Abstract
Low-level laser therapy (LLLT) is used in chronic wounds due to its healing effects. However, bacterial species may colonize these wounds and the optimal parameters for effective bacterial inhibition are not clear. The aim of this study was to analyze the effect of LLLT on bacterial growth in vitro. Bacterial strains including Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa were suspended in saline solution at a concentration of 103 cells/ml and exposed to laser irradiation at wavelengths of 660, 830, and 904 nm at fluences of 0 (control), 3, 6, 12, 18, and 24 J/cm2. An aliquot of the irradiated suspension was spread on the surface of petri plates and incubated at 37 °C for quantification of colony-forming unit after 24, 48, and 72 h. Laser irradiation inhibited the growth of S. aureus at all wavelengths and fluences higher than 12 J/cm2, showing a strong correlation between increase in fluence and bacterial inhibition. However, for P. aeruginosa, LLLT inhibited growth at all wavelengths only at a fluence of 24 J/cm2. E. coli had similar growth inhibition at a wavelength of 830 nm at fluences of 3, 6, 12, and 24 J/cm2. At wavelengths of 660 and 904 nm, growth inhibition was only observed at fluences of 12 and 18 J/cm2, respectively. LLLT inhibited bacterial growth at all wavelengths, for a maximum of 72 h after irradiation, indicating a correlation between bacterial species, fluence, and wavelength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial species can be detected in almost all chronic wounds. However, from this initial colonization, more serious problems can occur such as infections or even sepsis [1]. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli are among the main bacterial species that commonly colonize skin ulcers [2–4]. In a study conducted by Korber et al. [1], 107 ulcers were investigated and 191 pathogenic bacteria were identified, with 55.1 % of ulcers colonized by S. aureus, followed by P. aeruginosa (33.6 %) and E. coli (7.5 %). In a recent study, colonization by E. coli was detected in 116 patients with diabetic ulcers, and in 35.71 % of these, 75 % were resistant to ampicillin [5].
In developing countries, it is estimated that 1–2 % of the population will develop chronic ulcers during their lifetime. A North American estimate reports that the cost of treating only one chronic ulcer is approximately US$8000 per year, increasing to US$17,000 when it is infected. Worldwide, the costs generated by care of chronic ulcers are approximately US$13–15 billion per year [6].
Low-level laser therapy (LLLT) has been used as an adjuvant therapeutic approach in the healing process. Fulop et al. [7] conducted a meta-analysis of studies published between 2000 and 2007 and found that the use of phototherapy had positive results on tissue repair. Additionally, Peplow et al. [8], in a review of studies from 2002 to 2009, found that the photobiomodulator effect of LLLT helped stimulate the proliferation of human and animal cells in vitro. Important for healing, Nussbaum et al. [9] reported that LLLT also played an important role in bacterial inhibition. They demonstrated varying biomodulator effects using different wavelengths, fluences, and bacterial species and found that the results were dependent on wavelength, bacterial species, time of exposure to irradiation, and irradiation fluence. However, most studies that investigated the irradiation of bacterial cultures were conducted using different methods [9–20], which can affect the absorption of irradiation.
In the literature, few studies have used different wavelengths of LLLT, with different fluences and time, to analyze the growth inhibition of major bacterial species that commonly colonize skin ulcers. Therefore, the aim of this study was to analyze the effect of LLLT in the red and infrared light spectra on S. aureus, E. coli, and P. aeruginosa growth in vitro.
Material and methods
Cell culture
S. aureus ATCC 25923 (gram-positive), P. aeruginosa ATCC 27853 (gram-negative), and E. coli ATCC 25922 (gram-negative) were purchased from ATCC. Bacterial cultures were maintained in Mueller-Hinton culture medium (BD®, New Jersey, PA, USA), replated, and incubated for 24 h at a temperature of 37 °C. Cells were then suspended in saline solution (0.9 % NaCl), then serial dilutions were carried out which were spectrophotometrically monitored using a Densicheck™ Plus spectrophotometer (bioMérieux®, Durham, NC, USA). Turbidity remained between 0.5 and 0.63, corresponding to a concentration of 1.5 × 108 colony-forming units (CFU)/ml on the McFarland scale. The cells were then serially diluted to a concentration of 1.5 × 103 CFU/ml. A 300-μL aliquot of this suspension was then transferred to individual wells of a microtiter plate for irradiation.
Irradiation
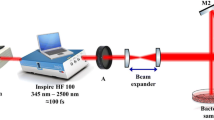
Bacterial suspensions were irradiated with a laserpulse (Ibramed®, Amparo, SP, Brazil) with diodes at wavelengths of 660 nm (red), 830 nm (infrared), and 904 nm (infrared) (Table 1). After irradiation, 100 μL of this suspension was transferred and spread over the surface of solid Mueller-Hinton culture medium in petri dishes (90 × 15 mm) and incubated at 37 °C for 24, 48, and 72 h, at which points the number of CFUs was determined using ImageJ 1.45® software (http://rsb.info.nih.gov/ij). All experimental procedures were performed in a sterile laminar flow hood. Each experiment was conducted in triplicate and repeated three times on different days, totaling 486 samples (3 wavelengths × 6 intensities × 3 series × 3 repetitions × 3 bacterial species). Approximately 20 % of samples were repeated due to contamination. Irradiation was conducted in a room with controlled temperature (23 ± 2 °C). The temperature of samples was monitored at different wavelengths and fluences (Table 2). The temperatures of two samples were measured in triplicate at each fluence and wavelength, using an infrared thermometer (Incoterm®, Porto Alegre, RS, Brazil).
Energy density (fluence) of irradiation was calculated based on the formula below:
where:
- ED :
-
energy density (J/cm2)
- P :
-
average power (W)
- T :
-
irradiation time (s)
- A :
-
irradiation area (cm2)
The energy density presented on the LLLT considered the diode’s irradiation area as a parameter for calculation. Because of the use of three separate diodes, with different areas and powers (Table 1), we made the decision to use the area of 1 cm2 corresponding to the area of individual wells of the microtiter plate that contained the bacterial samples in suspension; this ensured that energy density and radiated energy were equal for all diodes. Thus, to irradiate the bacterial species, fluences of 0 (control), 3, 6, 12, 18, and 24 J/cm2 were used for 0, 100, 200, 400, 600, and 800 s, respectively, at wavelengths of 660 and 830 nm. At 904 nm, the irradiation times were 0, 75, 150, 300, 450, and 600 s, respectively, at the same fluences. Control wells were not irradiated and were in ambient light. All irradiations occurred directly, punctually, and perpendicularly to the plate, and the emitter was fixed with the aid of a support, at a distance of 2 mm above the plate, over the area to be irradiated.
Measurement of diodes
The equipment was checked at the beginning and end of the experiment using a powermeter Field Max Top with PM3 sensor—0.5 mW to 2 W broadband sensor (RoHS) (Coherent®, Staunton, VA, USA) according to the method by Guirro and Weis [21]. Prior to beginning the irradiation experiments, we performed experiments to ensure uniformity of laser irradiation on bacteria in suspension. To do this, an experiment was conducted to analyze laser transmission in saline solution containing the bacterial species under study (at the same concentration and volume as our irradiation experiments). For this purpose, the bacterial sample was positioned between the diode and the powermeter and irradiated in a colorless solution (saline solution) to quantify the influence of the culture medium on light beam transmission. The results from this experiment showed that the light beam was slightly attenuated (Table 3).
Statistical analysis
Initially, the Shapiro-Wilk normality test was applied. Data on the number of CFU were analyzed using Kruskal-Wallis and post hoc Dunn’s tests. The diodes’ power, transmissivity, and temperature were analyzed using repeated measures ANOVA and post hoc Tukey’s test. The correlation between inhibition and fluence of irradiation was analyzed using Spearman’s correlation test. All tests were performed with the significance level of 5 % (p < 0.05) using SPSS version 17.0 software (IBM®, Chicago, IL, USA).
Results
Compared to the control, laser irradiation inhibited the growth of S. aureus at fluences higher than 12 J/cm2, regardless of the wavelength used (Fig. 1; Table 4). Moreover, a high negative correlation between fluence and bacterial inhibition was observed (Table 5). When fluences were compared, the results showed that a fluence of 24 J/cm2 led to greater inhibition than 3 J/cm2 at all wavelengths. When different wavelengths were compared at a fluence of 24 J/cm2, irradiation with red light (660 nm) led to greater inhibition of S. aureus than with infrared light (830 nm) (Table 4).
P. aeruginosa showed inhibition at 660 nm at fluences of 18 and 24 J/cm2 at all time points, with the highest inhibition observed at 24 J/cm2. At 830 nm, significant inhibition could be observed at fluences greater than 6 J/cm2. At 904 nm, inhibition could only be observed at 24 J/cm2 (Fig. 2; Table 4). Although different diodes and fluences led to varying degrees of inhibition, there was a negative correlation between bacterial inhibition and fluence at all wavelengths (Table 5).
As for E. coli, we showed that at a wavelength of 660 nm, inhibition could only be observed at 12 J/cm2. At a wavelength of 830 nm, inhibition could be observed at 3, 6, 12, and 24 J/cm2. At 904 nm, inhibition could be observed only at a fluence of 18 J/cm2; however, at 3 J/cm2, inhibition varied at 24 and 72 h after irradiation. Greater inhibition was observed at a wavelength of 830 than 660 nm at fluences of 3, 6, and 24 J/cm2, as well as 904 nm at fluences of 18 and 24 J/cm2 (Fig. 3; Table 4). For E. coli, a low negative correlation was observed between fluence and number of CFUs, for all wavelengths.
Discussion
As previously mentioned, there is no consensus regarding the most appropriate wavelength and fluence to use to inhibit bacterial growth. One explanation for this problem may be the variety of equipment used with varying parameters for beam emission area and power. These variations determine irradiation time and can directly affect experimental results [11]. While we took care to radiate the same energy density for the same area in this study, we did not observe an inhibition response that was dependent on fluence.
Among the wavelengths and bacterial species studied, red light (660 nm at 30 mW) led to the greatest bacterial inhibition, with reduced S. aureus growth at fluences of 12, 18, and 24 J/cm2. In contrast, Nussbaum et al. [9] conducted a study with the same wavelength (660 nm at 75 mW) and found no significant difference in bacterial growth.
As for infrared irradiation, our results showed that S. aureus growth was significantly inhibited at fluences greater than 12 J/cm2. These findings differ from our previous study [22], which showed growth inhibition at fluences of 1, 2, 3, 4, 5, and 16 J/cm2. Furthermore, in a study by Guffey and Wilborn [13], combining wavelengths of 405 and 880 nm inhibited S. aureus growth in a fluence-dependent manner whereby higher fluence led to greater inhibition.
Nussbaum et al. [9] used wavelengths that were very close to those of our study and found that S. aureus growth was inhibited at wavelengths of 810 and 905 nm (15 mW) at a fluence of 5 J/cm2. In contrast, a growth of 27 % was observed at a fluence of 50 J/cm2 at a wavelength of 905 nm. In another study, the same authors evaluated the effect of laser therapy (810 nm at15 mW) at different pulse frequencies (continuous, 26, 292, 1000, and 3800 Hz) and found increased growth of S. aureus at the two highest frequencies [10].
When analyzing the effect of laser irradiation on P. aeruginosa growth, we showed that infrared light (830 nm) resulted in significant growth inhibition at fluences greater than 6 J/cm2. However, at wavelengths of 660 and 904 nm, inhibition of P. aeruginosa occurred only at a fluence of 24 J/cm2. Corroborating these findings, Guffey and Wilborn [13] obtained 93.8 % inhibition of P. aeruginosa growth by combining blue laser (405 nm at 200 mW) with infrared laser (880 nm at 250 mW) at a fluence of 20 J/cm2. Similarly, Nussbaum et al. [9] obtained significant inhibition with a 810 nm laser at a fluence of 18 J/cm2 and at wavelengths of 630 and 660 nm; P. aeruginosa growth decreased by 27 % (1 J/cm2) and 18 % (5 J/cm2), respectively. In a subsequent study, these authors observed P. aeruginosa growth inhibition at fluences between 5 and 50 J/cm2 at the same wavelength and two different power settings (15 and 75 mW) [11]. However, these authors verified growth of P. aeruginosa at the same wavelength and fluences at different pulse frequencies (26, 1000, and 3800 Hz) [10].
After irradiation, E. coli growth was inhibited at a wavelength of 830 nm at fluences of 3, 6, 12, and 24 J/cm2; however, there was no difference among them. In contrast, Nussbaum et al. [10], in evaluating the effect of laser therapy (810 nm at 15 mW) in different pulse frequencies (continuous, pulsed at 26, 292, 1000, and 3800 Hz), observed growth of E. coli in continuous and the pulsed mode at 1000 Hz at fluences of 1, 2, 5, and 10 J/cm2. In another study, the same authors obtained similar results using the laser in continuous mode at the same wavelength and fluences [9]. Nussbaum et al. [11] evaluated the effect of power (15 and 30 mW) on E. coli growth using an 810 nm laser at different fluences (1, 2, 5, 10, 20, 30, 40, and 50 J/cm2) and observed growth at most fluences tested (1–20 J/cm2). These results support the hypothesis that, regardless of the light spectrum, elements such as power, pulse scheme, and frequency are also determining factors for growth inhibition.
In the present study, we showed that E. coli and P. aeruginosa growth were inhibited at all fluences tested, in contrast to S. aureus (Table 3). This can be explained by the fact that S. aureus are gram-positive bacteria and E. coli and P. aeruginosa are both gram-negative. Gram-positive bacteria have thick cell walls, whereas gram-negative bacteria have thinner cell walls. In addition, gram-negative bacteria have an outer membrane that contains lipopolysaccharide on the cell surface, whereas in gram-positive bacteria, the cell wall generally contains a variety of anionic substances that can bind strongly to peptides/cationic proteins, such as lipoteichoic acid (LTA) and peptidoglycans. LTA is known to be a compound of adherence in gram-positive bacteria. LTA carries the main virulence factors and initiates an inflammatory response when in contact with host cells [23]. These structural differences between gram-positive and gram-negative bacteria can impact the penetration of laser irradiation and mediate differences to irradiation susceptibility.
Another hypothesis that can be raised about the inhibitory effect of laser irradiation refers to photon absorption, by the possible chromophores present in the bacterial respiratory chain, and reactive oxygen species (ROS) can be produced, with no addition of exogenous photosensitizers preadipocytes (3T3-L1), prechondrocytes (ATDC5), myoblasts (C2C12), mesenchymal stromal cells (KUSA-A1), lung cancer cells (LLC), insulinoma cells (MIN6), fibroblasts (NIH 3T3), human cervix adenocarcinoma cells (HeLa), macrophages differentiated from lymphocytes (THP-1) after treatment with phorbol ester, and rat basophilic leukemia cells (RBL-2H3). Once, Kushibiki et al. [24] observed that intracellular chromophores have the ability to excite oxygen through the electron transport chain in eukaryotic cells. Intracellular porphyrin may also be responsible for ROS production [25], in particular hydroxyl radical (OH−) and singlet oxygen (1O2), which can promote toxic effects associated with oxidative stress, culminating in cell death [26].
Thus, these aspects should be investigated to understand morphological and physiological effects on different bacterial species, with laser irradiation at different wavelengths and fluences. Specifically, a wavelength of 450 nm should be investigated because this wavelength is known to inhibit bacterial growth [27]. These studies will be needed to reach a consensus with regard to the best parameters to be used to inhibit specific bacterial species.
Comparison of the results from this study may have been complicated by factors including the power of the diodes, irradiation time, and experimental design. In this study, the bacteria were suspended in a colorless liquid medium (saline solution) and, prior to the experiment, we showed that the saline solution led to a small decrease in irradiation transmissivity, which ensured that all content in the well of the microtiter plate was irradiated. Additionally, the temperature of the bacterial suspension remained unchanged despite a long irradiation time. However, in all studies mentioned, irradiation of bacteria was performed on culture medium of varied staining, and the laser beam absorption, refraction, and transmissivity levels were not stated [9–20]. These are important points that should be considered in future studies, since laser absorption or reflection by culture medium can affect bacterial growth and/or inhibition.
Conclusion
LLLT inhibited bacterial growth at 660, 830, and 904 nm up to 72 h after irradiation and was not time-dependent. Thus, we conclude that wavelengths of 660, 830, and 904 nm can be used to inhibit S. aureus, P. aeruginosa, and E. coli growth in vitro at fluences between 12 and 24 J/cm2.
References
Korber A, Schmid EN, Buer J, Klode J, Schadendorf D, Dissemond J (2010) Bacterial colonization of chronic leg ulcers: current results compared with data 5 years ago in a specialized dermatology department. J Eur Acad Dermatol Venereol 24(9):1017–1025
Demetriou M, Papanas N, Panopoulou M, Papatheodorou K, Maltezos E (2013) Determinants of microbial load in infected diabetic foot ulcers: a pilot study. Int J Endocrinol 2013:858206
Tascini C, Piaggesi A, Tagliaferri E, Iacopi E, Fondelli S, Tedeschi A, Rizzo L, Leonildi A, Menichetti F (2011) Microbiology at first visit of moderate-to-severe diabetic foot infection with antimicrobial activity and a survey of quinolone monotherapy. Diabetes Res Clin Pract 94(1):133–139
Jockenhofer F, Gollnick H, Herberger K, Isbary G, Renner R, Stucker M, Valesky E, Wollina U, Weichenthal M, Karrer S, Klode J, Dissemond J (2013) Bacteriological pathogen spectrum of chronic leg ulcers: results of a multicenter trial in dermatologic wound care centers differentiated by regions. J Dtsch Dermatol Ges 11(11):1057–1063
Shahi SK, Singh VK, Kumar A (2013) Detection of Escherichia coli and associated beta-lactamases genes from diabetic foot ulcers by multiplex PCR and molecular modeling and docking of SHV-1, TEM-1, and OXA-1 beta-lactamases with clindamycin and piperacillin-tazobactam. PLoS One 8(7), e68234. doi:10.1371/journal.pone.0068234
Siddiqui AR, Bernstein JM (2010) Chronic wound infection: facts and controversies. Clin Dermatol 28(5):519–526
Fulop AM, Dhimmer S, Deluca JR, Johanson DD, Lenz RV, Patel KB, Douris PC, Enwemeka CS (2009) A meta-analysis of the efficacy of phototherapy in tissue repair. Photomed Laser Surg 27(5):695–702
Peplow PV, Chung TY, Baxter GD (2010) Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies. Photomed Laser Surg 28(Suppl 1):S3–S40
Nussbaum EL, Lilge L, Mazzulli T (2002) Effects of 630-, 660-, 810-, and 905-nm laser irradiation delivering radiant exposure of 1-50 J/cm2 on three species of bacteria in vitro. J Clin Laser Med Surg 20(6):325–333
Nussbaum EL, Lilge L, Mazzulli T (2002) Effects of 810 nm laser irradiation on in vitro growth of bacteria: comparison of continuous wave and frequency modulated light. Lasers Surg Med 31(5):343–351
Nussbaum EL, Lilge L, Mazzulli T (2003) Effects of low-level laser therapy (LLLT) of 810 nm upon in vitro growth of bacteria: relevance of irradiance and radiant exposure. J Clin Laser Med Surg 21(5):283–290
Dadras S, Mohajerani E, Eftekhar F, Hosseini M (2006) Different photoresponses of Staphylococcus aureus and Pseudomonas aeruginosa to 514, 532, and 633 nm low level lasers in vitro. Curr Microbiol 53(4):282–286
Guffey JS, Wilborn J (2006) Effects of combined 405-nm and 880-nm light on Staphylococcus aureus and Pseudomonas aeruginosa in vitro. Photomed Laser Surg 24(6):680–683
Guffey JS, Payne W, Jones T, Martin K (2013) Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 nm light from a supraluminous diode array. Photomed Laser Surg 31(4):179–182
Guffey JS, Wilborn J (2006) In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed Laser Surg 24(6):684–688
Bumah VV, Masson-Meyers DS, Cashin SE, Enwemeka CS (2013) Wavelength and bacterial density influence the bactericidal effect of blue light on methicillin-resistant Staphylococcus aureus (MRSA). Photomed Laser Surg 31(11):547–553
Enwemeka CS, Williams D, Enwemeka SK, Hollosi S, Yens D (2009) Blue 470-nm light kills methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomed Laser Surg 27(2):221–226
Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK (2008) Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med 40(10):734–737
Bumah VV, Whelan HT, Masson-Meyers DS, Quirk B, Buchmann E, Enwemeka CS (2015) The bactericidal effect of 470-nm light and hyperbaric oxygen on methicillin-resistant Staphylococcus aureus (MRSA). Lasers Med Sci 30(3):1153–1159
Bumah VV, Masson‐Meyers DS, Cashin S, Enwemeka CS (2015) Optimization of the antimicrobial effect of blue light on methicillin‐resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med 47(3):266–272
Guirro RR, Weis LC (2009) Radiant power determination of low-level laser therapy equipment and characterization of its clinical use procedures. Photomed Laser Surg 27(4):633–639
Sousa NT, Guirro RR, Santana HF, Silva CC (2012) In vitro analysis of bacterial morphology by atomic force microscopy of low level laser therapy 660, 830 and 904 nm. Photomed Laser Surg 30(5):281–285
Morita S, Tagai C, Shiraishi T, Miyaji K, Iwamuro S (2013) Differential mode of antimicrobial actions of arginine-rich and lysine-rich histones against Gram-positive Staphylococcus aureus. Peptides 48:75–82
Kushibiki T, Hirasawa T, Okawa S, Ishihara M (2013) Blue laser irradiation generates intracellular reactive oxygen species in various types of cells. Photomed Laser Surg 31(3):95–104
Papageorgiou P, Katsambas A, Chu A (2000) Phototherapy with blue (415 nm) and red (660 nm) light in the treatment of acne vulgaris. Br J Dermatol 142(5):973–978
Vatansever F, de Melo WC, Avci P, Vecchio D, Sadasivam M, Gupta A, Chandran R, Karimi M, Parizotto NA, Yin R, Tegos GP, Hamblin MR (2013) Antimicrobial strategies centered around reactive oxygen species—bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol Rev 37(6):955–989
Sousa NTA, Santos MF, Gomes RC, Brandino HE, Martinez R, Guirro RRJ (2015) Blue laser inhibits bacterial growth of staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Photomed Laser Surg 33(5):278–2
Acknowledgments
The authors wish to thank the Coordination of Higher Education and Graduate Training (CAPES) for a master’s scholarship and the São Paulo Research Foundation (FAPESP) for grant 2011/22170-0 and the undergraduate research scholarship (2013/02974-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
de Sousa, N.T.A., Gomes, R.C., Santos, M.F. et al. Red and infrared laser therapy inhibits in vitro growth of major bacterial species that commonly colonize skin ulcers. Lasers Med Sci 31, 549–556 (2016). https://doi.org/10.1007/s10103-016-1907-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-1907-x