Abstract
The wastewaters produced from dyeing process in textile industries contain xenobiotic mixed reactive dyes that have negative impact on ecosystem when discharged as untreated/partially treated. The present study aims to degrade mixed dyes (reactive red 21 (RR21) and reactive orange 16 (RO16)) present in wastewater by Pseudomonas aeruginosa 23N1. The process optimization and the effect of experimental parameters (like amount of co-substrate, pH and temperature) on process performance are investigated through response surface methodology. To understand the extent of degradation of dye compounds, metabolites extracted from treated water samples were investigated using UV–visible, FTIR and GC-MS analyses. The results reveal that the bacteria could significantly reduce ADMI value of wastewater by ~ 87% against the initial mixed dyes containing aqueous solution (50 mg/L each of RR21 and RO16 dyes). The analysis of extracted metabolites from treated water sample indicates the utilization of dye compounds as nutrient by bacteria. The bacteria might exhibit satisfactory ADMI reduction in the presence of initial ≤ 100 mg/L Cr(VI) concentration. The bio-degradation is performed under microbial fuel cell in the presence of co-substrate yeast extract and found very much promising in terms of faster ADMI reduction and energy production. The maximum output voltage generation of 790 ± 5 mV and power density 940.61 ± 5 mW/m2 are recorded during decolourization of mixed dye-laden real wastewater in a microbial fuel cell. The bacteria studied here confirm the effective bio-decolourization of real textile wastewater.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The colourants are being used since prehistoric time for artistic, fibre additive, communicative and esthetical purposes (Paz et al. 2017). Earlier, colourants were extracted from flowering plants, alga and insects, which are considered as renewable, sustainable eco-friendly sources (Shahid et al. 2013). However, the ever increased demand of colourants has led to excessive exploitation of natural resources. Moreover, the biological colourants have certain drawbacks like less stable, low brightness and insufficient fixation capacity with fibres, which could be accomplished by the use of synthetic dyes (Chaudhari et al. 2017). The synthetic dyestuffs belong to major complex organic groups like azo, triphenylmethane and anthraquinone, which differ in physical and chemical properties related to their applications (Mishra and Maiti 2018a). Among the colourants, reactive azo dyes are widely used in textile dyeing process due to their better stability and fixation property with fibres (Maqbool and Abid 2016). Considerably, more than 15% of these reactive azo dye molecules could not fix with fibres and lost through effluents generated from industrial dying processes (Shafqat et al. 2017; Hameed and Ismail 2018). These dyes are carcinogenic and mutagenic, which have several detrimental effects on the surrounding ecosystem and human health on its exposure (Roy et al. 2018). Along with dyestuffs, the textile effluent contains high concentration of salt (sodium chloride ~ 4 g/L) and chromate (Cr(VI)) ions that are used to improvise the binding of dye molecules with fibres (Liu et al. 2017; Yaseen and Scholz 2019). Therefore, effective wastewater treatment technologies need to be investigated for detoxification and decolourization of such effluents loaded with dyes, salt and heavy metal ions. The conventional treatment processes are found to be insignificant for decolourization of synthetic reactive dyes, because these complex compounds might easily pass through this system and remain unaffected or partially altered (Keskin et al. 2015). Currently, the biological process involving with biotic organisms like bacteria, fungi and plants is used to degrade or detoxify the different dye compounds from wastewater (Huang et al. 2015; Hussain et al. 2017). Although this process is eco-friendly, inexpensive and efficient to treat textile wastewater, but fungi and plants show certain drawbacks as those require long period for satisfactory growth and bio-degradation of dyes (Chakraborty et al. 2018). Comparatively, the bacterial species could colonize and degrade wide classes of dyestuffs within short period of time and have better adaptability in adverse environments (Ma et al. 2014). The bacterial cells might utilize dye compounds as only nutritional source (Mishra and Maiti 2019a). The metabolic degradation of reactive azo dyes by bacterial species is followed through mineralization of dye molecules that reduces the chance of production of toxic secondary amines (Naseer et al. 2016). The bacterial enzymes like azoreductase, laccase and peroxidase are commonly involved in azo dye degradation (Telke et al. 2015; Mishra and Maiti 2019b, c). In most of the reported studies, azoreductase has been found as chief enzyme involved in cleavage of azo bonds (N = N) (Chacko and Subramaniam 2011). Moreover, the satisfactory activity of bacteria could be achieved under the optimal process condition. Therefore, the process parameters like temperature, concentration of nutrients and dissolve oxygen content should be optimized to enhance the growth and performance of bacterial cells for dye degradation (Garg et al. 2015). In this regard, response surface methodology (RSM) is commonly used to explore the integrative effects of process parameters on the interested process response.
Among all bacterial species investigated for azo dye degradation, Pseudomonas sp. has been found as more efficient bio-remediation agent (Bedekar et al. 2014; Mishra and Maiti 2018b). In recent trend, the industries demand of hybrid wastewater treatment and energy generation from the same treatment process might reduce the cost of treatment and provide energy for various industrial operations (Ilamathi and Jayapriya 2018). In this regard, the microbial fuel cells (MFCs) are being investigated for hybrid wastewater treatment with energy recovery (Lovley 2006; Miran et al. 2016). The microorganism promotes the oxidative conversion of chemical energy into electrical energy. The bio-conversion of organic compounds to bio-energy could vary due to physicochemical composition, concentration of the organic/inorganic compounds and source of wastewater (Mohan et al. 2010).
In this study, P. aeruginosa 23N1 is investigated to decolourize the wastewater containing mixed azo reactive orange 16 (RO16) and reactive red 21 (RR21) dyes. Meanwhile, these toxic reactive azo dyes are generally used for colouring the fabrics in textile industries. Previously, optimization of process parameters for effective decolourization of individual RR21 (Mishra and Maiti 2018c), and RO16 (Mishra and Maiti 2018d) dye has been reported from our laboratory. In this study, the optimization of process condition to decolourize the wastewater containing mixed RR21 and RO16 dyes is carried out using RSM-based central composite design (CCD) method. Progressing further, it is the first kind of the study that reports the degradation mechanism of individual and mixed reactive RR21 and RO16 dyes by strain 23N1, which is elaborately discussed on the basis of analysis of end metabolites through FTIR and GC-MS techniques. Furthermore, concentration of total dissolved carbon (TOC) of untreated and treated wastewaters containing dye molecules is estimated to verify the extent of utilization of dye as carbon source by bacteria. The effect of Cr(VI) on performance of remediation system is investigated to ascertain a good response of bacteria even in the presence of toxic heavy metal ions. The two-chambered microbial fuel cell (MFC) is examined to generate the energy during mixed dye-laden wastewater treatment process, which could be a useful application for reduction in overall operating cost of water treatment. Thus, bio-treatment of textile wastewater as well as production of electrical energy could be a promising approach for wastewater treatment. Various co-substrates have been used to promote decolourization of dye solutions, but role of co-substrates in the process bio-decolourization coupled with microbial fuel cell is not explored in the literature. In this study, the role of co-substrate in power generation from MFCs has been explored. Further, few studies might report the bio-decolourization using wastewater containing mixed dyes and heavy metal ions. The toxicity analysis of the end products of bio-treatment is performed by estimating the germination rate of Vigna radiata seeds, to assess the toxicity of treated water. The capability of bacteria to decolourize real textile wastewater is assessed to ensure its feasibility to treat industrial effluents.
Materials and methods
Dyes and chemicals
The reactive azo dye chemicals used in this study, namely reactive orange 16 (RO16, CAS number 12225-83-1), reactive red 21 (RR21, CAS number 11099-79-9) and 99% K2Cr2O7, were provided by Sisco Research Laboratories Pvt. Ltd., Delhi, India. The other chemicals (such as NaCl and yeast extract) were supplied from Himedia Laboratories, Mumbai, India. The individual dye stock solution of 1000 mg/L was prepared by dissolving 1 g of dye powder in 1000 mL double distilled water (DDW), while the stock solution of Cr(VI) was prepared by dissolving 2.835 g of K2Cr2O7 in 1 L DDW. The mixed dyes/Cr(VI) solution for respective experimental steps was prepared by mixing and diluting the required volume of each dye stock solution in 1000 mL DDW, for example: 50 mL of RR21 dye stock solution is mixed with the same volume of RO16 dye stock solution and diluted to 1000 mL DDW, assuming individual dye concentration would be obtained as 50 mg/L each. The initial pH of experimental dye solutions was maintained by using 1 N solutions of NaOH and HCl.
Bacterial culture conditions
The pure culture of bacteria P. aeruginosa 23N1 is supplied by National Centre for Cell Sciences (NCCS), Pune, India. The bacterial cells were cultured in HK34b nutrient agar medium. The bacterial inoculums for experimental study were routinely cultured in Erlenmeyer flask (volume 100 mL) supplemented with 50 mL nutrient broth (yeast extract 0.35% (w/v) and peptone 2% (w/v)) and incubated at 30 °C for 24 h under orbital shaking at 150 rpm (Mishra and Maiti 2018c). This bacterial inoculation volume is used in % (v/v) for subsequent experiments. The experiments for optimization of dye decolourization process condition were carried out in 100-mL Erlenmeyer flask and incubated for 48 h under static-isothermal condition.
Instrumental analysis and data evaluation
It should be noted that there is no standard analytical technique available to estimate the individual dye concentration from mixed dyes solution. In this regard, American dyes manufactures institutes (ADMI) index value of mixed dyes solution is evaluated, which is based on UV–visible transmittance data of coloured solution. The samples were collected from cultured experimental flask and then centrifuged at ~ 10080 RCF for 20 min to separate the bacterial biomass from supernatant. The supernatant solution was analysed through UV–visible spectroscopy to obtain the transmittance of residual dye solution. The change in ADMI value (in %) to treated dye aqueous solution compared to untreated dye solution signifies the extent of decolourization, which can be calculated by using the following formula:
where ADMIin represents the initial ADMI value of untreated dyes solution and ADMIf denotes the final ADMI value of treated dyes solution. The diphenylcarbazide method (given as per APHA 2011) is followed to analyse the concentration of Cr(VI) at 540 nm (λmax) in the experimental samples using UV–visible spectroscopy. TOC concentration of the experimental dye solutions was analysed using TOC analyser (model TOC-L, Shimadzu). To confirm the degradation of dye molecules, the experimental solutions were analysed using FTIR and GC-MS techniques. In this regard, supernatant solution was mixed in equivalent volume of ethyl acetate to extract the metabolites. This extract was separated and dried over anhydrous Na2SO4. The ethyl acetate was removed to obtain powder metabolites using rotary evaporator at 72 °C. Thereafter, the extracted metabolites powder was dissolved in methanol (HPLC grade) for further analysis. These prepared solutions were analysed using FTIR and GC-MS.
Toxicity analysis of metabolites
The dye-contaminated untreated and treated water samples were used to germinate the seeds of V. radiata, under laboratory condition. The healthy seeds were collected from local market, which were primarily washed with DDW before use. These seeds were laid on a filter paper dipped in 4 mL of the corresponding water samples, in petri plates (size 100 × 15 mm). The seeds containing petri plates were kept in the dark at temperature 24 ± 1 °C for 120 h, to promote the germination of seeds. In addition, an equal number of seeds were grown in the control experiment (only DDW) to compare the analysis. The phytotoxicity of treated water sample was estimated in relation to normal seed germination rate using the methodology as reported by Kurade et al. (2016) and Priac et al. (2017).
Statistical analysis
The optimization of process condition for mixed dyes decolourization was executed based on experimental matrix obtained through RSM-based CCD method using Design-Expert software (version 7.1.6, Stat-Ease Inc., Minneapolis, USA). Small type CCD with 5 centre points, one axial point, one factorial point, along with block value of 1, and alpha value of 2 were selected in this study. For optimization of process condition, the analysis of variance (ANOVA) was performed for experimental decolourization response data of experimental trials. The numeric parameters were varied over five levels as: alpha − 2 and + 2 (axial point), one centre point, and + 1 and − 1 (factorial points), as shown in Table 1.
Bio-energy generation
The generation of bio-energy during decolourization of mixed reactive dye-laden wastewater by strain 23N1 is carried out in “H”-shaped MFC chamber. This MFC was designed using 0.5-cm-thick Plexiglas material. Two cylindrical chambers of equal working volume of 900 mL with dimension (15 cm height × 10 cm diameter) were used in MFC, as shown in Fig. S6. Two graphite rods of dimension (17 cm length × 1.2 cm diameter) were used electrodes in MFC. The proton exchange membrane “Nafion-117 from DuPont” was used in this study. The membrane was pre-treated in solution of H2O2 (3%) for 1 h at 80 °C and then in solution of 0.5 M H2SO4 for 1 h at 80 °C. Open-circuit voltage was monitored using digital multimeter (Mashtech India Pvt. Ltd.), which was connected to 100 Ω external resistances (R) in series. Power density was calculated using the following equation:
where V signifies the voltage (mV); A signifies the total surface area of the electrode (m2); and R signifies the resistance. The real textile wastewater used in this study was collected from textile industry located in the district Saharanpur of Uttar Pradesh, India. The schematic diagram of overall experimentation method adopted to carry out this study is shown in Fig. 1.
Results and discussion
In the reported literature, the decolourization of individual RR21 (Mishra and Maiti 2018c) and RO16 (Mishra and Maiti 2018d) dyes by strain 23N1 has been found to be effective under static-isothermal condition. However, these dyes are found in mixed state in the wastewater generated from dyeing industries. Considering these reported findings, the decolourization of mixed (RR21 and RO16) dyes and optimization of process condition are carried out under static-isothermal condition.
Optimization of dye decolourization process condition
To investigate the ADMI reduction of mixed dyes containing aqueous solutions and to study the effect of process parameters on the performance of bio-decolourization, the experimental scheme is produced through CCD, which exhibits total 41 experimental trials, as shown in Table 2. The lower and higher limits of process parameters are defined as per the result obtained during the preliminary study (reported in Mishra and Maiti 2018c, d). The ADMI reduction percentages for all experimental trials are obtained in the range 3.5% (lowest) to 81.1% (highest). These response data are used in analysis of variance (ANOVA) to assess the reliability of derived quadratic model equation to predict the ADMI reduction percentage of wastewater containing mixed dyes under defined process condition. The experimental data are found to be strongly correlated with model-predicted values of respective experimental trial, with correlation coefficient (R2) value of 0.998 that ensures the well fitness of data for practical use. The ANOVA analysis of experimental data is shown in Table 3, which signifies that the response model is statistically significant with F value and p value of 59.08 and 0.0001, respectively. The adjusted R2 value, predicted R2 and adequacy precision value of model are obtained as 0.981, 0.953 and 25.04, respectively, which indicate better accuracy of model for prediction of ADMI reduction percentage of mixed reactive dyes solution.
The major influential model terms are found as A, C, E, F, AE, AC, AB, AG, BE, BG, CE, FG, A2, B2, F2, D2, E2 and G2. The quadratic model equation produced through multiple regression analysis of experimental response data is as follows:
The diagnostic plots give better understanding of the feasibility of model and are shown in Fig. S1 of supplementary information (SI). Moreover, the insignificant model terms from the above equation are ignored through backward elimination regression method and alpha out value 0.1. The resultant model equation is obtained as follows:
During the regression analysis, the coefficient of factor F is obtained as 17.23, which is highest among all individual process parameters, shown in Table S1 of SI. The insignificant factors are removed through backward elimination regression method using alpha value of 0.1 to exit, and the modified model equation is obtained as:
Perturbation plot shows the steeper increment in the slope of factor F, as shown in Fig. S1f of SI, which reveals that the amount of yeast extract has major influence on ADMI reduction of mixed reactive azo dyes solution. However, the decrement in perturbation slope and negative coefficient values of factor C (− 12.12) and D (− 1.61) reveal that the increase in dye concentration significantly reduces the response. This might be due to the suppressed growth and activity of bacteria at higher toxicity level of medium. The pH (factor A) and salinity (factor E) of the medium have shown positive effect on the response (coefficient values of 5.86 and 6.35, respectively), while among the interactive terms, factor AE has shown the highest positive coefficient of 22.41.
The interactive effect of model terms could be better analysed through contour plots constructed through ANOVA analysis as shown in Fig. 2. From Fig. 2a, it is revealed that the strain 23N1 is salt tolerant and exhibits higher dye decolourization even in alkaline medium, which has proved here as a beneficial characteristic (capability to treat alkaline textile wastewater) of biotic agent. The significant elliptical plot of interaction between salt and temperature is shown in Fig. 2b, which signifies that these bacteria express its capability to reduce ADMI value under salty environment, when incubation temperature of medium is maintained around 30 °C (suitable temperature for microbial growth and activity).
Similar interactive trend is obtained between pH and temperature, as shown in Fig. 2c. These illustrations are reinforced by interactive effect of inoculation volume with pH and temperature on ADMI reduction percentage as shown in Fig. 2d, e, respectively. It could be observed from Fig. 2d, e that the ADMI reduction percentage increases with decrease in inoculation volume at alkaline pH and at the temperature range of 30–35 °C. This signifies that the small inoculation volume (~ 3%) could be sufficient to achieve higher ADMI reduction in alkaline culture medium, when incubated at the temperature range of 30–35 °C. The co-substrate in the culture medium enhances the bacterial growth and its activity (Imran et al. 2016). The requirement of lower amount of co-substrate reduces the operational cost of treatment. The interaction behaviour of inoculation volume and yeast extract is represented in Fig. 2f, which reveals that ADMI reduction percentage increases with increment in amount of yeast extract, which is supplied as co-substrate in dye decolourization medium. In a culture condition, the low ADMI reduction response of system with large inoculation volume might be due to the achievement of early metabolic and growth saturation stage by bacteria without consuming dye molecules as food supplement.
The suitability of quadratic model equation for prediction of ADMI reduction percentage of mixed RR21 and RO16 dyes solution in a given condition is confirmed through the confirmatory experiments. A total of five confirmatory experiments are investigated to compare with the corresponding model-predicted response values, which are enlisted in Table 4. The actual experimental ADMI response data are found in strong correlation with the corresponding model-predicted values.
Degradation of reactive azo dyes
The mechanism of dye decolourization is investigated through the analysis of metabolites extracted from treated dye water samples and compared with the corresponding raw solutions. Initially, the decolourization of dye is confirmed through analysis of change in UV–visible absorbance spectra between treated and untreated dye solutions used to validate derived quadratic model for RR21 decolourization (confirmatory experiments 1, reported by Mishra and Maiti 2018c) and RO16 dye decolourization (confirmatory experiments 2, reported by Mishra and Maiti 2018d) as shown in Fig. 3a, b, respectively. It could be clearly observed that the absorbance peak at maximum wavelength of RR21 (λmax at 537 nm) and RO16 dye (λmax at 493 nm) obtained during analysis of untreated dye solution is completely diminished for treated solution and no other absorbance peak appeared. Evidence of mixed dyes degradation could be confirmed with the change in UV–visible transmittance spectra between treated and untreated dye solutions as represented in Fig. 3c. These results indicate that the dye decolourization could be either due to bio-transformation/degradation or bio-accumulation of dye by bacteria. Although it is well known that during the bio-accumulation of dye molecules by bacteria, coloured biomass cells appear at the end (Holkar et al. 2018). However, it is observed in this study that the bacterial cells do not exhibit any change in morphological colour as compared to biomass cells in control experiments (without dye). Hence, the metabolite present in supernatant of treated water is investigated through FTIR and GC-MS techniques, to assess the extent of degradation of initial dye molecules. The FTIR transmittance spectra of RR21, RO16 and mixed dyes solution are shown in Fig. 3d, e, f, respectively, which shows similar peaks in all six samples. The major transmittance peaks in FTIR spectra of untreated sample are found as: 2950–2750 cm−1 (C–H stretch), 3500–3200 cm−1 (N–H stretch), 1600–1700 cm−1 (aromatic C = C stretch and C = N stretch), 1450–1200 cm−1 (phenol C–O stretch), 2160 cm−1 (C–S or S–O stretch), 750–850 cm−1 (C–Cl stretch) and 1050–1150 cm−1 (alkoxy C–O stretch and S = O stretch). After treatment, FTIR transmittance peaks of aromatic C = C, C–H stretch, N–H stretch, C = N stretch, C–Cl stretch and S = O stretch are drastically reduced, while the peaks of phenol C–O stretch, S–H stretch and S–O stretch almost disappear. The FTIR analysis of treated mixed dyes aqueous solution after 96 h of incubation period exhibits small transmittance peak of C–O stretch, while other peaks have almost disappeared, shown in Fig. 3f. The disappearance of functional group peaks in treated solution confirms the bio-degradation of dye molecules by strain 23N1. Furthermore, to confirm the bio-degradation, the treated dye solution collected after incubation period of 48 h (used for FTIR analysis) is used for GC-MS analysis. The GC-MS analysis of untreated and treated RR21 dye water samples have shown several transmittance peaks at different retention time as shown in Fig. S2 of SI. To verify the degradation of dye chromophore, GC-MS analyses of yeast extract and salt containing water samples are used for comparative assessment of m/z spectra as shown in Fig. S3 of SI.
Change in absorption and transmittance spectra of untreated and treated dye solutions: a UV–visible absorption spectra of RR21 dye; b UV–visible absorption spectra of RO16 dye; c UV–visible transmittance spectra of mixed dyes; d FTIR spectra of RR21 dye; e FTIR spectra of RO16 dye; f FTIR spectra of mixed dyes
The GC-MS analysis of raw RR21 dye solution exhibits m/z spectra peak at retention time 4.2 min, which corresponds to m/z spectra of un-metabolized RR21 dye molecule. These peaks completely disappear after treatment of RR21 dye solution, and several new peaks of unidentified metabolites appear near by the retention time of raw dye molecule. In addition, unidentified peaks at retention time 5 and 5.8 min are detected for untreated samples, which do not appear after bio-degradation of dye. During the analysis of treated RR21 dye solution, several other peaks at retention time 19.9, 20.8, 23.4, 24.2, 30.7 and 32.6 min are detected, which indicate the formation of new lower molecular weight metabolites. GC-MS analysis of untreated and treated RO16 dye solution exhibits several m/z spectra peaks at different retention time as shown in Fig. S4 of SI. The GC-MS analysis of raw RO16 dye solution has showed peaks at retention time 40 min, which could be the m/z spectra of RO16 dye. These peaks disappear and new peaks appear near by the retention time after treatment. The other peaks of unidentified compositions are also observed at retention time 6.3, 8.0, and 42.3 min, which are not obtained after treatment of dye solution. Interestingly, several new peaks appeared after treatment at retention time 4, 24.7 and 27.2, which could be due to formation of new lower molecular weight metabolite through bio-degradation of RO16 dye. Bouraie and Din (2016) have reported that the microbial decolourization of reactive azo dyes begins with reduction of azo chemical bond or mineralization of dye molecule and then complete degradation of intermediate aromatic amines, when incubated under static-isothermal condition. In this study, the GC-MS analyses of treated azo dye samples reveal that RR21 and RO16 dye compounds have been most likely broken down into unidentified lower molecular weight compounds. The dye chromophores are degraded due to breaking of azo bond and other bonds, which leads to the decolourization of water samples by bacteria. The intermediate metabolite formed after degradation of RR21 dye might be 3-acetoxy-5-acetoxymethyl-4-cyano-2-meth, while the intermediates formed during degradation of RO16 dye might be pentan-2-one, 4-(2-naphthylsulphonyl)-4-M. Bedekar et al. (2014) have investigated the RO16 dye bio-degradation pathway mediated by Lysinibacillus sp. RGS. They have proposed that the RO16 is catabolized into end metabolites of 4-hydroxy-5,8-dihydronaphthalene-2-sulphonate and 2-[(3-aminophenyl) sulphonyl] ethanol by bacteria, which confirmed the detoxification of RO16 dye. Furthermore, the GC-MS analysis of metabolites extracted from the treated mixed dyes solution (confirmatory experiment 5) exhibits m/z spectra at retention time 5.2, 11.6, 13.4, 15.3, 17.2, 20.3, 24.1, 31.3, 33.6, 34.7 and 35.2 min (shown in Fig. S6 of SI), which indicate the conversion of mixed dyes compound into smaller metabolites. Comparative analysis of m/z spectra of untreated and treated mixed dyes samples indicates that disappearance of peaks and reduction in intensity of the existing peaks after degradation of dyes by strain 23N1 as shown in Fig. S4 of SI.
The involvement of azoreductase enzyme in degradation of RR21 and RO16 dyes can be ensured through the analysis of cell-free supernatant solution of treated samples, as per the experimental protocol reported by Telke et al. (2010). The 2 mL reaction mixture containing methyl red (4.45 μM), NADH (100 μM) and potassium phosphate buffer (1.7 mL, 20 mM, pH 7.5) is inoculated by 0.1 mL supernatant solution, followed by incubation at 30 °C for 12 h. The UV–visible absorbance spectral analysis of colour reaction mixture at λmax 430 nm indicates the decrease in absorbance of inoculated sample after 12-h incubation, which confirms the involvement of azoreductase enzyme in degradation of dyes. Furthermore, the TOC concentration of initial untreated mixed dyes water supplemented with yeast extract (confirmatory experiment 5) is analysed as 3492 mg/L, which is reduced by 45% after treatment. This indicates the utilization of dye as a carbon source by bacteria during treatment process. The remaining TOC concentration could be removed through ultra/nanofiltration membrane technique, whose study is under progress and the details will be reported in future from our laboratory.
Biomass growth in dye containing culture media
The biomass growth of strain 23N1 in three culture media for different dyes combinations is investigated through measurement of optical density at 600 nm (OD600) using UV–visible spectroscope. The OD600 measurement methodology is a well-known technique to estimate the density of bacterial cell in culture media. The biomass growth is assessed in 50 mg/L solutions of individual RR21 and RO16 dyes. The process condition is adopted as: temperature 40 °C, pH 9, yeast extract 8 g/L, salt 4 g/L and 0.5% inoculation volume (v/v). The change in OD600 value of dye containing culture media with respect to time (h) is illustrated in Fig. 4. In RR21 dye solution, the maximum absorbance is obtained as 2.09, while it is 2.25 for RO16 dye solution. Comparatively, the bacterial density is higher for RO16 containing sample than RR21 dye solution, which indicates that RR21 dye is more toxic as compared to RR21 dye.
Effect of chromate
The chromium salt is generally used, as mordant in the industrial dyeing process. The co-existence of chromate (Cr(VI)) with dye in textile wastewater increases the toxicity and makes it resistant to microbial growth. The effect of Cr(VI) (initial concentration range: 25–150 mg/L) on ADMI reduction performance of strain 23N1 is assessed using process condition of confirmatory experiment 5 (mentioned in Table 4). The change in UV–visible transmittance spectra of mixed dyes containing water sample due to the presence of Cr(VI) ions is shown in Fig. 5, which clearly indicates the decrease in transmittance of treated water containing Cr(VI). The initial and final ADMI values with reductions of ADMI values in percentages of experiments (confirmatory experiment 5) containing different Cr(VI) concentrations are illustrated in Table 5.
Change in UV–visible transmittance spectra due to effect of Cr(VI) on ADMI reduction of mixed dyes water sample. Note: Initial untreated sample denotes the spectra of mixed dyes water sample; treated 1, 2, 3, 4, 5, 6 and 7 represent the spectra of treated water sample containing initial Cr(VI) concentration 0 mg/L, 25 mg/L, 50 mg/L, 75 mg/L, 100 mg/L, 125 mg/L and 150 mg/L, respectively
The ADMI reduction percentage decreases from 81.07 to 63.16% with respect to an increase in initial Cr(VI) concentration from 25 to 100 mg/L. The presence of Cr(VI) concentration ≥ 125 mg/L could be lethal and exhibits only 10.76% ADMI reduction, which might be due to unfavourable higher toxicity of medium for bacterial growth and its activity. This result indicates that the bacterial strain 23N1 could sustain even if initial Cr(VI) concentration lies between 0 and 100 mg/L and exhibits satisfactory ADMI reduction. The literature shows that the Cr(VI) concentration of < 50 mg/L is commonly found in textile wastewater (Chaudhari et al. 2017); thus, strain 23N1 could be used satisfactorily for textile wastewater treatment.
Simultaneous decolourization of wastewater and production of bio-energy using microbial fuel cell (MFC)
In MFCs, the decolourization of synthetic mixed dyes aqueous solution and real industrial wastewater is carried out in chamber 1 at room temperature (~ 30 °C) for 120 h under static condition, while chamber 2 is filled with 100% DDW as shown in Fig. S6 of SI. The bio-decolourization process produces protons (H+) and electrons (e−) through redox reactions that facilitates the power generation (Ilamathi and Jayapriya 2018). The defined process conditions selected for the four different experiments are as: sample “MDY” represents mixed dyes aqueous solution containing yeast extract and salt (same as confirmatory experiment number 5); sample “RW1” represents real wastewater (physiochemical characteristics are enlisted in Table 6); sample “RW2” represents real textile wastewater containing Cr(VI); sample “RW2Y” represents real wastewater supplemented with 8 g/L yeast extract as co-substrate.
Each experimental sample (above stated) is inoculated with 4% (V/V) bacterial inoculums. The output voltage is monitored using digital voltmeter at the time interval of 4 h as shown in Fig. 6a. The power density is evaluated using voltage data, shown in Fig. 6b. The maximum voltage and power density in the experiment using sample MDY is recorded as 624 mV and 586.85 mW/m2 after 88 h of incubation period, which is probably obtained through degradation of dyes in saline medium by bacteria. The bio-electricity generation potential of bacteria is estimated through MFC using two different samples of RW1 and RW2, from which the maximum voltages for two water samples are recorded as 790 mV (power density 940.61 mW/m2) after 88 h and 550 mV (power density 455.91 mW/m2) after 64 h treatment, respectively. This variation in voltage generation between samples RW1 and RW2 might be due to different composition of dyes and other ionic constituents. It has been observed that the reduction % of ADMI values for experiment using samples RW1 and RW2 is obtained as 76.0% after 120 h and 71.4% after 108 h of incubation period, respectively. In order to achieve satisfactory ADMI reduction percentage and estimate the bio-electricity generation potential of bacteria, the voltage generation is examined using sample RW2 supplemented with yeast extract (coded as: RW2Y). The maximum voltage in RW2Y is recorded as 515 mV (power density 399.73 mW/m2) after 48 h with ADMI reduction of 77.32 ± 0.4%, which reveals that bacteria enhance the bio-decolourization activity in the presence of co-substrate and promotes the generation of energy within short period of time. Previously, Sun et al. 2013 have reported the increase in power generation and decolourization of Congo red dye on the addition of redox mediator in bacteria-driven MFC. However, the amount of power generation and decolourization of dyes vary with different co-substrates and the effluent composition (Cao et al. 2010; Nayak et al. 2018). The decline in power generation after maximum gain could be due to reduction in generation of electrons in chamber 1. It is well known that the glucose, acetate and ethanol are most commonly used co-substrate in such processes (Sun et al. 2013). However, in our study, the glucose has shown negative influence on decolourization response of reactive dyes by bacterial strain 23N1 (Mishra and Maiti 2018c, d). This study reveals that the yeast extract as co-substrate promotes the decolourization of mixed dyes as well and enhances the rate of power generation though MFCs.
Although the selection of co-substrate for effective decolourization of dyes depends on the dye composition in the water, strain 23N1 requires peptone with yeast extract for decolourization of MO dye (reported in earlier study of Mishra and Maiti 2018b). Thus, this study suggests that the degradation of mixed dyes (RR21 and RO16) compounds by strain 23N1 could be coupled with power generation in the presence of yeast extract as only co-substrate. However, a detailed study is required to optimize the process condition for high-power generation with faster decolourization of textile dye wastewater, which might be explored in the separate study.
Furthermore, the decolourization of RR21, RO16 and other unknown dyes containing wastewater RW1 is investigated under optimal process condition as: yeast extract 9 g/L, pH 9, inoculations volume 4% and incubation time 48 h at temperature 40 °C under static condition. The considerable changes in UV–visible transmittance spectra of treated water sample are observed compared raw feed, as shown in Fig. 7. The wider peaks observed at wavelength range 450–550 nm (includes both λmax of RR21 at 537 nm and λmax of RO16 at 493 nm) for untreated sample completely diminish in treated sample, which indicates the decolourization of dyes. The ADMI value for treated water sample obtained from this experiment and ADMI reduction percentage is estimated are 142.12 ± 0.8 and 69.63% ± 0.5, respectively. This result signifies the feasibility of strain 23N1 for treatment of industrial wastewater along with electrical power generation.
Toxicity analysis of metabolites
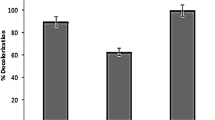
The treated mixed synthetic aqueous dye solution (confirmatory experiment 5) and real textile wastewater RW2 are used to investigate the germination rate of V. radiata seeds under controlled laboratory conditions. During the analysis of germination rate, it is observed that treated aqueous dye solution and treated RW2 water promote the germination of seeds, which exhibit 100% germination rate compared to control test as shown in Fig. S7a and S7b, respectively, of SI. The growing seeds do not express any morphological disorder, which indicates non-toxicity of end metabolites in the treated water samples. The average length of radicals germinated in the presence of treated waters from synthetic water and raw wastewater samples is estimated as 5.8 and 5.5 cm, respectively, which is comparatively better than radical grown in control (average length 4.4 cm). Thus, it confirms that the end metabolites produced after treatment of mixed dyes sample are non-toxic and might support in the growth of vegetation.
The few studies related to decolourization of mixed dyes (of different chromophore) by bacterial species have been reported in recent past, shown in Table 7. Joe et al. (2008) have investigated the capability of Clostridium bifermentans SL186 to bio-decolourize mixed dyes (reactive black-5, reactive yellow 3G-P and reactive red 3B-A) in anaerobic condition. They have found that the bacteria could effectively reduce ADMI value by 90% of mixed dyes solutions in wide range of pH (6–12). However, they suggested that the bacteria require additional glucose as carbon source along with yeast extract in dye decolourization medium that increases chemical cost of the process. Kurade et al. (2011) used Brevibacillus laterosporus MTCC 2298 to decolourize the mixture of structurally dissimilar dyes (rubine GFL, Remazol red, brown 3-REL, golden yellow HER, scarlet RR, brilliant blue GL and methyl red) in static micro-aerobic medium containing yeast extract, peptone and additional carbon sources. The authors reported that the bacteria could reduce ADMI value by 87% of dye chromophore through cellular oxidative–reductive metabolism involving enzymes like veratryl alcohol oxidase, tyrosinase, NADH-DCIP reductase and azo reductase. Lucious et al. (2014) carried out the decolourization of mixed dyes (acid blue and acid red) using P. aeruginosa in glucose-supplemented medium under aerobic condition. The authors reported that the bacteria exhibited maximum 90% ADMI reduction efficiency. Maheswari and Sivagami (2016) investigated the decolourization of mixed dyes (reactive red M5B, malachite green, reactive purple and reactive pink MB) from textile industrial effluent by Bacillus subtilis and B. cereus in aerobic condition. They reported that the B. cereus exhibited comparably higher dye degradation performance (70.8%) than B. subtilis. Mishra et al. (2019) investigated the decolourization of mixed dyes (RR21and RO16) solution by P. aeruginosa in jack fruit seed powder-supplemented medium. The authors reported that bacterial could significantly reduce ADMI by 90.2% after 96 h. However, the same bacterial growth and decolourization activity were significantly reduced in the presence of metal ions in the culture medium. Based on the literature available, it has been observed that the bacterial species require additional carbon sources to sustain the dye toxicity stress of the culture medium. The reported bacterial species exhibit certain limitations like requirement of additional carbon source, negative effect of metal ions and long incubation period for effective decolourization of dyes. In this study, the bacteria strain 23N1 exhibits satisfactory dye decolourization efficiency in the absence of additional carbon source other than yeast extract and the presence of metals ions than other bacterial species. This study would give good idea to researchers for investigating potential bacterial species and explore the optimization of process condition for wastewater treatment along energy generation.
Conclusion
This study is carried out to optimize the decolourization process condition of mixed (reactive red 21 (RR21) and reactive orange 16 (RO16)) dyes containing aqueous solutions by P. aeruginosa 23N1, through response surface methodology. Further, the metabolites extracted from the treated water sample are used to investigate the extent of degradation of RR21 and RO16 dyes in water samples containing either individual dye or mixed dyes. The non-toxicity of end metabolites is assessed through germination test of V. radiata seeds in medium supplemented with treated mixed dyes water sample. The quadratic model equation generated through analysis of variance for mixed dyes ADMI reduction percentage data is found to be statistically significant (p < 0.05) and suitable to predict the bio-decolourization efficiency of the process. The strain 23N1 exhibits maximum ADMI reduction percentage of 81.08 ± 0.4% for mixed dye-laden water sample. The yeast extract is required to be used as co-substrate by bacteria to decolourize dyes, which is highly salt tolerant (~ 6 g/L NaCl) and exhibits better performance in alkaline medium. The strain 23N1 could sustain Cr(VI) concentration up to 50 mg/L and exhibit satisfactory ADMI reduction of ~ 67%. The yeast extract as co-substrate is found to be very effective to increase the rate of bio-degradation of dyes and to achieve maximum voltage generation in microbial fuel cell within very short period of time. The maximum voltage generation of 790 mV and power density 940.61 mW/m2 are noticed during degradation of mixed dye-laden real wastewater in microbial fuel cell. The results reveal that the both complex dyes are utilized as nutrient by bacteria and converted into simpler organic compounds, which could promote the 100% healthy germination rate of V. radiata seeds that signify the non-toxicity of end metabolites. Thus, the microbial fuel cell could be a promising technology for bio-decolourization of textile wastewater as well as bio-energy generation.
References
APHA (2011) AWWA and WPCF standard methods for the examination of waters and waste waters, 22nd edn. American Public Health Association (APHA), Washington
Bedekar PA, Saratale RG, Saratale GD, Govindwar SP (2014) Oxidative stress response in dye degrading bacterium Lysinibacillus sp. RGS exposed to Reactive Orange 16, degradation of RO16 and evaluation of toxicity. Environ Sci Pollut Res 21:11075–11085
Bouraie ME, Din WSE (2016) Biodegradation of Reactive Black 5 by Aeromonas hydrophila strain isolated from dye-contaminated textile wastewater. Sustain Environ Res 2:209–216
Cao Y, Hu Y, Sun J, Hou B (2010) Explore various co-substrates for simultaneous electricity generation and Congo red degradation in air-cathode single-chamber microbial fuel cell. Bioelectrochemistry 79:71–76
Chacko JT, Subramaniam K (2011) Enzymatic degradation of azo dyes—a review. Int J Environ Sci 1:6
Chakraborty J, Mallick S, Raj R, Das S (2018) Functionalization of extracellular polymers of Pseudomonas aeruginosa N6P6 for synthesis of CdS nanoparticles and cadmium bioadsorption. J Polym Environ 26:3097–3108
Chaudhari AU, Paul D, Dhotre D, Kodam KM (2017) Effective biotransformation and detoxification of anthraquinone dye reactive blue 4 by using aerobic bacterial granules. Water Res 122:603–613
Garg SK, Tripathi M, Lal N (2015) Response surface methodology for optimization of process variable for reactive orange 4 dye discoloration by Pseudomonas putida SKG-1 strain and bioreactor trial for its possible use in large-scale bioremediation. Desalination Water Treat 54(11):3122–3133. https://doi.org/10.1080/19443994.2014.905975
Hameed BB, Ismail ZZ (2018) Decolorization, biodegradation and detoxification of reactive red azo dye using non-adapted immobilized mixed cells. Biochem Eng J 137:71–77
Holkar CR, Arora H, Halder D, Pinjari DV (2018) Biodegradation of reactive blue 19 with simultaneous electricity generation by the newly isolated electrogenic Klebsiella sp. C NCIM 5546 bacterium in a microbial fuel cell. Int Biodeterior Biodegrad 133:194–201
Huang G, Wang W, Liu G (2015) Simultaneous chromate reduction and azo dye decolourization by Lactobacillus paracaseae CL1107 isolated from deep sea sediment. J Environ Manag 157:297–302
Hussain S, Quinn L, Li J, Casey E, Murphy CD (2017) Simultaneous removal of malachite green and hexavalent chromium by Cunninghamella elegans biofilm in a semi-continuous system. Int Biodeterior Biodegrad 125:142–149
Ilamathi R, Jayapriya J (2018) Microbial fuel cells for dye decolorization. Environ Chem Lett 16:239–250
Imran M, Arshad M, Negmd F, Khalid A, Shaharoona B, Hussain S, Nadeem SM, Crowley DE (2016) Yeast extract promotes decolorization of azo dyes by stimulating azoreductase activity in Shewanella sp. strain IFN4. Ecotoxicol Environ Saf 124:42–49
Joe MH, Lim SY, Kim DH, Lee IS (2008) Decolorization of reactive dyes by Clostridium bifermentans SL186 isolated from contaminated soil. World J Microbiol Biotechnol 24(10):2221–2226
Keskin NOS, Celebioglu A, Sarioglu OF, Ozkan AD, Uyar T, Tekinay T (2015) Removal of a reactive dye and hexavalent chromium by a reusable bacteria attached electrospun nanofibrous web. RSC Adv 5:86867
Kurade MB, Waghmode TR, Govindwar SP (2011) Preferential biodegradation of structurally dissimilar dyes from a mixture by Brevibacillus laterosporus. J Hazard Mater 192(3):1746–1755
Kurade MB, Waghmode TR, Khandare RV, Jeon BH, Govindwar SP (2016) Biodegradation and detoxification of textile dye Disperse Red 54 by Brevibacillus laterosporus and determination of its metabolic fate. J Biosci Bioeng 121:442–449
Liu W, Liu C, Liu L, You Y, Jiang J, Zhou Z, Dong Z (2017) Simultaneous decolorization of sulfonated azo dyes and reduction of hexavalent chromium under high salt condition by a newly isolated salt tolerant strain Bacillus circulans BWL1061. Ecotoxicol Environ Saf 141:9–16
Lovley DR (2006) Microbial fuel cells: novel microbial physiologies and engineering approaches. Curr Opin Biotechnol 17:327–332
Lucious S, Reddy ES, Anuradha V, Yogananth N, Ali MYS, Vijaya P, Rajan R, Parveen PMK (2014) Decolorization of acid dyes by B. cereus and P. aeruginosa isolated from effluent of dyeing industry. Int J Pure Appl Biosci 2(3):23–29
Ma L, Zhuo R, Liu H, Yu D, Jiang M, Zhang X, Yang Y (2014) Efficient decolorization and detoxification of the sulfonated azo dye Reactive Orange 16 and simulated textile wastewater containing Reactive Orange 16 by the white-rot fungus Ganoderma sp. En3 isolated from the forest of Tzu-chin Mountain in China. Biochem Eng J 82:1–9
Maheswari NU, Sivagami S (2016) Biological degradation of textile dyes using marine Bacillus species. Int J Pure Appl Biosci 4(4):123–128
Maqbool Z, Abid M (2016) Characterization of a salt resistant bacterial strain Proteus sp. NA6 capable of decolorizing reactive dyes in presence of multi-metal stress. World J Microbiol Biotechnol 32:181
Miran W, Nawaz M, Jang J, Lee DS (2016) Sustainable electricity generation by biodegradation of low-cost lemon peel biomass in a dual chamber microbial fuel cell. Int Biodeterior Biodegrad 106:75–79
Mishra S, Maiti A (2018a) The efficacy of bacterial species to decolourise reactive azo, anthroquinone and triphenylmethane dyes from wastewater: a review. Environ Sci Pollut Res 25:8286–8314
Mishra S, Maiti A (2018b) Process optimization for effective bio-discolouration of methyl orange by Pseudomonas aeruginosa 23N1 using chemometric methodology. Can J Chem Eng. https://doi.org/10.1002/cjce.23410
Mishra S, Maiti A (2018c) Optimization of process parameters to enhance the bio-decolorization of Reactive Red 21 by Pseudomonas aeruginosa 23N1. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-018-2023-1
Mishra S, Maiti A (2018d) Process optimization for effective bio-discolouration of reactive orange 16 using chemometric methods. Int J Environ Sci Health Part A. https://doi.org/10.1080/10934529.2018.1541383
Mishra S, Maiti A (2019a) Effectual bio-decolourization of anthraquinone dye reactive blue-19 containing wastewater by Bacillus cohnii LAP217: process optimization. Bioremediation J. https://doi.org/10.1080/10889868.2019.1671793
Mishra S, Maiti A (2019b) Applicability of enzymes produced from different biotic species for biodegradation of textile dyes. Clean Technol Environ Policy 21(4):763–781
Mishra S, Maiti A (2019c) Study of simultaneous bioremediation of mixed reactive dyes and Cr(VI) containing wastewater through designed experiments. Environ Monit Assess 191:766
Mishra S, Mohanty P, Maiti A (2019) Bacterial mediated bio-decolourization of wastewater containing mixed reactive dyes using jack-fruit seed as co-substrate: process optimization. J Clean Prod 235:21–33
Mohan SV, Mohananakrishna G, Velvizhi G, Babu VSPNL (2010) Bio-catalyzed electrochemical treatment of real field dairy wastewater with simultaneous power generation. Biochem Eng J 51:32–39
Naseer A, Nosheen S, Kiran S, Kamal S, Javaid MA, Mustafa M, Tahir A (2016) Degradation and detoxification of Navy Blue CBF dye by native bacterial communities: an environmental bioremedial approach. Desalination Water Treat 57:24070–24082
Nayak JK, Amit Ghosh UK (2018) An innovative mixotrophic approach of distillery spent wash with sewage wastewater for biodegradation and bioelectricity generation using microbial fuel cell. J Water Process Eng 23:306–313
Paz A, Carballo J, Perez MJ, Domínguez JM (2017) Biological treatment of model dyes and textile wastewaters. Chemosphere 181:168–177
Priac A, Badot PM, Crini G (2017) Treated wastewater phytotoxicity assessment using Lactuca sativa: focus on germination and root elongation test parameters. C R Biol 340:188–194
Roy U, Sengupta S, Banerjee P, Das P, Bhowal A, Datta S (2018) Assessment on the decolourization of textile dye (reactive yellow) using Pseudomonas sp. immobilized on fly ash: response surface methodology optimization and toxicity evaluation. J Environ Manag 223:185–195
Shafqat M, Khalid A, Mahmood T, Siddique MT, Hand J, Habteselassie MY (2017) Evaluation of bacteria isolated from textile wastewater and rhizosphere to simultaneously degrade azo dyes and promote plant growth. J Chem Technol Biotechnol 92:2760–2768
Shahid M, Islam SU, Mohammad F (2013) Recent advancements in natural dye applications: a review. J Clean Prod 53:310–331. https://doi.org/10.1016/j.jclepro.2013.03.031
Sun J, Li W, Li Y, Hu Y, Zhang Y (2013) Redox mediator enhanced simultaneous decolorization of azo dye and bioelectricity generation in air-cathode microbial fuel cell. Bioresour Technol 142:407–414
Telke AA, Joshi SM, Jadhav SU, Tamboli DP, Govindwar SP (2010) Decolorization and detoxification of Congo red and textile industry effluent by an isolated bacterium Pseudomonas sp. SU-EBT. Biodegradation 21:283–296
Telke AA, Kadam AA, Govindwar SP (2015) Bacterial enzymes and their role in decolorization of azo dyes. In: Singh S (ed) Microbial degradation of synthetic dyes in wastewaters. Springer, New York. https://doi.org/10.1007/978-3-319-10942-8_7
Yaseen DA, Scholz M (2019) Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review. Int J Environ Sci Tech 16:1193–1226
Acknowledgements
The authors acknowledge the financial support from Ministry of Human Resource Development (MHRD), Government of India, for student scholarship and funding support through project (IMPRINT scheme, Project number: 6125) along with Ministry of Urban Development, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mishra, S., Nayak, J.K. & Maiti, A. Bacteria-mediated bio-degradation of reactive azo dyes coupled with bio-energy generation from model wastewater. Clean Techn Environ Policy 22, 651–667 (2020). https://doi.org/10.1007/s10098-020-01809-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-020-01809-y