Abstract
In the previous study, environmental impacts of lithium-ion batteries (LIBs) have become a concern due the large-scale production and application. The present paper aims to quantify the potential environmental impacts of LIBs in terms of life cycle assessment. Three different batteries are compared in this study: lithium iron phosphate (LFP) batteries, lithium nickel cobalt manganese oxide (NCM) 811 batteries and NCM622 batteries. The results show that the environmental impacts caused by LIBs are mainly reflected in five aspects from eleven evaluation indexes: Abiotic depletion (fossil fuels), Global warming, Human toxicity potential (HTP), Freshwater ecotoxicity potential (FETP) and Marine ecotoxicity potential (METP). The production phase and assembly phase of LIBs are the main sources of Greenhouse gas (GHG) emission, the GHG emissions of NCM622 battery is 156.73 kg CO2-eq/kWh, which accounts for 35.40% of the total GHG emissions. The study shows that the hydrometallurgy method in the recycling phase may not always be environmentally friendly for NCM batteries, it can increase the indicators of HTP, FETP and METP. The precursor materials in NCM batteries and the electricity consumption of LFP batteries are sensitive factors to environmental impacts, which can be effectively improved by improving the process and optimizing the electricity mixes. Suggestions for process optimization of China’s LIBs industry are proposed based on the future electricity mixes.
Graphical abstract

Life cycle assessment of three typical lithium-ion batteries and sensitivity analysis of main contributors
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, China is the leading producer of lithium-ion batteries (LIBs), which rely on essential components such as lithium, cobalt and graphite. The number of battery electric vehicles (BEVs) on the road is predicted to reach over 130 million globally by 2030 (Kapustin and Grushevenko 2020). The requirement for LIBs is anticipated to increase significantly, with an estimated global demand of 9300 GWh by 2030 (Lai, Chen et al. 2022). This growth in the BEV market is expected to be driven largely by China, which in the near future will have significant impacts on the production and the disposal of LIBs. China’s regulations on lithium waste are also being updated in response to the rapid updating of electronics. China faces greater energy pressure compared to developed countries in particularly (Chen, Lai et al. 2022a, b). Therefore, it is critical to accurately estimate the life cycle resource consumption of various LIBs in China to mitigate potential environmental pressures.
Promoting BEVs will help to solve our nation’s problem with energy security (Haustein and Jensen 2018). BEVs use a variety of chemistries and battery architectures, including Li-ion, nickel metal hydride and sodium-nickel chloride (Asef, Milan et al. 2021). At the same time, the advancement of battery technology and commercial potential suggests that LIBs are better option for the growth of the BEVs industry due to their technological maturity and lower manufacturing costs (Diaz-Ramirez, Ferreira et al. 2020). In both mobile and permanent energy storage systems, LIBs are regarded as a crucial component to assist in lowering harmful emissions from the transportation sector by enabling electric mobility (Tourlomousis and Chang 2017).
Currently, there are various types of LIBs available, with lithium iron phosphate (LFP) batteries and lithium nickel cobalt manganese oxide (NCM) batteries being used extensively in BEVs (Shu et al. 2021). In the context of electric passenger vehicles, the predominant LIB chemistry used in China is NCM 622 battery, although there are other types such as NCM 111 and NCM 811 available (Yang, Mu et al. 2020). However, the production and application of these batteries have the potential to emit greenhouse gas (GHG) and environmental damage during their whole life cycle (Manh-Kien, Mevawala et al. 2020). One promising strategy for reducing the environmental impacts of LIBs is secondary use, which can delay disposal and provide commercial and environmental benefits (Ahmadi et al. 2015). It is important to examine environmental effects across entire life cycle, including long lifespan and multiple stages (Kelly et al. 2020).
Additionally, the environmental impacts of LIBs varies depending on the use of various materials, industrial procedures, manufacturing strategies and recycling methods (Du et al. 2017). Du et al. selected 7 distribution methods to study the production and recycling of LIB on the basis of the same data, and proposed that the recycling of lithium-ion batteries must consider the influence of modeling methods and distribution methods (Du et al. 2022a, b). To meet the demands of the LIBs industry, new cathode materials with greater density of energy and capacity are constantly being created (Wang, Wu et al. 2019a, 2019b). Currently, LFP is used as the positive electrode in LFP batteries, while the positive electrodes of NCM batteries consist of nickel, cobalt, and aluminum (Yang et al. 2021). Nevertheless, there is a persistent effort to develop cathode materials with greater energy density and capacity to meet the requirements of LIBs (Chakraborty, Banerjee et al. 2018).
There has been considerable attention given to conducting life cycle assessment (LCA) research on LIBs in recent years, particularly in terms of the resource utilization and environmental impacts (Liu, Liu et al. 2021). However, many studies have mainly focused on the production phase of the battery and have not taken into account the potential impacts of recycling phase (Nordelof et al. 2016). Additionally, few studies have investigated the overall environmental impacts of LIBs throughout the entire life cycle (Wang, Deng et al. 2020). Instead, most of the available research have concentrated on a single stage of the life cycle. For example, some studies have examined the environmental impacts of NCM batteries, indicating that the material development phase contributes the most to energy utilization, potential for global warming and acidification (Sun, Luo et al. 2020). The production of NCM111 batteries, which involves cathode materials and aluminum manufacturing has been identified as a primary source of contamination by researchers (Dai, Kelly et al. 2019).
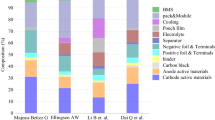
Scholars have also studied the environmental effects of various LIBs. MajeauBettez et al. assessed the environmental impacts of NCM batteries, NiMH batteries and LFP batteries during the manufacturing process and found that NiMH batteries posed the greatest environmental load (Majeau-Bettez et al. 2011). Smith et al. proved that solid-state batteries exhibit lower carbon emissions than LIBs during the manufacturing stage (Smith, Ibn-Mohammed et al. 2021). Oliveira et al. used SimaPro software to evaluate several environmental variables of the LMO batteries and LFP batteries (Oliveira et al. 2015). Besides, several studies examining the environmental effects of LIBs from other angles. For instance, Zackrisson et al. assessed the effect of LFP batteries produced using various solvents on the environment, which revealing that water exhibits superior performance than N-methyl-2-pyrrolidone (NMP) (Zackrisson et al. 2010). While numerous research have looked into the effects on the environment of LIBs, less focus has been placed on the long-term environmental impacts of different types of LIBs throughout their life cycles.
The LCA method is employed in this studies to quantify possible environmental impacts of three typical batteries, namely LFP batteries, NCM 811 batteries and NCM622 batteries. This methodology comprehensively evaluates the environmental hotpots, which describes a technique for systematic analysis used to quantify the collection of raw materials, manufacturing, use of resources, transportation, disposal and any potential environmental effects from recycling (Arshad, Lin et al. 2022). Energy consumption is calculated based on battery capacity decay and cycle life during the use phase. The potential contributions of this article are as follows:
-
(1)
The “cradle-to-grave” LCA is conducted for three typical LIBs in China, providing a complete and transparent life cycle list for all life cycle stages and understand which LIBs are more environmentally friendly for BEVs.
-
(2)
Through sensitivity analysis to evaluation indicators that have great influence for LIBs on the environmental impacts. Besides, the GHG emissions of the current and future LIBs production are calculated under hybrid energy structure.
-
(3)
Further exploring the environmental performance of the recycling processes for LFP and NCM batteries, providing technical support for related green manufacturing and full life cycle management.
Methodology
The LCA technique is employed to the extent of ISO 14040–14044 standards to analyze the whole life cycle of the product, process, service or activity, and estimates its environmental effects in a quantitative manner (Daniele Landi 2022). The models are developed by SimaPro 9.0 software, which integrates the Ecoinvent 3.0 database (Suh et al. 2016) and provides midpoint and endpoint impact assessment methods, such as Eco-indicator 99, Impact 2002+, CML IA and ReCiPe to analyze the environmental impacts. Each of method includes several categories of effects, such as climate change and acidification which are widely used in LCA. In this study, the CML-IA v3.05 method (Silvestri, Forcina et al. 2020) is used to assess the environmental impacts. The evaluation will strictly adhere to the international standard ISO 14044 to ensure the accuracy of the findings (Hua, Zhou et al. 2020).
Goal and scope
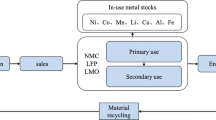
As shown in Fig. 1, scope of “cradle-to-grave” is employed in this study to evaluate the environmental effects of three types of LIBs, considering battery recycling and utilization. The performance parameters of the three battery systems are shown in Table 1. The power capacity of the LFP battery system is almost identical to the NCM battery system. The energy density of LFP battery is 121 Wh/kg, the energy density of NCM622 battery is 149 Wh/kg, and NCM811 battery have an energy density of 154 Wh/kg. In this study, the total mass of key components of the battery is calculated by setting the mass of 1 kWh LFP battery pack at 7.49 kg, the 1 kWh NCM622 battery pack at 5.76 kg, and 1 kWh NCM 811 battery pack at 5.33 kg.
The functional unit (FU) is established as the rated capacity of 1 kWh battery pack, which is commonly utilized unit in previous LCA studies. To make the environmental effects of various batteries comparable, all the gathered data must be converted to FU (Wu et al. 2021). The LIB is made up of the single cell, shell, wire and battery management system. The cathode, anode, electrolyte and diaphragm are the four most crucial components of the LIB used in BEVs (Tian, Qin et al. 2020). The weight of the NCM622 battery is 161.3 kg, the overall mass of the LFP battery is 209.7 kg, and the total mass of the NCM811 battery is 149.2 kg. Table 2 shows the mass ratios of different components of three types of batteries.
The system boundary is shown in Fig. 2, which includes the raw material extraction, component production, battery manufacturing, transportation, use and end of life stage. The battery production phase requires the preparation of raw materials, including the production of cathode materials and the refining of lithium. Then the battery cells, copper rods, battery management system, etc. are assembled to form the battery system. This process includes winding, cutting, winding shaping, laminating, and other processes (Wang, Yu et al. 2019a, 2019b). The transportation stage includes the transport of raw materials from the origin to the manufacturing factory and the transport of used batteries from the collection center to the recycling plant. According to (Zackrisson et al. 2016), it can be assumed that a common waste battery collection center is 500 km away from the recycling plant in the European context. The same assumption is made for the average distance between Chinese cities. Truck transportation is the primary means of transportation.
The energy consumption in the use stage is mainly caused by the decrease in charge and discharge efficiency and quality loss (Pellow, Ambrose et al. 2020). Meanwhile, the charge and discharge efficiency of the battery will decrease with the increase in the number of cycles (Picatoste, Justel et al. 2022). The total driving distance (L) of the electric vehicle in the use phase is expressed with the initial driving distance per charge (D0), the capacity decay rate (r) and the number of cycles (c). The total driving distance (L) of the BEVs in the use phase is defined as follows (Deng et al. 2019):
The total energy consumption (E) in the use stage is related to factors such as battery efficiency (\(\eta_{{{\text{battery}}}}\)), battery charging efficiency (\(\eta_{{{\text{charger}}}}\)), discharged energy (\(E_{{{\text{discharged}}}}\)), and total driving distance (L). To simplify the study, it is assumed that the charge and discharge efficiency in the use stage is 80% and will not decrease with the increase of the number of cycles. The total energy consumption in the use stage is expressed as follows (Deng et al. 2019):
The mainstream process of recycling LIBs in Chinese enterprises is hydrometallurgy method, which has the characteristics of low energy consumption, high recovery rate and relatively mature process (Du et al. 2022a, b). The hydrometallurgical process can be divided into three different steps: Pretreatment, leaching and metal recovery. After the battery is discharged, disassembled and crushed, the final product is obtained by hydrometallurgical technology through low temperature leaching, purification and separation. The hydrometallurgy recycling method is used in this research. The production and recycling of other electric vehicle components are not considered in this study.
Life cycle inventory
Life cycle inventory (LCI) analysis is the crucial step in constructing an input and output list for a product system to analyze its environmental impacts (Qiao et al. 2017). LCI data can be obtained directly through experimentation (Bobba et al. 2018), cooperation with companies (Koroma, Costa et al. 2021), indirectly through sources of literature, LCA software and databases (Wernet et al. 2016). To ensure data completeness and representation, we have conducted a field survey of three leading LIB factories and two recycling enterprises in China to collect primary LCI data. The data used in this study are mainly from technical data sheets and literature readings (Sun et al. 2017). Ecoinvent 3.0 database has supplemented the inventory data. Data sources for three types of LIBs are listed in Table 3, and the detailed date list of three types of LIBs can be found in Tables 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 and 18.
Cathode material is an important part of LIBs, which directly determined its energy density and service life. The cathode material of 1 kWh NCM811 battery is 1.63 kg, accounting for 26.3% of the total material input. The cathode material of 1 kWh NCM622 battery is 1.77 kg, accounting for 26.8% of the total material input. The cathode material of NCM battery is mainly obtained by hydrothermal method from ternary precursor and lithium hydroxide (LiOH), in which LiOH derives from lithium ore (Soge, Willoughby et al. 2021). The cathode material of 1 kWh LFP battery is 2.11 kg, accounting for 25.2% of the total input material. The cathode material of LFP battery is mainly obtained by ferric sulfate, LiOH and phosphoric acid (H3PO4) under constant temperature condition. The anode material is graphite. The selected binder material is polyvinylidene fluoride (PVDF). The electrolyte consisted of lithium hexafluorophosphate (LiPF6) and ethylene carbonate (EC). The main component of the shell is aluminum. Cathode collector has the function of loop formation and stable control performance. Aluminum foil is used for cathode collector. Anode collector is made of copper foil by pyrometallurgy.
The molded cathode and anode materials are coated, dried and rolled. The energy consumption in the battery assembly stage is shown in Table 4, among which the energy consumption in the drying stage accounts for the largest proportion. The transportation phase is carried by truck, and the study assumes that the transport distance is 500 km. In the context of this study, considering the capacity of 28 kWh LIBs with efficiency of 80% are used for 2500 days in BEVs. The modeling information mainly refers to the battery cycle number and capacity fade experiment of BEVs (Dong, Fu et al. 2020).
Life cycle impact assessment
Due to the fact that LIBs have become the growing issue in recent years, the assessment of its environmental impacts on the whole life cycle is helpful to provide the LIBs industries with an appropriate reference value. The production of LIBs is a complicated process that uses resources, produces waste and releases different exhaust gases. Based on the above methods, this study uses SimaPro 9.0 software to construct the LCA model. To fully assess the environmental effects of LIBs, the CML-IA v3.05 (Silvestri, Forcina et al. 2020) is used to assess the impact of the three types of battery systems on the environment.
Interpretation
By life cycle interpretation, the primary contributing factors and essential components of LIBs are discovered. The environmental indexes of CML-IA method are discussed: Abiotic depletion (AD), Abiotic depletion (fossil fuels) (ADF), Global warming (GWP), Ozone layer depletion (ODP), Human toxicity potential (HTP), Freshwater ecotoxicity potential (FETP), Marine ecotoxicity potential (METP), Terrestrial ecotoxicity potential (TETP), Photochemical ozone creation potential (POCP), Acidification potential (AP) and Eutrophication potential (EP). The sensitivity analysis is carried out to identify the most sensitive substances to environmental impacts. By quantifying the energy consumption in the production phase, the change in environmental indicators caused by the increase of 10% in the baseline case is explored. Besides, this paper creates the scenario analysis based on the future electricity mixes to provide reference for realizing the China’s carbon peaking and carbon neutrality goals.
Results and discussion
Life cycle assessment results
Table 5 displays the comparison of 11 ecological indicators across the entire life cycle of three types of LIBs, which highlights the major contributing processes of the production phase and assembly phase. The transportation process is the major contributor to METP, GWP and ADF. Furthermore, the LFP batteries consume more energy than the NCM batteries. This is because the energy density of LFP batteries is lower than NCM batteries, which consumes more energy during the transport phase. As the development of LIBs moves toward high nickel content, it is important to consider the ecological indexes of AP and POCP, this is because the preparation of raw materials for ternary precursors, such as nickel sulfate (NiSO4) and cobalt sulfate (CoSO4), involves the use of concentrated sulfuric acid. The reaction of concentrated sulfuric acid results in the production of SO2, and NiSO4 can be used as catalyst to improve the rate and efficiency of hydrogenation and oxidation reactions, which are the main component affecting AP and POCP. The EP of LFP batteries is higher than NCM622 and NCM811 batteries. This is because the primary raw material for the positive electrode generates ammonium-containing waste liquid during the production phase. Besides, in the case of LFP battery systems, almost all ADF and TETP occur during the use phase and the GHG emissions in the assembly stage is higher than the production stage, where the main energy is electricity.
The LCA findings reveal that the environmental impacts caused by LIBs is mainly reflected in five aspects from eleven evaluation indexes: HTP, FETP, GWP, ADF and METP. The three LIBs have the most substantial environmental contamination during the production phase of the five stages, which is followed by the use phase, assembly phase and transport phase. In addition, the study shows that the hydrometallurgy recycling of LFP batteries can effectively relieve the environmental impacts. As for the NCM622 batteries and NCM811 batteries, the hydrometallurgy recycling results in greater HTP, FETP and METP. The main reason is that NCM batteries contain heavy metals, during the recycling phase, toxic and harmful metal elements and substances can cause pollution to human health and the environment. This proved that the recycling stage is not always a stage of offsetting environmental pollution.
The research finding reveals that NCM622 batteries have higher GHG emissions than NCM811 batteries, as demonstrated by Fig. 3. In contrast, LFP batteries exhibit significantly lower GHG emissions at 136.79 kg CO2-eq/kWh, indicating that Prioritizing LFP battery utilization can effectively cut GHG emissions. Moreover, the study founds the GHG emissions contribution of various components in the production of LIBs. The results indicate that the assembly of NCM811 batteries resulted in GHG emissions of 47.21 kg CO2-eq/kWh, accounting for 31.63% of the total GHG emissions. Notably, the production phase and assembly phase are the primary contributors to GHG emissions across all phases of LIBs. The main reason is that the precursor of NCM batteries require heavy metal salts such as NiSO4 and CoSO4 will generate a large amount of GHG. The main energy consumed during the battery assembly stage is electricity.
To ensure the accuracy of the experimental results, we compare the GHG emissions with previous studies in Fig. 4. The GHG emissions of different LIBs are significantly different, ranging from 49 to 124 kg CO2-eq/kWh. In this research, the GHG emissions of NCM811 battery during assembly are 47.52 kg CO2-eq/kWh. This is because NCM811 battery have higher nickel content, which requires more energy to extract and process than cobalt and manganese. The difference in the GHG emissions during battery assembly phase is mainly due to difference in grid structure. The difference in the GHG emissions of the rest of the battery is due to the difference in the background data source and the choice of production equipment. As shown in Fig. 4, GHG results in the production stage are in the middle of literature estimates.
Abiotic Depletion (ADP fossil) (ADF) is an evaluation index to measure the consumption of fossil energy (Yang, Lan et al. 2022). Figure 5 shows the ADF values for the three types of LIBs. Compared with LFP batteries, NCM811 and NCM622 batteries produce more severe ADF burden. The ADF of LIBs is mainly produced in the battery production, assembly and use stage. This is because the battery production and assembly stages consume huge amounts of energy, such as coal, oil and electricity to make metals such as steel, lithium and cobalt. Besides, coal power is still the main way used in China.
Human toxicity potential (HTP) is an evaluation index that measures how hazardous substances affect the human body (Smith et al. 2020). Figure 6 shows the HTP values for the three types of LIBs. The production phase is the main source of HTP for NCM622 and NCM811 batteries, the HTP of NCM battery mainly comes from the thermal radiation produced by the heavy metal in the cathode and aluminum shell. On the other hand, the HTP of LFP batteries mainly comes from the assembly phase, because the thermal radiation comes from energy consumption, it is dominated by electricity.
Marine ecotoxicity potential (METP) refers to the adverse effects of toxic chemical emissions on marine ecosystems, including the accumulation and discharge of substances like mercury, copper, lead and radioactive elements (Jackson et al. 2016). Figure 7 illustrates the METP impact of three types of LIBs. The results reveal that the highest METP impact is during the production and assembly stages of cathode materials. This is due to the presence of heavy metal pollutants in cathode production and toxic pollutants produced during the assembly stage. The precursor of the NCM battery contains heavy metals such as nickel, cobalt and manganese. At the same time, during the production process, the LIBs will produce waste water, which may contain heavy metals, organic substance, acid and alkali and other harmful substances, causing certain pollution to the soil and water resources.
As shown in Fig. 7, negative values represent existence of enviromental benefits under corresponding impact category. During the recycling stage, the environmental benefit of LFP batteries is higher than NCM batteries. The main reason is that the hydrometallurgical recycling Li, Fe and Al from the spent LFP batteries can alleviate the METP. Pyrolysis is considered as an alternative recovery method to pretreatment, which can decompose organic substances such as PVDF under high temperature conditions and facilitate subsequent hydrometallurgical processes. However, the pyrolysis separation electrode may affect the recycling of materials and produce wastewater.
Freshwater ecotoxicity potential (FETP) is a measure of the impact of pollutants on freshwater ecology. It refers to the interdependent relationship between all biological communities and physical and chemical environment within the given water area, formed through the cycling of substances (Aurisano, Albizzati et al. 2019). Figure 8 presents the FETP impact of three types of LIBs. The causes of FETP during the battery “Production stage” are consistent with those of METP, which involve the presence of heavy metal pollutants and toxic substances.
Sensitivity analysis
The sensitivity analysis highlights that the production stage of LIBs materials has the most significant impact on environmental sustainability, particularly for cathode materials. To ensure the accuracy of the model, expert consultation is utilized to assume that the cathode active material’s mass and battery energy density remain unchanged despite variations in the material’s chemical properties. By analyzing the sensitivities of LFP batteries and NCM batteries over their life cycles, identifying the key parameters that have high sensitivity and impact the model output.
In this study, the 10% floating interval is used to quantify the battery input and present the results in Fig. 9 and Fig. 10. For LFP batteries, eight sensitive parameters are selected according to the floating ratio of environmental indicators by quantifying input list, namely NMP, electricity, glucose, H3PO4, ethylene carbonate, lithium chloride, aluminum ingot, and battery separator. For NCM622 and NCM811 batteries, eight sensitive parameters are selected according to the floating ratio of environmental indicators, namely electricity, lithium carbonate (Li2CO3), aluminum, oxygen, sodium hydroxide (NaOH), sheet rolling, battery separator and NiSO4, among which input of nickel sulfate is the key parameter in the production stage. The findings reveal that electricity is the most sensitive factor throughout the entire life cycle of LFP batteries. For NCM batteries, the precursor material in the cathode material is identified as the most sensitive factor. This is because increasing the NiSO4 content during the production of precursor material will lead to higher consumption of steam, LiOH and oxygen.
Scenario analysis
Table 6 indicates the main sources for electricity production in the next 30 years in China, which are thermal power (67.9%), hydropower (17.0%), wind power (6.0%), nuclear power (5.6%) and photovoltaic power (3.5%) in 2020 (CEC 2021). In particular, coal plays a dominant role in thermal power. Consequently, the authors assume that thermal power is completely fueled by coal. The energy consumption and environmental emissions are calculated using the ecoinvent 3.0 database. It is predicted that wind power and photovoltaic power will be the main power generation methods in China by 2050. As demonstrated in Fig. 11, the study reveals that wind power has the greatest potential for reducing greenhouse gas emissions. Besides, increasing the proportion of clean energy sources in the production of LFP batteries can effectively mitigate environmental impacts, especially for METP and GWP. The study has found that TETP did not change significantly as the proportion of clean energy increased, this is because during the construction of power stations, the surface environment is destroyed and reformed, involving the emission of carbon dioxide, methane and other gases buried below the surface.
Currently, various methods are used to prepare cathode materials for NCM batteries, such as coprecipitation, hydrothermal, sol–gel, solid phase and combustion methods. The coprecipitation method involves dissolving the metal salt solution with nickel, cobalt and manganese, precipitating each component according to the stoichiometric ratio, and obtaining the precursor through extraction and filtration treatment. The precursor is transformed into the ternary cathode material through high-temperature burning. In this study, the sol–gel method is employed to prepare the positive electrode material of ternary lithium battery, using lithium acetate, nickel acetate, manganese acetate and acetic acid as raw materials. The optimization diagram of the two ternary lithium batteries are shown in Fig. 12. The results indicates that the sol–gel method has significant reduction in five environmental impacts indexes, including AD, POCP, AP, FETP and HTP.
Conclusions
In this paper, the material consumption and energy input lists of three LIBs used in BEVs are collected and sorted out. The life cycle resource consumption index and environmental impact index of LFP, NCM622 and NCM811 batteries are calculated by using the SimaPro software and CML-IA method. The main conclusions are summarized as follows:
-
1
The environmental impacts of three types of LIBs are mainly generated in the production and assembly stage, followed by the use stage. The production phase and assembly phase of LIBs are the main sources of GHG emission, the GHG emissions of NCM622 battery is 156.73 kg CO2-eq/kWh, which accounts for 35.40% of the total GHG emissions. The transportation stage has the least environmental impacts, and is the main contributor to METP, GWP and ADF. The environmental damage in the whole life cycle of three batteries is mainly reflected in the following five aspects: METP, GWP, HTP, ADF and FETP. It is found that hydrometallurgy recycling of LFP batteries can effectively relieve the environmental pressure. NCM622 and NCM811 batteries have obvious environmental benefits on METP, HTP, GWP and ADF, but at the same time increase the environmental impacts of HTP, FETP and METP.
-
2
The environmental impacts of LFP batteries under different power combinations over the next 30 years is studied. It is found that increasing the proportion of clean energy use in production Stage could effectively ease the environmental burden, especially METP and GWP. TETP are the lowest change rates among the 11 indexes, which is mainly related to the way of energy development and utilization. Giving priority to the use of wind power has positive effect on China’s goal of carbon neutrality. Sensitivity analysis shows that the precursor of NCM battery is the key factor to produce environmental impacts. In this study, the sol–gel method is used instead of hydrothermal method to prepare cathode materials, which could effectively improve the environmental impacts.
-
3
Given that China’s electricity mix is still dominated by coal, LFP batteries are more environmentally friendly for BEVs. This study provides a reference for understanding the environmental risks of LIBs for BEVs, and provides a path reference for realizing the goal of carbon neutrality of LIBs in China: (a) Using green electricity and increasing the proportion of clean energy; (b) Optimizing the production process of LIBs, especially the production of cathode materials; (c) Strengthening the technical management of recycling phase, which can effectively reduce resource and energy consumption.
NCM batteries rely on precious metals such as nickel, cobalt and manganese, have achieved high energy density but pose significant environmental risks. On the other hand, LFP batteries are more eco-friendly but still have lower energy density compared to ternary lithium batteries. In future, priority should be given to green electricity and optimizing production processes to alleviate the environmental impacts of LIBs.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AD:
-
Abiotic depletion
- ADF:
-
Abiotic depletion (fossil fuels)
- AP:
-
Acidification potential
- BEVs:
-
Battery electric vehicles
- CoSO4 :
-
Cobalt sulfate
- EC:
-
Ethylene carbonate
- EP:
-
Eutrophication potential
- FETP:
-
Freshwater ecotoxicity potential
- FEP:
-
Freshwater eutrophication potential
- FU:
-
Functional unit
- GHG:
-
Greenhouse gas
- GWP:
-
Global warming potential
- H3PO4 :
-
Phosphoric acid
- HTP:
-
Human toxicity potential
- LCA:
-
Life cycle assessment
- LCI:
-
Life cycle inventory
- LCIA:
-
Life cycle impact assessment
- LFP:
-
Lithium iron phosphate
- LIB:
-
Lithium-ion battery
- Li2CO3 :
-
Lithium carbonate
- LiOH:
-
Lithium hydroxide
- LiPF6 :
-
Lithium hexafluorophosphate
- LMO:
-
Lithium manganese oxide
- METP:
-
Marine ecotoxicity potential
- NaOH:
-
Sodium hydroxide
- NCM:
-
Lithium nickel cobalt manganese oxide
- NiSO4 :
-
Nickel sulfate
- NMP:
-
N-Methyl-2-pyrrolidone
- ODP:
-
Ozone layer depletion
- POCP:
-
Photochemical ozone creation potential
- PVDF:
-
Polyvinylidene fluoride
- TETP:
-
Terrestrial ecotoxicity potential
References
Ahmadi L, Young SB, Fowler M, Fraser RA, Achachlouei MA (2015) A cascaded life cycle: reuse of electric vehicle lithium-ion battery packs in energy storage systems. Int J Life Cycle Assess 22(1):111–124. https://doi.org/10.1007/s11367-015-0959-7
Arshad F, Lin J, Manurkar N, Fan E, Ahmad A, M-u-N WuF, Tariq R, Li CL (2022) Life cycle assessment of lithium-ion batteries: a critical review. Resour Conserv Recycl. https://doi.org/10.1016/j.resconrec.2022.106164
Asef P, Milan M, Lapthorn A, Padmanaban S (2021) Future trends and aging analysis of battery energy storage systems for electric vehicles. Sustainability. https://doi.org/10.3390/su132413779
Aurisano N, Albizzati PF, Hauschild M, and Fantke P (2019) Extrapolation factors for characterizing freshwater ecotoxicity effects. Environ Toxicol Chem 38(11): 2568–2582. https://doi.org/10.1002/etc.4564
Bobba S, Mathieux F, Ardente F, Blengini GA, Cusenza MA, Podias A, Pfrang A (2018) Life Cycle Assessment of repurposed electric vehicle batteries: an adapted method based on modelling energy flows. J Energy Storage 19:213–225. https://doi.org/10.1016/j.est.2018.07.008
CEC (2021) Annual report on development of China power industry 2021. From https://www.cec.org.cn/menu/index.html?688. Accessed Jan 2022
Chakraborty S, Banerjee A, Watcharatharapong T, Araujo RB, Ahuja R (2018) Current computational trends in polyanionic cathode materials for Li and Na batteries. J Phys-Condens Matter. https://doi.org/10.1088/1361-648X/aac62d
Chen Q, Lai X, Gu H, Tang X, Gao F, Han X, Zheng Y (2022) Investigating carbon footprint and carbon reduction potential using a cradle-to-cradle LCA approach on lithium-ion batteries for electric vehicles in China. J Clean Prod. https://doi.org/10.1016/j.jclepro.2022.133342
Dai Q, Kelly JC, Gaines L, Wang M (2019) Life cycle analysis of lithium-ion batteries for automotive applications. Batteries. https://doi.org/10.3390/batteries5020048
Daniele Landi, MM, Pietronib G (2022) Comparative life cycle assessment of two different battery technologies: lithium iron phosphate and sodium-sulfur. Procedia CIRP. https://doi.org/10.1016/j.procir.2022.02.080
Deng Y, Ma L, Li T, Li J, Yuan C (2019) Life cycle assessment of silicon-nanotube-based lithium ion battery for electric vehicles. Acs Sustain Chem Eng 7(1):599–610. https://doi.org/10.1021/acssuschemeng.8b04136
Diaz-Ramirez MC, Ferreira VJ, Garcia-Armingol T, Lopez-Sabiron AM, Ferreira G (2020) Battery manufacturing resource assessment to minimise component production environmental impacts. Sustainability. https://doi.org/10.3390/su12176840
Dong H, Fu J, Zhao Z, Liu Q, Li Y, Liu J (2020) A comparative study on the energy flow of a conventional gasoline-powered vehicle and a new dual clutch parallel-series plug-in hybrid electric vehicle under NEDC. Energy Convers Manag. https://doi.org/10.1016/j.enconman.2020.113019
Du J, Ouyang M, Chen J (2017) Prospects for Chinese electric vehicle technologies in 2016–2020: ambition and rationality. Energy 120:584–596. https://doi.org/10.1016/j.energy.2016.11.114
Du S, Gao F, Nie Z, Liu Y, Sun B, Gong X (2022a) Comparison of electric vehicle lithium-ion battery recycling allocation methods. Environ Sci Technol 56(24):17977–17987. https://doi.org/10.1021/acs.est.2c05755
Du S, Gao F, Nie Z, Liu Y, Sun B, Gong X (2022b) Life cycle assessment of recycled NiCoMn ternary cathode materials prepared by hydrometallurgical technology for power batteries in China. J Clean Prod. https://doi.org/10.1016/j.jclepro.2022.130798
Haustein S, Jensen AF (2018) Factors of electric vehicle adoption: a comparison of conventional and electric car users based on an extended theory of planned behavior. Int J Sustain Transp 12(7):484–496. https://doi.org/10.1080/15568318.2017.1398790
Hua Y, Zhou S, Huang Y, Liu X, Ling H, Zhou X, Zhang C, Yang S (2020) Sustainable value chain of retired lithium-ion batteries for electric vehicles. J Power Sour. https://doi.org/10.1016/j.jpowsour.2020.228753
Jackson M, Eadsforth C, Schowanek D, Delfosse T, Riddle A, Budgen N (2016) Comprehensive review of several surfactants in marine environments: fate and ecotoxicity. Environ Toxicol Chem 35(5):1077–1086. https://doi.org/10.1002/etc.3297
Kapustin NO, Grushevenko DA (2020) Long-term electric vehicles outlook and their potential impact on electric grid. Energy Policy. https://doi.org/10.1016/j.enpol.2019.111103
Kelly JC, Dai Q, Wang M (2020) Globally regional life cycle analysis of automotive lithium-ion nickel manganese cobalt batteries (vol 13, pg 514, 2020). Mitig Adapt Strat Glob Change 25(3):495–495. https://doi.org/10.1007/s11027-020-09913-6
Koroma MS, Costa D, Cardellini G, Messagie M, Ieee (2021) Life Cycle Assessment of Lithium-ion Battery Pack: Implications of Second-life and Changes in Charging Electricity. In: 18th IEEE vehicle power and propulsion conference (IEEE VPPC), Electr Network
Lai X, Chen Q, Tang X, Zhou Y, Gao F, Guo Y, Bhagat R, Zheng Y (2022a) Critical review of life cycle assessment of lithium-ion batteries for electric vehicles: a lifespan perspective. eTransportation. https://doi.org/10.1016/j.etran.2022.100169
Lai X, Gu H, Chen Q, Tang X, Zhou Y, Gao F, Han X, Guo Y, Bhagat R, Zheng Y (2022b) Investigating greenhouse gas emissions and environmental impacts from the production of lithium-ion batteries in China. J Clean Prod. https://doi.org/10.1016/j.jclepro.2022.133756
Liu W, Liu H, Liu W, Cui Z (2021) Life cycle assessment of power batteries used in electric bicycles in China. Renew Sustain Energy Rev. https://doi.org/10.1016/j.rser.2020.110596
Majeau-Bettez G, Hawkins TR, Stromman AH (2011) Life cycle environmental assessment of lithium-ion and nickel metal hydride batteries for plug-in hybrid and battery electric vehicles. Environ Sci Technol 45(10):4548–4554. https://doi.org/10.1021/es103607c
Manh-Kien T, Mevawala A, Panchal S, Raahemifar K, Fowler M, Fraser R (2020) Effect of integrating the hysteresis component to the equivalent circuit model of lithium-ion battery for dynamic and non-dynamic applications. J Energy Storage. https://doi.org/10.1016/j.est.2020.101785
Nordelof A, Messagie M, Tillman A-M, Soderman ML, Van Mierlo J (2016) Environmental impacts of hybrid, plug-in hybrid, and battery electric vehicles-what can we learn from life cycle assessment? (vol 19, pg 1866, 2014). Int J Life Cycle Assess 21(1):134–135. https://doi.org/10.1007/s11367-015-1004-6
Oliveira L, Messagie M, Rangaraju S, Sanfelix J, Rivas MH, Van Mierlo J (2015) Key issues of lithium-ion batteries - from resource depletion to environmental performance indicators. J Clean Prod 108:354–362. https://doi.org/10.1016/j.jclepro.2015.06.021
Pellow MA, Ambrose H, Mulvaney D, Betita R, Shaw S (2020) Research gaps in environmental life cycle assessments of lithium ion batteries for grid-scale stationary energy storage systems: end-of-life options and other issues. Sustain Mater Technol. https://doi.org/10.1016/j.susmat.2019.e00120
Picatoste A, Justel D, Mendoza JMF (2022) Circularity and life cycle environmental impact assessment of batteries for electric vehicles: industrial challenges, best practices and research guidelines. Renew Sustain Energy Rev. https://doi.org/10.1016/j.rser.2022.112941
Qiao Q, Zhao F, Liu Z, Jiang S, Hao H (2017) Cradle-to-gate greenhouse gas emissions of battery electric and internal combustion engine vehicles in China. Appl Energy 204:1399–1411. https://doi.org/10.1016/j.apenergy.2017.05.041
Raugei M, Winfield P (2019) Prospective LCA of the production and EoL recycling of a novel type of Li-ion battery for electric vehicles. J Clean Prod 213:926–932. https://doi.org/10.1016/j.jclepro.2018.12.237
Shu X, Guo Y, Yang W, Wei K, Zhu G (2021) Life-cycle assessment of the environmental impact of the batteries used in pure electric passenger cars. Energy Rep 7:2302–2315. https://doi.org/10.1016/j.egyr.2021.04.038
Silvestri L, Forcina A, Arcese G, Bella G (2020) Recycling technologies of nickel–metal hydride batteries: an LCA based analysis. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.123083
Smith C, Hill AK, Torrente-Murciano L (2020) Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ Sci 13(2):331–344. https://doi.org/10.1039/c9ee02873k
Smith L, Ibn-Mohammed T, Astudillo D, Brown S, Reaney IM, Koh SCL (2021) The role of cycle life on the environmental impact of Li6.4La3Zr1.4Ta0.6O12 based solid-state batteries. Adv Sustain Syst. https://doi.org/10.1002/adsu.202000241
Soge AO, Willoughby AA, Dairo OF, Onatoyinbo OO (2021) Cathode materials for lithium-ion batteries: a brief review. J New Mater Electrochem Syst 24(4):229–246
Suh S, Leighton M, Tomar S, Chen C (2016) Interoperability between ecoinvent ver. 3 and US LCI database: a case study. Int J Life Cycle Assess 21(9):1290–1298. https://doi.org/10.1007/s11367-013-0592-2
Sun X, Luo X, Zhang Z, Meng F, Yang J (2020) Life cycle assessment of lithium nickel cobalt manganese oxide (NCM) batteries for electric passenger vehicles. J Clean Prod 273:123006. https://doi.org/10.1016/j.jclepro.2020.123006
Sun X, Zheng JH, Zhang P, Zhao MN, Wu HX, Yan YT (2017) Comparative life cycle assessment of Chinese radial passenger vehicle tire. Mater Sci Forum 898:2432–2445. https://doi.org/10.4028/www.scientific.net/MSF.898.2432
Tian H, Qin P, Li K, Zhao Z (2020) A review of the state of health for lithium-ion batteries: research status and suggestions. J Clean Prod 261:120813. https://doi.org/10.1016/j.jclepro.2020.120813
Tourlomousis F, Chang RC (2017) Dimensional metrology of cell-matrix interactions in 3D microscale fibrous substrates. Procedia CIRP 65:32–37. https://doi.org/10.1016/j.procir.2017.04.009
Wang F, Deng Y, Yuan C (2020) Life cycle assessment of lithium oxygen battery for electric vehicles. J Clean Prod 264:121339. https://doi.org/10.1016/j.jclepro.2020.121339
Wang L, Wu H, Hu Y, Yu Y, Huang K (2019a) Environmental sustainability assessment of typical cathode materials of lithium-ion battery based on three LCA approaches. Processes 7(2):83. https://doi.org/10.3390/pr7020083
Wang Y, Yu Y, Huang K, Tang B (2019b) From the perspective of battery production: energy-environment-economy (3e) analysis of lithium-ion batteries in China. Sustainability 11(24):6941. https://doi.org/10.3390/su11246941
Wernet G, Bauer C, Steubing B, Reinhard J, Moreno-Ruiz E, Weidema B (2016) The ecoinvent database version 3 (part I): overview and methodology. Int J Life Cycle Assess 21(9):1218–1230. https://doi.org/10.1007/s11367-016-1087-8
Wu H, Hu Y, Yu Y, Huang K, Wang L (2021) The environmental footprint of electric vehicle battery packs during the production and use phases with different functional units. Int J Life Cycle Assess 26(1):97–113. https://doi.org/10.1007/s11367-020-01836-3
Yang Gu, Guo and Chen, (2019) Comparative life cycle assessment of mobile power banks with lithium-ion battery and lithium-ion polymer battery. Sustainability 11(19):5148. https://doi.org/10.3390/su11195148
Yang J, Mu D, Li X, I. O. P. Publishing (2020) A system dynamics analysis about the recycling and reuse of new energy vehicle power batteries: an insight of closed-loop supply chain. In: 6th International conference on energy materials and environment engineering (ICEMEE), Electr Network
Yang X-G, Liu T, Wang C-Y (2021) Thermally modulated lithium iron phosphate batteries for mass-market electric vehicles. Nat Energy 6(2):176–185. https://doi.org/10.1038/s41560-020-00757-7
Yang Y, Lan L, Hao Z, Zhao J, Luo G, Fu P, Chen Y (2022) Life cycle prediction assessment of battery electrical vehicles with special focus on different lithium-ion power batteries in China. Energies 15(15):5321. https://doi.org/10.3390/en15155321
Yao Y, Zhu M, Zhao Z, Tong B, Fan Y, Hua Z (2018) Hydrometallurgical processes for recycling spent lithium-ion batteries: a critical review. Acs Sustain Chem Eng 6(11):13611–13627. https://doi.org/10.1021/acssuschemeng.8b03545
Yuan C, Cao H, Shen K, Deng Y, Zeng D, Dong Y, Hauschild M (2021) Water-based manufacturing of lithium ion battery for life cycle impact mitigation. CIRP Ann Manuf Technol 70(1):25–28. https://doi.org/10.1016/j.cirp.2021.04.038
Zackrisson M, Avellán L, Orlenius J (2010) Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles—critical issues. J Clean Prod 18(15):1519–1529. https://doi.org/10.1016/j.jclepro.2010.06.004
Zackrisson M, Fransson K, Hildenbrand J, Lampic G, O’Dwyer C (2016) Life cycle assessment of lithium-air battery cells. J Clean Prod 135:299–311. https://doi.org/10.1016/j.jclepro.2016.06.104
Zhao S, You F (2019) Comparative life-cycle assessment of Li-ion batteries through process-based and integrated hybrid approaches. ACS Sustain Chem Eng 7(5):5082–5094. https://doi.org/10.1021/acssuschemeng.8b05902
Acknowledgements
This work is supported by Major Science and Technology Program for Development and Demonstration on Key Technologies for Urban Advanced Sewage Treatment (ZJWM-KJYF-2021-A-01) from CCCC Sinobioway Environmental Protection Co., Ltd., Jinan China.
Funding
Solid Waste’s Special Project for Soil Pollution Prevention and Control (GXZC2023-G3-000790-GSZX) from Central Government of the People’s Republic of China.
Author information
Authors and Affiliations
Contributions
TJ: investigation, resources, data analysis, writing and review; HW: Data collection, data analysis, software, writing-review, editing and visualization; QJ: supervision, conceptualization and methodology. All authors of this paper have directly participated in this study’s writing, editing, planning, execution, and analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
We affim that all authors have agreed on the submission of the paper to Clean Technologies and Environmental Policy and are fully aware of ethical responsibilities.
Consent for publication
We affirm that all authors have agreed on the submission of the paper to Clean Technologies and Environmental Policy and are fully aware of ethical responsibilities.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Tables 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 and 18.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, T., Wang, H. & Jin, Q. Comparison of three typical lithium-ion batteries for pure electric vehicles from the perspective of life cycle assessment. Clean Techn Environ Policy 26, 331–350 (2024). https://doi.org/10.1007/s10098-023-02629-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-023-02629-6