Provided that the primary tumor has been treated surgically with no local recurrence and the patient is in adequate cardiopulmonary condition, the physician can consider surgical treatment of lung metastases. However, a prerequisite for this procedure is that all tumors are entirely resectable (R0). Long-term survival is greatly compromised by an R1 situation [2]. An R0 resection is often only possible via an anatomical resection such as a lobectomy when dealing with a centrally located metastasis [3]. On the other hand, surgeons removing lung metastases must try to spare as much as possible of the healthy parenchyma [4]. In our opinion, this goal is best achievable with laser technology. Our department uses the Nd:YAG Laser LIMAX® 120 (Gebrüder Martin GmbH & Co KG, Tuttlingen, Germany) with a maximum output of 120 W for this indication. During lung surgery, we throttle the laser down to 100 W. This setting ensures a quick and particularly hemorrhage-sparing surgical intervention. When the laser beam hits the parenchyma, the temperature in that tissue area rises to over 900 °C, leading to local vaporization. Beneath the vaporization zone, an area of coagulation is formed due to lower temperatures. The temperatures in the vaporization area’s periphery drop quickly so that no further tissue damage occurs. The advantage of the laser effect on the lung parenchyma is the combination of tissue cutting and coagulation [5]. To date it is unclear, whether centrally sited lung metastases lying near the larger vessels and bronchi are accessible to high-power laser surgery because of the delicate anatomical environment with its fragile great vessels. Experienced surgeons have informed us that they are particularly reticent in such situations due to the lack of evidence on laser surgery at this anatomical site [6]. In any case, before applying the laser in these pulmonary regions, the central pulmonary artery and veins must be wrapped and protected by a vessel loop so that if a massive life-threatening bleeding occurs, it can be controlled. We have conducted many resections of central metastases with the laser and have witnessed no appreciable bleeding or bronchial opening. The discrepancy between our surgical colleagues’ reticence and our positive experiences inspired us to carry out these experiments to determine whether the effect of a 100-W laser on the bronchi and pulmonary artery would cause serious tissue damage.
Material and methods
We conducted our experiments on bronchi and pulmonary artery specimens from freshly slaughtered pigs (n = 12, weighing 100 kg). After the animals’ death, the swine’s thorax was opened and the heart and lungs were removed en bloc. From these specimens, we removed cylinders from the trachea, main bronchi, and lobar bronchi. We also separated the left main pulmonary artery from the rest of the tissues and surrounding vessels and removed them as cylinders as well. The bronchi were separated from the peribronchial tissue and like the vessel specimens, wrapped in moist compresses (sodium chloride 0.9%) and transported to the laboratory.
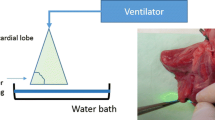
The cylinders of the trachea, main bronchi, and lobar bronchi (each n = 12) were then fixed consecutively in a row on a styrolene working surface. The cylinders of the vessels were cut, and we fixed pieces of the vessel wall (n = 12) on the styrolene working surface. The laser (the diode-pump Nd:YAG Laser LIMAX® 120 (Gebrüder Martin GmbH & Co KG, Tuttlingen, Germany) functioned at a consistent power of 100 W during all the experiments. The laser always worked in a continuous mode. The diameter of the laser fiber was 400 μm. The light was led in the center of the fiber. The laser’s handle was clamped into a hydraulic feed unit so that the visible laser beam was focused sharply at a constant distance (3 cm) perpendicular to the tissue and moved at constant velocity of 10 mm/s over the specimens (Fig. 1). This is the usual working velocity when operating on the lung parenchyma. In surgical practice when we use the laser in more central regions of the lung, we reduce the laser power and work more slowly (with a speed of approximately of 5 mm/s). The spot area at the tissue surface was 0.00635 cm2. With a working laser power of 100 W, the energy density was 15.72 kw/cm2. In case of a constant velocity of 10 mm/s, the laser has an effect for approximately 0.09 s on each spot area. As a consequence, an energy density of 1417.3 j/cm2 resulted. The handheld had an integrated work air flow of 50 l/min. The laser beam was moved only one time over the surface of each specimen (for more details please see Table 1).
The specimens were inspected after the laser procedure for visible damage like coagulation or vaporization zones and especially for wall perforations. Finally, they were formalin fixed and paraffin embedded using routine procedures and subjected to histological processing and examination. After Hematoxylin–Eosin staining, the specimens were assessed for laser-typical damage caused by vaporization and coagulation. The depth of the coagulation zone was measured using the Image J software (Version 1.47, National Institute of Health, USA).
At the end of our experiments, we measured the time it would take for a tracheal wall perforation when at a laser power of 100 W the laser beam remained at a defined local point without moving.
Results
Visible inspection revealed no signs of wall perforation in any of the trachea, main bronchi, or lobar bronchi. The specimen surfaces displayed a striated, approximately 2-mm-wide coagulated area with no integral signs of wall defects. Histology revealed a very superficial area of coagulation (Figs. 2 and 4). We observed no relevant tissue defects such as a vaporization zone. The cartilage in all specimens was completely intact, showing no signs of destruction. Even when the laser was applied in a region between two cartilage junctions, we noted only superficial coagulation with a maximum depth of 2.1 ± 0.8 mm (Fig. 3). The cartilage junctions withstood the laser effect completely; none of the specimens displayed any injury (Fig. 4).
Looking at the walls of the pulmonary artery, all specimens were entirely intact as well, revealing no signs of perforation. Visual inspection displayed striated coagulations approximately 2 mm wide of the adventitia. The histology results confirmed our macroscopic findings. We identified no vaporization effects in any specimen. The laser effect revealed itself as an approximately 2 mm broad, loosely organized area resembling coagulation, a lesion reproducible in all specimens. The vessel walls demonstrated no other kinds of damage. There is no risk of a secondary wall perforation due to such limited damage. The laser effect depends on both the wattage and the duration of application. We achieved the same vessel wall defect by reducing the output to 40 W and halving the application time to 5 mm/s (Fig. 5). However, the histologically evident lesions were the same in those cases: an approx. 2 mm broad area with a loosely organized and vacuolized structure as a result of coagulation by the laser and no signs of any further changes in the vessel wall.
a HE staining of the pulmonary artery with laser lesions (40 W, velocity 5 mm/s), 25× magnification. b Macroscopic view of the laser lesion on the pulmonary artery wall (100 W, velocity 10 mm/s). c HE staining of the pulmonary artery with laser lesion (100 watts, velocity 10 mm/s), 25× magnification
We tried to determine the time needed for complete wall perforation at 100-W laser output by stopping the hydraulic feed device and applying the laser beam to one area alone. In all examined 12 cases with a laser output power of 100 W, after 5 s, we observed a complete perforation of the wall into the lumen (Fig. 6). This corresponds to an energy density of 78,740 j/cm2 at this laser power.
Discussion
With the progress of laser technology, the surgeon is able to resect centrally located lung metastases in a non-anatomical fashion. Each metastasis is resected under palpatory control, abiding a safety margin of 5 mm around each lesion. Additionally, the new Nd:YAG laser generation has a higher laser output power and thus enables faster resection, significantly reducing the duration of the surgical procedure. Among surgeons, however, there is reluctance for the use of high-power lasers for central and non-anatomical lung resections mostly because of the risk involved with respect to large vessel or bronchial perforation.
To address this issue, we developed an ex vivo model. During surgery, the laser is manually swept with a velocity of 10 mm/s. In our experiments, we simulated the working velocity of 10 mm/s and observed no wall perforations whatsoever in any of the tracheal, main bronchi, or lobar bronchi specimens, not even in the central pulmonary artery. The laser effect consisted of a striated coagulation zone 2 mm wide with no anatomical or histological signs of deeper penetration. This is in contrast to the use of an electrocautery device which causes more severe macroscopic and cytostructural tissue damage [7].
Microscopic examination of the bronchial walls revealed no vaporization damage, in contrast to the effects seen in lung tissue resections [8], only an approx. 2-mm-deep coagulated lesion, and no other sign of a deeper effect. All of the cartilage junctions were unscathed. These results are also very interesting for all endoscopic debulking operations in case of malignant airway obstruction [9]. According to a study by Perin et al. [10], there is some risk of perforation of the bronchial wall with consecutive development of a pneumothorax, a pneumomediastinum, or a bronchoesophalgeal fistula during endobronchial recanalization. So in these situations, the use of the laser seems to be very safe.
The walls of the pulmonary arteries were all intact. The laser loosened up an area measuring about 2 mm, revealing its immediate effect; the remaining vessel wall was entirely unchanged. In our opinion, this procedure entails no risk of secondary vessel rupture. However, we are unaware of any similar investigations. These findings especially underline the possibility to laser-resect multiple and deeper located nodules in areas where the density of greater vessels is higher [11]. Additionally, Lesser [12] showed in a study that a thoracoscopic laser resection of subpleural nodules is feasible and safe. We think that based on our results it is also safe to resect deeper nodules in lung parenchyma with the laser.
While a single laser application on central structures carries a minimal risk for complications and it is effective to resect lung metastases in the central region at a high laser output, we would like to draw the reader’s attention to the fact that as the laser output rises and the application time is reduced, so does the risk of bronchial perforation increase exponentially. We determined that 5 s of laser application at 100 W will lead to a complete wall perforation (Fig. 6). However, this risk exists at lower laser output with increased application time as well. However, when maintaining a standard working velocity, the application of the laser beam has to be considered safe.
In this study, we successfully demonstrated that laser damage on the central bronchi and pulmonary artery is reproducible and that, provided perivascular or peribronchial tissue is still in situ, the laser effect is additionally attenuated. We were both surprised and pleased to observe that the laser exerts only a mild effect on the bronchi and vessels, and owing to their higher tissue density compared to the lung tissue, the therapeutic window is wide enough to both effectively resect lung tissue and at the same time spare any damage on the bronchi and vessels. Thus, should the laser touch the central bronchi or vessels briefly and at a usual working velocity despite a high-power output, the perforation risk is not increased. The damage triggered by the laser is minor and superficial and does not lead to secondary damage later.
Conclusion
At a usual working velocity and despite a high-power output, the laser does not perforate the central bronchi or vessels. Even in the central lung area, there is nothing to impede laser therapy.
References
Internullo E et al (2008) Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 3(11):1257–1266
Osei-Agyemang T, Ploenes T, Passlick B (2012) Pulmonary metastasectomy: indication and technique. Zentralbl Chir 137(3):234–241
Erhunmwunsee L, D’Amico TA (2009) Surgical management of pulmonary metastases. Ann Thorac Surg 88(6):2052–2060
Pfannschmidt J, Dienemann H (2009) Current surgical management of pulmonary metastases. Zentralbl Chir 134(5):418–424
Rolle A, Kozlowski M (2005) Laser resection of lung parenchyma—a new technical and clinical approach. Rocz Akad Med Bialymst 50:193–196
Treasure T (2007) Pulmonary metastasectomy: a common practice based on weak evidence. Ann R Coll Surg Engl 89(8):744–748
Scanagatta P et al (2012) Pulmonary resections: cytostructural effects of different-wavelength lasers versus electrocautery. Tumori 98(1):90–93
Kirschbaum A et al (2012) Local effects of high-powered neodymium-doped yttrium aluminium garnet laser systems on the pulmonary parenchyma: an experimental study on the isolated perfused pig lung lobe. Interact Cardiovasc Thorac Surg 15(2):191–193
Kawano R et al (2009) Primary lung cancer protruding into right main bronchus, successfully treated with endoscopic neodymium yttrium aluminum garnet (Nd-YAG) laser. Kyobu Geka 62(9):807–811
Perin B et al (2012) Patient-related independent clinical risk factors for early complications following Nd: YAG laser resection of lung cancer. Ann Thorac Med 7(4):233–237
Vodicka J et al (2009) Use of the KLS Martin Nd:YAG laser MY 40 13 in lung parenchyma surgery. Rozhl Chir 88(5):248–252
Lesser TG (2012) Laser application enables awake thoracoscopic resection of pulmonary nodules with minimal access. Surg Endosc 26(4):1181–1186
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were performed on slaughtered animals. An ethical approval was not needed.
Informed consent
Informed consent was not needed.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kirschbaum, A., Rexin, P., Bartsch, D.K. et al. Effect of high laser output on the central bronchi and pulmonary artery. Lasers Med Sci 32, 881–886 (2017). https://doi.org/10.1007/s10103-017-2188-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2188-8