Abstract
The aim of this study was to evaluate the effects of simulated pulpal pressure (SPP) on the variation of intrapulpal temperature (ΔT) and microtensile bond strength (μTBS) to dentin submitted to an adhesive technique using laser irradiation. One hundred sound human molars were randomly divided into two groups (n = 50), according to the presence or absence of SPP (15 cm H2O). Each group was divided into five subgroups (n = 10) according to Nd:YAG laser energy (60, 80, 100, 120, 140 mJ/pulse). The samples were sequentially treated with the following: 37 % phosphoric acid, adhesive (Scotchbond Universal), irradiation with Nd:YAG laser (60 s), and light curing (10 s). ΔT was evaluated during laser irradiation using a type K thermocouple. Next, a composite resin block was build up onto the irradiated area. After 48 h, samples were submitted to microtensile test (10 kgf load cell, 0.5 mm/min). Data were analyzed by two-way ANOVA and Tukey tests (p = 0.05). ANOVA revealed significant differences for ΔT and TBS in the presence of SPP. For ΔT, the highest mean (14.3 ± 3.23 °C)A was observed in 140 mJ and without SPP. For μTBS, the highest mean (33.4 ± 4.15 MPa)A was observed in 140 mJ and without SPP. SPP significantly reduced both ΔT and μTBS during adhesive procedures, lower laser energy parameters resulted in smaller ΔT, and the laser parameters did not influence the μTBS values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dentistry is passing through a relatively stagnant period with regard to adhesive procedures. Current adhesive systems cannot promote effective long-term adhesion with the inherent variability of dentin substrate, whether using the all-etch hybridization mechanism introduced by Fusayama et al. [1] or the self-etch integration mechanism initiated by Sano et al. in 1994 [2].
Adhesion to the dentin is more complex when compared to bonding to enamel because of the structural variability in the dentin, which is due to a greater proportion of organic components; the presence of water; different ratios of peritubular, intertubular, and sclerotic dentin; differing percentages of tubules; and varying levels of communication with the pulp [3–8]. The density, diameter, and orientation of tubules vary according to dentin depth, that is, the quantity and diameter of tubules increase when moving closer to the pulp while peritubular dentin thickness decreases [9, 10].
The dentin tubules are filled with interstitial fluid that is similar to blood plasma [11–13]. The dentinal fluid can move through the tubules using capillary action, driven by hydrostatic intrapulpal pressure. This pulpal fluid displacement towards the external medium accounts for the moistness of the dentin surface [5, 14].
Accordingly, dentin moisture and pulp fluid flow should be considered during adhesive techniques, as excessive amounts of water might dilute the hydrophilic adhesive monomers; promoting separate phases of adhesive system; and affecting the polymer structure before photopolymerization [15–17].
To minimize these effects of dentin wetness on adhesive procedures, many studies have been conducted to verify the action of several types of lasers, including Nd:YAG (neodymium yttrium aluminum garnet), Er:YAG (erbium), Ho:YAG (holmium), and CO2 in the treatment of dental tissues and for adhesive purposes [8, 18–20].
The first Nd:YAG laser applications were performed prior to applying the adhesive system, which resulted in a reduction in bond strength when compared to non-irradiated cavities in most studies. This occurred because the neodymium laser caused denaturation of the dentin organic components due to heat generation, obliteration of dentin tubules due to fusion, and recrystallization of the inorganic components [18, 20].
In 1999, Gonçalves and others [8] evaluated the irradiation of the dentin substrate using a neodymium laser after etching with phosphoric acid and applying the adhesive system. Those authors found fusion and recrystallization of dentin in the presence of resin monomers, which resulted in an increase in bond strength. Based on this methodology, several studies regarding the effects of laser irradiation after adhesive system application and before photopolymerization were conducted and showed better results when compared to those using the conventional adhesive technique [18, 19, 21–24].
However, treatment with laser irradiation might cause a temperature increase of the dentin-pulp complex, depending on the thickness of dentin section, surface cooling, and the laser parameters [11, 25–27]. Thermal damage to irradiated and adjacent tissues might be prevented by evaluating the tissue temperature, since the Nd:YAG laser tips do not have an associated cooling system associated with it. Consequently, questions have been raised regarding the effects of the laser on the pulp chamber.
Considering the previously mentioned observation of the influence of fluid flow on the dentin substrate and its effect on adhesion when associated with a laser, this study evaluated the effects of simulated pulpal pressure on the variation of intrapulpal temperature and on the microtensile bond strength after applying an adhesive and laser irradiation. The null hypotheses tested were the following: (I) the simulated pulpal pressure does not significantly influence the intrapulpal temperature; (II) changes in the parameters of the Nd:YAG laser energy do not result in significant differences in intrapulpal temperature; (III) the simulated pulpal pressure does not significantly influence the bond strength; (IV) changes in the parameters of the Nd:YAG laser energy do not result in significant differences in the bond strength.
Materials and methods
Preparation of samples
One hundred sound human molars that were extracted for therapeutic reasons under approval of the Research Committee at the Sao Jose dos Campos School of Dentistry were used in this study. The teeth were cleaned using periodontal curettes and stored in deionized water at 4 °C for a maximum period of 6 months [28].
The teeth were fixed into an acrylic holder (2.5 cm/diameter and 2 cm/height) using dental wax and sectioned using a low-speed laboratory cutting machine (Labcut 1010, Extec Technologies Inc., CT, USA), under water cooling. Two horizontal sections were performed: (1) parallel to the occlusal surface to expose dentin; (2) 1 mm below the enamel-cementum junction to separate the crown from the roots, which were discarded. The pulpal soft tissues were removed using curettes.
The dentin specimens were standardized at approximately 2 mm thickness from the highest pulp horn [29], as measured with a caliper (Otto-Arminger & Cia Ltda., Rio Grande do Sul, Brazil). Dentin surfaces were polished using 600-grit aluminum oxide abrasive disks (Extec Corp., CT, USA) in a polishing device (DP-10, Panambra, São Paulo, Brazil), under water cooling.
The one hundred samples were randomly divided into two groups (n = 50) according to the following treatments: group 1 (PRESENCE): presence of simulated pulpal pressure; and group 2 (ABSENCE): absence of simulated pulpal pressure.
Simulated pulpal pressure
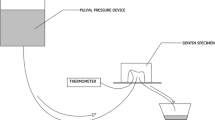
In group 1, self-cured acrylic resin holders (Jet, Artigos Odontológico Clássico, São Paulo, Brazil) were constructed measuring 1.5 × 1.5 × 0.5 cm. Three holes were drilled into these holders: two holes simulating water fluid flow inside the pulp chamber and one hole to install the thermocouple inside the highest pulp horn.
Intrapulpal pressure simulation was achieved by positioning two hypodermic needles (0.7 × 10 mm) into the center of the acrylic holder perpendicular to its base. The first needle was positioned so that its upper tip communicated with the pulp chamber; its lower tip was linked to a hydrostatic pressure device [11]. The second needle was placed with its upper tip inside the pulp chamber; the lower tip was linked to silicone tubes enabling the water fluid to flow towards the other reservoir (Fig. 1). The hydrostatic pressure device had a reservoir filled with deionized water at 37 °C [11, 27, 30], placed at 15 cm above the level of the pulp chamber [31]. Before simulated intrapulpal pressure, deionized water was injected into the pulp chamber to avoid air bubbles and to assure its total filling by the liquid.
Adhesive procedure
The dentin surfaces were etched with 37 % phosphoric acid (Scotchbond Universal Etchant Etching Gel, 3 M ESPE, MN, USA) for 15 s, rinsed for 30 s, and gently dried with absorbent paper to remove excess water. Next, the adhesive system (Scotchbond Universal; 3 M ESPE, MN, USA) was applied for 20 s, according to the manufacturer’s instructions. Before photopolymerization, the samples were irradiated with Nd:YAG laser. Each group was subdivided into five subgroups (n = 10), according to the variations in Nd:YAG laser energy parameters:
-
Subgroup 1 (60 mJ): tissue irradiation with Nd:YAG laser using 60 mJ of energy;
-
Subgroup 2 (80 mJ): tissue irradiation with Nd:YAG laser using 80 mJ of energy;
-
Subgroup 3 (100 mJ): tissue irradiation with Nd:YAG laser using 100 mJ of energy;
-
Subgroup 4 (120 mJ): tissue irradiation with Nd:YAG laser using 120 mJ of energy;
-
Subgroup 5 (140 mJ): tissue irradiation with Nd:YAG laser using 140 mJ of energy.
The adhesive system was light cured for 10 s after laser irradiation (LED Light Curing System, Demi Plus, Kerr Corporation, WI, USA), with a power density of 1200 mW/cm2.
Nd:YAG laser
Samples were irradiated using an Nd:YAG laser (neodymium yttrium aluminum garnet; Pulse Master 600 IQ; American Dental Technologies, USA), at a wavelength of 1064 nm, 100 μs pulse width, and a 400 μm diameter optical fiber. The frequency of 10 Hz was applied using varying energy parameters: 60, 80, 100, 120, and 140 mJ/pulse; and varying energy densities: 48, 64, 80, 95, and 111 J/cm2, respectively. Laser irradiation was applied freehand, in noncontact mode, and with surface scanning for 60 s. During laser irradiation, the laser fiber tip was positioned perpendicular to the sample surface and at a distance of approximately 1 mm. The laser irradiation was executed by the same calibrated operator.
Temperature measurement
To measure the variation of temperature, a digital thermometer with a type K thermocouple sensor (MT-507, Minipa Indústria e Comércio Ltda., São Paulo, Brazil) was used. The thermocouple sensor monitored the temperature inside the simulated pulp chambers during laser irradiation. The initial measurement was performed before irradiation and the maximum temperature peaks (°C) were registered in the first 60 s.
In group 1 (PRESENCE; n = 50), the samples were fixed into the holders to simulate intrapulpal pressure. In this group, the thermocouple sensor was placed below the roof of the pulpal chamber of the highest pulp horn, in contact with the dentin and in the presence of water.
In group 2 (ABSENCE; n = 50), the pulp chamber was filled with thermal paste (Implastec, São Paulo, Brazil) [30], in order to increase the thermocouple contact. This enabled the determination of temperature changes within the pulp chamber during laser application, without the interference of the external medium.
Restorative procedure
Composite resin blocks (4 mm/diameter and 2 mm/height) were built up on the irradiated dentin. Composite resin (Z350 XT, 3 M ESPE, MN, USA) was inserted in two increments of about 2 mm each, with each increment light cured for 20 s (LED Light Curing System, Demi Plus, Kerr Corporation, WI, USA). All samples were immersed in deionized water at 37 °C for 48 h.
Microtensile bond strength analysis
After 48 h, the samples were sectioned into dentin-composite resin sticks (1 mm2) suitable for the microtensile bond strength testing, using a low-speed laboratory cutting machine (Labcut 1010, Extec Technologies Inc., CT, USA) under water cooling. The sticks were stored in individual and identified tubes (Eppendorf, São Paulo, Brazil) containing deionized water for 24 h, at 37 °C, before the bond strength test. The microtensile bond strength test was performed in a universal testing machine (EMIC DL-1000, Equipamentos e Sistemas Ltda., Paraná, Brazil) at a crosshead speed of 0.5 mm/min and using a 10 kgf load cell. The microtensile bond strength values from the sticks of the same tooth sample were averaged and the mean bond strength was used as one unit for statistical analysis.
Statistical analysis
Concerning the variation of intrapulpal temperature, in degrees Celsius (°C), obtained during laser irradiation, the difference between the maximum value and the initial temperature (before irradiation) was calculated, using the formula: ∆T = T maximum − T initial. The microtensile bond strength values were expressed in MPa, and the mean of the sticks for each molar was calculated. Data were submitted to two-way analysis of variance (ANOVA; pulpal pressure and laser energy) and Tukey tests, using a level of significance of 5 % (p < 0.05).
Results
Pulpal temperature
According to the two-way ANOVA (Table 1), the presence of simulated pulpal pressure (group 1) significantly affected (p = 0.0001) the variation of intrapulpal temperature when compared with the absence of simulated pulpal pressure (group 2). Laser energy parameters (p = 0.0001) and the interaction of factors (p = 0.0018) also showed statistically significant influence on the variation of intrapulpal temperature.
Table 2 presents the mean (±standard deviation) values and Tukey’s test for all groups analyzed. The significantly lowest variation of temperature means was exhibited in the groups with simulated pulpal pressures irradiated at 100 mJ (5.00 ± 2.26)B, 80 mJ (4.80 ± 2.61)B, and 60 mJ (3.90 ± 1.91)B when compared with the other energy parameters.
Figure 2 shows the variation of temperature means of each group, in the presence (p) or absence (a) of simulated pulpal pressure, based on the Nd:YAG laser energy parameters. It showed a transition zone in the curve behavior in the graph, before and after the parameter of 100 mJ. The presence of simulated pulpal pressure reduced the variation of temperature mean values (°C) after irradiation through the laser energy parameters. However, despite this reduction, greater laser energy parameters induced greater variations of temperature.
Microtensile bond strength
According to the two-way ANOVA (Table 3), the presence of simulated pulpal pressure (group 1) significantly affected the microtensile bond strength values (p = 0.0001) when compared with those of the group without simulated pulpal pressure (group 2).
Table 4 presents the mean (±standard deviation) values and Tukey’s test for all groups/subgroups. The groups restored without simulated pulpal pressure exhibited significantly higher bond strength values when compared to those groups with simulated pulpal pressure, regardless of the laser energy parameters, except for the control groups.
Concerning the failure types, the groups restored without simulated pulpal pressure exhibited more cohesive resin failures. The groups restored with simulated pulpal pressure exhibited more mixed failures.
Discussion
This present study analyzed the dentin substrate adhesive technique proposed by Gonçalves and others [8], while simulating the pulp fluid flow, to evaluate the variation of temperature and microtensile bond strength, with the aim of contributing to standardizing the best laser energy parameters to be used for in vivo conditions.
Gonçalves and others [8] recommended laser irradiation for the dentin surface after etching and applying an adhesive system but before photopolymerization of the bonding system. Dentin surface modifications using the Nd:YLF laser in the presence of non-polymerized adhesive represented greater hybridization, significantly increasing the bond strength by improving adhesive infiltration and resin tag formation. In the studies of Matos and others [18], Nd:YAG laser irradiation on non-polymerized adhesive system also increased the bond strength. Despite the differences in laser type (Nd:YLF and Nd:YAG), the technique of laser irradiation on non-polymerized adhesive itself was found to be effective. Based on the same methodology, other studies showed favorable results in relation to bond strength [19, 20, 23] and marginal sealing [21]. Likely, these results occurred due to higher evaporation of the solvent from the adhesive systems, increased hybrid layer thickness, and possible chemical interactions [32].
According to the results of this present study, the null hypotheses tested for the variation of temperature were rejected: (I) SPP resulted in significant pulpal temperature reduction; (II) lower laser energy parameters resulted in smaller ΔT when compared to those of higher energy parameters.
Group 2 exhibited variations of temperature means (12.1 °C) greater than 5.5 °C for all energy parameters used. When a laser is used in adhesive procedures, intrapulpal temperature is directly proportional to the amount of energy applied. According to previous studies, temperatures increasing above 5.5 °C provoke pulpal necrosis; while temperatures above 3.3 °C provoked reversible pulpal injuries [33]. Some studies have suggested that variations of temperature in the root surface should be lower than 5 °C to avoid damage to the periodontium [34].
Notwithstanding, in vivo intrapulpal temperature increases might be lower than those obtained through in vitro studies due to the presence of an intact periodontal ligament, cortical bone, and pulpal blood flow, which potentially reduces increased temperatures [35]. Pulp blood flow dissipates the heat applied before the cells are damaged [36]. Moreover, the presence of the root further reduced the temperature because of the tooth structure mass [27, 35]. Thus, increases in in vivo temperatures would likely be lower.
The presence of SPP significantly interfered with the temperature by significantly reducing the ΔT for the 60, 80, and 100 mJ energy parameters (reduction of approximately 40 % in temperature when compared to group 2). The presence of liquids within the pulp chamber altered the absorption of heat generated by laser energy [25, 37].
Additionally, the presence of the smear layer and smear plugs might limit transudation on the dentin surface [31, 38, 39]. Given the restorative procedures, in vivo dentin acid etching raises the intrinsic water volume from 22 to 70 % [40] and smear layer removal unblocks the dentinal tubule lumen, increasing the tissue permeability [31, 41]. Therefore, the protocol of acid etching associated with SPP may have contributed to the reduction of the final temperature of the pulp wall when the dentin crown was irradiated with the Nd:YAG laser, similarly to the in vivo situation.
Dentinal fluid flow occurs by capillary action, driven by hydrostatic pulpal pressure. The hydrostatic pulpal pressure in this study was a 15-cm column of deionized water [29, 31, 38], simulating the physiological pressure of a human tooth at 1.47 KPa [31]. Moreover, the circuit created for liquid input and output permitted a fluid movement generated by a pressure gradient. Accordingly, heat dissipation occurred similarly to the in vivo condition through renewing the liquid inside the pulp chamber.
In the results of this study, the energy parameters of 120 and 140 mJ provoked ΔT means above 10 °C on the pulp chamber wall, even when using SPP, which could irreversibly damage both the pulp and periodontium [33, 34, 42]. These results demonstrated that SPP did not allow heat dissipation inside pulp chamber below biologically accepted thresholds for the energy parameters of 120 and 140 mJ. The results of this present study are in agreement with previous study [43], which demonstrated that increasing Nd:YAG laser energy parameters caused greater dentinal tissue overheating and, consequently, greater pulp chamber heating.
According to the findings of bond strength values of the present study, the third null hypothesis was rejected for μTBS: (III) SPP resulted in a significant bond strength reduction. However, the fourth null hypothesis was accepted for μTBS: (IV) the laser energy parameters did not influence the bond strength values.
A reduction in μTBS of approximately 48 % occurred in the presence of fluid. To establish an effective hybridization, the resin monomers should flow within tubules filled by water and diffuse through interfibrillar spaces to enable the polymerization and formation of resin tags [15–17, 31, 38]. Notwithstanding, significant amounts of water might dilute and/or prevent polymerization of the hydrophilic monomer from adhesive systems, separate the phases (hydrophilic/hydrophobic), and affect the polymer structure [15–17, 31, 38]. The presence of water might degrade the mechanical properties of the adhesive, such as bond strength and modulus of elasticity [44]. The fracture types also demonstrated an interaction with SPP. The dentin with less humidity was more susceptible to cohesive fracture of the substrate.
Dentin wetness generated by intrapulpal pressure might have limited the infiltration of resin monomers inside the tubules and demineralized collagen, damaging the formation of resin tags and hybrid layer [16, 31], in comparison with the group without SPP. Dentin surface wettability and the presence of SPP are the critical variables during adhesive procedures, especially for in vitro studies intended to simulate in vivo conditions [16].
The variations of Nd:YAG laser energy parameters significantly affected the μTBS in the current study. These current results demonstrated the possibility of using less substantial laser irradiation parameters on dentin surface by the technique proposed by Gonçalves and others, reducing the risks of possible permanent damage to pulpal and periodontal tissues, without compromising the bond strength to the substrate.
By predicting the in vivo conditions, the effects of temperature variations would be less damaging. The depth used in this study was 2 mm from the highest pulp horn and the time of irradiation was 60 s, these results offer an important understanding about the variables involved. Even in the presence of pulpal fluid, the temperature in the pulp chamber was higher than 5.5 °C for all parameters used. For this reason, the protocol for clinical application requires more investigations.
This present study also determined that SPP generated a significant reduction of ΔT and μTBS. As the absence of fluid is a non-vital condition, the protocol of SPP in in vitro studies should be routinely considered during adhesive procedures to better mimic in vivo conditions [31, 38]. It is important to point out that the results found in this laboratory study have some limitations: (1) difficulty in standardizing the dentin thickness across the uniform surface, due to anatomical variation of the pulp chamber; (2) unfeasibility to repeat the laser irradiation on the surface in other times, because the surface had already changed by laser irradiation from the first irradiation; (3) in vivo condition, the tooth has pulsatory flow (pressure differences) and, in the present study, there was no pressure variation.
Conclusions
Based on the current study, it can be concluded that (I) SPP significantly reduced the ΔT on a dentin substrate during laser irradiation, (II) SPP significantly reduced the μTBS to dentin substrate, (III) lower laser energy parameters resulted in smaller ΔT when compared to those of higher energy parameters, and (IV) the laser energy parameters did not influence the bond strength values.
References
Fusayama T, Nakamura M, Kurosaki N, Iwaku M (1979) Non-pressure adhesion of a new adhesive restorative resin. J Dent Res 58:1364–1370
Sano H, Shono T, Sonoda H et al (1994) Relationship between surface area for adhesion and tensile bond strength—evaluation of a micro-tensile bond test. Dent Mater 10:236–240
Oilo G, Olsson S (1990) Tensile bond strength of dentin adhesives: a comparison of materials and methods. Dent Mater 6:138–144
Garberoglio R, Brannstrom M (1976) Scanning electron microscopic investigation of human dentinal tubules. Arch Oral Biol 21:355–362
Tao L, Pashley DH (1976) The relationship between dentin bond strengths and dentin permeability. Dent Mater 5:133–139
Nakabayashi N, Nakamura M, Yasuda N (1991) Hybrid layer as a dentin-bonding mechanism. J Esthet Dent 3:133–138
Perdigao J, Swift EJ Jr, Denehy GE, Wefel JS, Donly KJ (1994) In vitro bond strengths and SEM evaluation of dentin bonding systems to different dentin substrates. J Dent Res 73:44–55
Goncalves SE, de Araujo MA, Damiao AJ (1999) Dentin bond strength: influence of laser irradiation, acid etching, and hypermineralization. J Clin Laser Med Surg 17:77–85
Marshall GW Jr (1993) Dentin: microstructure and characterization. Quintessence Int 24:606–617
Weiner S, Veis A, Beniash E et al (1999) Peritubular dentin formation: crystal organization and the macromolecular constituents in human teeth. J Struct Biol 126:27–41
Goodis HE, White JM, Marshall GW Jr et al (1999) Effects of Nd: and Ho:yttrium-aluminium-garnet lasers on human dentine fluid flow and dental pulp-chamber temperature in vitro. Arch Oral Biol 42:845–854
Perdigao J (2010) Dentin bonding-variables related to the clinical situation and the substrate treatment. Dent Mater 26:e24–e37
Haldi J, Wynn W (1963) Protein fractions of the blood plasma and dental-pulp fluid of the dog. J Dent Res 42:1217–1221
Brannstrom M, Linden LA, Astrom A (1967) The hydrodynamics of the dental tubule and of pulp fluid. A discussion of its significance in relation to dentinal sensitivity. Caries Res 1:310–317
Spencer P, Wang Y (2002) Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res 62:447–456
Sauro S, Pashley DH, Montanari M et al (2007) Effect of simulated pulpal pressure on dentin permeability and adhesion of self-etch adhesives. Dent Mater 23:705–713
Park J, Ye Q, Topp EM et al (2010) Effect of photoinitiator system and water content on dynamic mechanical properties of a light-cured bisGMA/HEMA dental resin. J Biomed Mater Res A 93:1245–1251
Matos AB, Oliveira DC, Kuramoto M Jr, Eduardo CP, Matson E (1999) Nd:YAG laser influence on sound dentin bond strength. J Clin Laser Med Surg 17:165–169
Matos AB, Oliveira DC, Navarro RS, de Eduardo CP, Matson E (2000) Nd:YAG laser influence on tensile bond strength of self-etching adhesive systems. J Clin Laser Med Surg 18:253–257
Franke M, Taylor AW, Lago A, Fredel MC (2006) Influence of Nd:YAG laser irradiation on an adhesive restorative procedure. Oper Dent 31:604–609
Araujo RM, Eduardo CP, Duarte Junior SL, Araujo MA, Loffredo LC (2001) Microleakage and nanoleakage: influence of laser in cavity preparation and dentin pretreatment. J Clin Laser Med Surg 19:325–332
Ferreira LS, Francci C, Navarro RS, Calheiros FC, Eduardo CP (2009) Effects of Nd:YAG laser irradiation on the hybrid layer of different adhesive systems. J Adhes Dent 11:117–125
Marimoto AK, Cunha LA, Yui KC et al (2013) Influence of Nd:YAG laser on the bond strength of self-etching and conventional adhesive systems to dental hard tissues. Oper Dent 38:447–455
Castro FL, Andrade MF, Hebling J, Lizarelli RF (2012) Nd:YAG laser irradiation of etched/unetched dentin through an uncured two-step etch-and-rinse adhesive and its effect on microtensile bond strength. J Adhes Dent 14:137–145
Yu D, Powell GL, Higuchi WI, Fox JL (1993) Comparison of three lasers on dental pulp chamber temperature change. J Clin Laser Med Surg 11:119–122
Anic I, Vidovic D, Luic M, Tudja M (1992) Laser induced molar tooth pulp chamber temperature changes. Caries Res 26:165–169
White JM, Fagan MC, Goodis HE (1994) Intrapulpal temperatures during pulsed Nd:YAG laser treatment of dentin, in vitro. J Periodontol 65:255–259
Ghiggi PC, Dall Agnol RJ, Burnett LH Jr, Borges GA, Spohr AM (2010) Effect of the Nd:YAG and the Er:YAG laser on the adhesive-dentin interface: a scanning electron microscopy study. Photomed Laser Surg 28:195–200
Belli R, Sartori N, Peruchi LD et al (2010) Slow progression of dentin bond degradation during one-year water storage under simulated pulpal pressure. J Dent 38:802–810
Lizarelli RF, Moriyama LT, Bagnato VS (2006) Temperature response in the pulpal chamber of primary human teeth exposed to Nd:YAG laser using a picosecond pulsed regime. Photomed Laser Surg 24:610–615
Ozok AR, Wu MK, De Gee AJ, Wesselink PR (2004) Effect of dentin perfusion on the sealing ability and microtensile bond strengths of a total-etch versus an all-in-one adhesive. Dent Mater 20:479–486
Batista GR, Barcellos DC, Torres CRG et al (2015) Effect of Nd:YAG laser on the solvent evaporation of adhesive systems. Eur J Esthet Dent 10(4):598–609
Zach L, Cohen G (1965) Pulp response to externally applied heat. Oral Surg Oral Med Oral Pathol 19:515–530
Blankenau RJ, Kelsey WP, Powell GL, Cavel WT, Anderson DM (1994) Power density and external temperature of laser-treated root canals. J Clin Laser Med Surg 12:17–19
Turkmen C, Gunday M, Karacorlu M, Basaran B (2000) Effect of CO2, Nd:YAG, and ArF excimer lasers on dentin morphology and pulp chamber temperature: an in vitro study. J Endod 26:644–648
Sulieman M, Rees JS, Addy M (2006) Surface and pulp chamber temperature rises during tooth bleaching using a diode laser: a study in vitro. Br Dent J 200:631–634
Secilmis A, Bulbul M, Sari T, Usumez A (2013) Effects of different dentin thicknesses and air cooling on pulpal temperature rise during laser welding. Lasers Med Sci 28:167–170
Mazzitelli C, Monticelli F, Osorio R et al (2008) Effect of simulated pulpal pressure on self-adhesive cements bonding to dentin. Dent Mater 24:1156–1163
Gregoire G, Joniot S, Guignes P, Millas A (2003) Dentin permeability: self-etching and one-bottle dentin bonding systems. J Prosthet Dent 90:42–49
Spencer P, Ye Q, Park J et al (2012) Durable bonds at the adhesive/dentin interface: an impossible mission or simply a moving target? Braz Dent Sci 15:4–18
Pashley DH (1984) Smear layer: physiological considerations. Oper Dent 3:13–29
Sauk JJ, Norris K, Foster R, Moehring J, Somerman MJ (1988) Expression of heat stress proteins by human periodontal ligament cells. J Oral Pathol 17:496–499
Moriyama EH, Zangaro RA, Lobo PD et al (2003) Optothermal transfer simulation in laser-irradiated human dentin. J Biomed Opt 8:298–302
Yiu CK, King NM, Carrilho MR et al (2006) Effect of resin hydrophilicity and temperature on water sorption of dental adhesive resins. Biomaterials 27:1695–1703
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, T.M., Gonçalves, L.L., Fonseca, B.M. et al. Influence of Nd:YAG laser on intrapulpal temperature and bond strength of human dentin under simulated pulpal pressure. Lasers Med Sci 31, 49–56 (2016). https://doi.org/10.1007/s10103-015-1827-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1827-1