Abstract
Among the different methods of H2S removal, the wet desulfurization is widely used because of its unique advantages. Wet desulfurization usually employs alkaline Na2CO3–NaHCO3 buffer solution as desulfurization agent, which can react with H2S in absorber tower to produce HS−. Then, HS− is oxidized into sulfur in the presence of a catalyst such as phthalocyanine dicaryon sulfonates or other desulfurization catalysts. But unfortunately, Na2S2O3, Na2SO3, NaSCN and other by-product salts are inevitably generated in the oxidation process. However, the desulfurization pH and the physical properties of the solution such as surface tension, density and viscosity are notably affected by the content in Na2CO3 and by-product salts. Therefore, it is necessary to investigate the influence of by-product salts and Na2CO3 on desulfurization efficiency. In this work, the effect of different by-product salts and Na2CO3 content on the total volumetric mass transfer coefficient KGa of H2S absorption process is experimentally explored. The results revealed that the increase in the by-product salts content results in a decrease in KGa and pH values. On the contrary, with the increase in Na2CO3 content, KGa and pH values increased. A mathematical model of the absorption process is proposed and analyzed; the mass transfer coefficient in gas phase kG and in liquid phase kL as well as secondary reaction constant k2 is calculated. The results showed that KGa was changed mainly by k2 which reflects the effect of pH. The surface tension, density and viscosity had relatively small effect on KGa. The present results provide the required theoretical guidance for practical industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The current China concentration standard for H2S in the air is less than or equal to 10 mg/m3 (GBZ of China 2007). In addition, the purification requirements in the oil refinery waste gas call for H2S concentration in the range of 10–20 mg/m3. H2S possesses a serious threat to people’s health (Bao et al. 2008). The increase in H2S concentration in the atmosphere along with the increase in exposure time could result to serious health issues, involving headache, dizziness, nausea, vomiting and even to death depending on its concentration and the duration of exposure. Moreover, serious environmental problems are related with the H2S, such as acid rain, corrosion of concrete and metals, among others. Zhao et al. (2006) studied the factors affecting the desulfurization in water gas (water gas is produced by the reaction of water vapor with hot anthracite or coke, consisting mainly from hydrogen and carbon monoxide) and semi-water gas (mixture of water gas and air gas) systems. It was revealed that the combustion products and the gas emitted from industrial plants can have significant effects in human health and environment. In addition, H2S in water gas not only lead to a severe corrosion of equipment, but also to catalyst deactivation in subsequent processes (Wang 2001). Pang et al. (2013) studied the application of MSQ-3 catalyst in the desulfurization of semi-water gas and concluded that the insufficient removal of H2S could cause a series of problems such as equipment corrosion and catalyst poisoning.
Among the different methods applied for H2S from water gas, dry desulfurization and wet desulfurization are the most common. Kawase and Otaka (2013) carried out a study on the purification of synthesis gas during biomass gasification by using the molten carbonate method. Yang et al. (2003) proposed a new method for the absorption of CO2 and H2S by using industrial waste alkaline solutions; the basic parameters affecting CO2 and H2S adsorption were analyzed, and a mass transfer model was established. A wet catalytic oxidation method at room temperature, employing a solution containing ferric, ferrous and cupric ions for H2S removal, was also investigated (Zhang 2006). Zhang (2005) introduced a method for H2S removal from coke oven gas by using catalyst composite of phthalocyanine dicaryon sulfonates (PDS) and tannin extraction. Wet desulfurization usually employs a desulfurization solution to remove H2S from water gas by chemical absorption into liquid phase. This method has many advantages such as easy operation, high desulfurization efficiency and low cost, and thus has been widely applied (Yang et al. 2012). The wet flue gas desulfurization (FGD) in the coal-fired power plants is the most widely applied method because of its high SO2 removal efficiency, reliability and low utility consumption (Dou et al. 2009). Alkaline Na2CO3–NaHCO3 buffer solution is usually used as a desulfurization solution where the H2S is reacted with OH− and converted to HS−. The purification efficiency of this particular process, which is usually carried out in an adsorption tower, is largely affected by the pH value of the solution. In order to achieve the required purification efficiency, a careful adjustment of pH range is needed. The solution absorbing H2S is usually called rich solution, contained in a regeneration tank. In regeneration tank, HS− is oxidized by O2 into sulfur in the presence of a desulfurization catalyst such as PDS (Yang et al. 1991) or 888 (an improved desulfurization catalyst based on PDS, which is composed of a trinuclear cobalt phthalocyanine ammonium sulfonate metal organic compound) (Qi et al. 2006). The rich solution without sulfur is called barren solution (Zhang et al. 2015), which is recycled to the absorption tower.
During the oxidization process, side reactions are inevitable and various by-products such as Na2S2O3, Na2SO3, NaSCN and Na2SO4 are in addition produced. These by-products have a significant effect on the physical properties of the desulfurization solution and its pH value. Na2CO3 should be added regularly to the desulfurization solution to maintain a stable pH value (Zhang and Chen 2008). However, Na2CO3 addition in the desulfurization solution results in an increase in by-product salts, which may lead to salt blockage on the desulfurization tower (Luo and Liu 2006). In addition, the increase in by-product salts can lead to equipment corrosion and to degradation of desulfurization efficiency. In summary, in order to achieve a stable operation of desulfurization process, the pH value of desulfurization solution should be maintained in a specific range (8.60–8.80) and the salts content should be closely monitored (Wang et al. 2007). Extensive research efforts have been put on the absorption of H2S by alkaline solutions. Different types of absorption liquid, reaction devices and absorption models have been employed toward optimizing the desulfurization efficiency (Yi et al. 2008). Shang (2012) analyzed the process of H2S absorption in a sodium hydroxide solution. Yang et al. (2012) proposed a method of treating acid gas pollution by the absorption of H2S with sodium hydroxide solution, and the purified NaHS liquid product was obtained by purification. The process of H2S absorption by sodium carbonate solution in a wetted-disk and a wetted-wall column was also proposed and analyzed (Garner et al. 1958). Shimizu et al. (1997) investigated gas and liquid absorption reactions in a wet flue gas desulfurization device. Zhao et al. (2009) used a swirl nozzle and a spiral nozzle in a wet-type desulfurization spray tower under single-layer spray conditions. Bontozoglou and Karabelas (1991) established a numerical model for the simultaneous absorption of CO2 and H2S by NaOH solution, which confirmed the role of CO2 dissociation in improving the dynamic selectivity of hydrogen sulfide absorption. Wallin and Olausson (1993) established a model for predicting the absorption rate of H2S and CO2 in sodium carbonate solution, exploring the influence of carbonate concentration, gas flow velocity and temperature on the removal efficiency of H2S and CO2. Park and Kang (1995) developed a new computer program for the simulation of chemical absorption of H2S and CO2 by using a hot potassium carbonate solution, MEA or DEA. Kiil et al. (1998) developed a detailed model for a wet FGD pilot plant based on a packed tower concept and conducted a parameter analysis toward validating assumptions and extracting information on wet FGD systems. However, the influence of by-product salts on the desulfurization efficiency has been rarely investigated. Meng and Li (2010), taking into consideration three parameters (surface tension, density and viscosity) investigated the effect of by-product salts on the desulfurization efficiency of tannin extract desulfurization liquid. Wang et al. (2008) explored only the influence of by-product salts on the density of tannin extract desulfurization liquid. Literature studies on the effect of by-product salts and Na2CO3 on the desulfurization of H2S in water gas by using the Na2CO3–NaHCO3 buffer solution with PDS as the catalyst are limited.
The objective of this study is to systematically explore how the by-product salts affect the desulfurization efficiency. In this work, the influence of by-product salts and Na2CO3 on the gas–liquid mass transfer in the H2S absorption process was investigated by experimental methods. The obtained results can provide valuable insights in relation to the control of by-product concentration and pH values for efficient desulfurization in real practical applications.
Experimental device and method
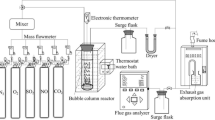
Figure 1 schematically illustrates the absorption tower and the setup used in the present study. The tower was filled with Raschig ring packing (material, ceramics; diameter × height × thickness is 3 mm × 3 mm × 1 mm). The inner diameter of the tower is 25 mm, and the packing layer is 360 mm high. The tower has a circulating warm water jacket to regulate the temperature of H2S absorption process. In order to ensure the reliability of the experimental device and the repeatability of the experiment, the necessary verification was carried out before starting the experiment. The average error of verification is lower than 5%. Each sample was analyzed every 30 min, and three measurements were obtained for each sample to take the average.
The desulfurization solution of a certain composition was prepared, and its physical properties such as surface tension (Rotenberg et al. 1983), density, viscosity (Tao et al. 2012) and pH (Yang et al. 2014) were determined. The flow of the desulfurization solution into the absorption tower was measured by a rotor flowmeter; the jacket of the tower was provided with a circulating warm water at 37 °C. The mixture of N2 and H2S was used to simulate the processed gas with H2S composition of about 2000 ppm. The mixture gas was measured by rotor flowmeter and went into the tower bottom. The desulfurization solution contacted with the gas during countercurrent movement in the tower to provide the necessary mass transfer for absorption. The intake and exhaust gas samples were taken, and H2S content was analyzed by gas chromatography. During the experiment, the gas flow rate was fixed at 180 L/h, while the desulfurization solution flow rate was fixed at 8.4 L/h (Yue et al. 2017).
Preparation of desulfurization solution
In areal production process, the mass ratio of the by-product salts produced by different raw materials is varied. In this work, the mass ratio used to prepare the desulfurization solution is: M (Na2S2O3):M (NaSCN):M (Na2SO3):M (Na2SO4) = 1:0.110:0.076:0.074, determined via continuous tracking analysis in a water gas purification device. The content of NaHCO3 and desulfurization catalyst PDS in desulfurization solution is constant and equal to 38 and 0.033 g/L, respectively.
Calculation of the total volumetric mass transfer coefficient K G a
KGa is an important parameter to characterize gas–liquid mass transfer device. The gas-phase component i (H2S) diffuses from the gas phase to the gas surface and liquid film and then into the liquid phase where it reacts with the absorbent. The mass transfer flux can be expressed as (Treybal 1980):
According to the mass conservation law, for a mass transfer unit at the packing height of dz, there is a differential equation:
When the fraction of component i in gas phase is relatively low, yi/(1 − yi) ≈ yi, VI ≈ V. In this work, the equilibrium partial pressure is relatively small and can be neglected. Thus, formula (2) can be approximately expressed as:
The definite integral of formula (3) is:
The total volumetric mass transfer coefficient KGa is:
The effective mass transfer area (a) is calculated by formula (6) (Onda et al. 1968):
Effects of various experimental factors on K G a
The influence of Na2CO3 content
According to the study of Aleboyeh et al. (2008), the Na2CO3 content has a significant effect on KGa. Two desulfurization solutions were prepared with different concentration in Na2CO3 and by-product salts. The Na2S2O3 content in the two different solutions is 51.00 and 100.00 g/L, respectively, whereas the other by-product salts content is proportionally calculated (Yue et al. 2017), as shown in Table 1.
In the as-prepared desulfurization solutions, the Na2CO3 content was changed in the range of 1–9 g/L, and the corresponding physical parameters of the solution (surface tension, density, viscosity, pH) were measured (Yue et al. 2017). The effect of Na2CO3 content on absorption (the total volumetric mass transfer coefficient KGa) was experimentally investigated. The results are shown in Figs. 2 and 3.
With the increase in Na2CO3 content, the pH of the two desulfurization solutions increases almost linearly. The pH value of the solution with lower content in by-product salts is slightly larger at the same Na2CO3 content, which indicates that the increase in the by-product salts content leads to a decrease in the pH value. With the increase in Na2CO3 content, KGa significantly increases, following, however, the same trend in the two different solutions. The KGa of the solution with lower content in by-products is larger at the same Na2CO3 content, implying that the presence of by-product salts hinders the gas–liquid mass transfer.
Effect of by-product salts content
The Na2CO3 content was fixed at 6 g/L, whereas by-product salts content is changed as shown in Table 2. The effect of by-product salts on pH value and KGa was experimentally investigated, and the results are shown in Fig. 4.
It is obvious that both the KGa and pH are monotonically decreasing as a function of by-product salts content. In particular, the pH decreased from 8.82 to 8.41 and KGa from 2 to 0.97 kmol/(m3 h kPa) upon increasing by-products content from 0 to 120 g/L, respectively (Fig. 4). The increase in the by-product salts content leads to the change of pH value, density, viscosity and surface tension of the desulfurization solution, which in turn results in an increase in the liquid film resistance accompanied by a substantial reduction of KGa (Yue et al. 2017).
The effect of pH values
Figure 5 depicts the influence of pH on KGa, under different by-product salts content.
It is evident that KGa slightly changes when the pH varies from 8.1 to about 8.4. However, for pH values higher than 8.4 the KGa is increased rapidly in all cases. According to Fig. 5, the pH value of desulfurization solution should be kept more than 8.65 to maintain a higher and more stable absorption effect. Figure 5 also depicts the influence of the by-product salts content on KGa under the same pH value, which is induced by the different physical properties of the desulfurization solution. In general, at the same pH, higher KGa values are obtained for lower by-product salts content. It is also of worth noticing that the influence of by-products content on KGa is strongly depended on pH values, being more intense for values higher than 8.5. It is considered that two main factors affect the mass transfer process during desulfurization. The first one is the pH of the solution, which affects the chemical adsorption. The second one is the by-product salts content, which alters the physical properties of the desulfurization solution. The impact of these particular parameters on mass transfer coefficient is analyzed in the following section.
Mass transfer coefficient
In the gas–liquid mass transfer processes, H2S transferred into the solution through the gas-phase and liquid-phase boundary resistance layers. The mass transfer flux can be expressed as:
The total mass transfer resistance is the sum of the liquid-phase boundary resistance and the gas-phase boundary resistance. For chemical absorption, the enhancement factor E is used to represent the influence of chemical reaction on the total mass transfer coefficient. KG, kL and kG are related through the equation:
kL and kG are computed by using the correlation proposed by Onda et al. (1968):
The enhancement factor E (Shi et al. 1996) is calculated as follows:
Ei is the enhancement factor of the instantaneous reaction (Shi et al. 1996):
In Eq. (12), Ha is Hatta number, calculated by the following equation (Shi et al. 1996):
The k2 in Eq. (14) is the rate constant of a second-order reaction.
Effect of Na2CO3 and by-product salts on k G and k L
According to the physical properties data and the experimental absorption parameters such as gas and liquid phase flow, the kG and kL of H2S absorption process can be calculated by Eqs. (9) and (10). The influence of Na2CO3 and by-product salts content on kG is shown in Figs. 6 and 7, respectively. The kG value remains constant, indicating that the composition of the desulfurization solution does not affect kG.
Figure 8 depicts the influence of Na2CO3 content on kL for the two different solutions (51 and 100 g/L Na2S2O3). A similar trend is observed in both solutions; kL is gradually decreased with the increase in Na2CO3 content. Figure 9 shows the influence of the by-product salts content on kL when the Na2CO3 content is fixed. The kL is decreased linearly upon increasing the by-product salts content. It can be therefore argued that both the Na2CO3 and by-product salts content can alter the properties of the desulfurization solution and in turn the kL.
The influence of pH value on the enhancement factor E
As shown in Fig. 8, the results reveal that the physical properties of the solution can affect the kL (and thus KGa). However, the enhancement factor E which reflects the effect of chemical reaction on the absorption process is not included in kL. The effect of chemical reaction is essentially manifested by the constant rate k2 of the second-order reaction in formula (14).
According to Eqs. (8)–(14) and the KGa values determined experimentally, the corresponding k2 values in H2S absorption process can be calculated (Table 3). It can be seen that the variation trend of k2 is basically the same as that of KGa. Therefore, compared with kL and kG, the chemical reaction enhancement factor E is the main factor affecting KGa. When the pH value is less than 8.52, the k2 of the different desulfurization solutions is basically the same. In contrast, when the pH value is larger than 8.52, the k2 is strongly depended on the content of desulfurization solution in by-product salts.
Conclusion
In this work, the influence of by-product salts on desulfurization efficiency was investigated. The results showed by changing Na2CO3 and by-product salts content, the physical properties (density, viscosity and surface tension) and the pH of desulfurization solution are notably altered, which in turn affects the total volumetric mass transfer coefficient KGa in the H2S absorption process. Compared with the physical properties of the desulfurization solution, pH has a greater effect on KGa.
The results also showed that the composition of the desulfurization solution does not affect kG, whereas Na2CO3 and by-product salts content has no significant effect in kL. Upon increasing the pH, the constant rate k2 representing the chemical absorption is changed accordingly to KGa, implying that the mass transfer coefficient is mainly affected by the k2. Therefore, compared with kL and kG, the chemical reaction enhancement factor E is the main factor affecting KGa.
The effective control of the H2S content in the air is of major importance toward improving air quality, preventing ecological damage and protecting human health. The results of this study can provide valuable insights for the effective control of H2S in practical industrial applications. The work for removal of H2S can effectively avoid and solve the potential environmental problems.
Abbreviations
- a :
-
Effective mass transfer area per unit volume of packing (m2/m3)
- a t :
-
Surface area per unit volume of packing (m2/m3)
- C :
-
Constant
- C Ai :
-
The concentration of the solute H2S at the interface (kmol/m3)
- C BL :
-
The concentration of the solute Na2CO3 in the liquid-phase body (kmol/m3)
- D G :
-
Diffusion coefficient in the gas phase (m2/h)
- D L :
-
Diffusion coefficient in the liquid phase (m2/h)
- d p :
-
Nominal diameter of the packing (m)
- G :
-
Mass velocity of the gas (kg/(m2 h))
- g :
-
Gravitational acceleration (m/s2)
- H :
-
Henry constant (kmol/(m3 kPa))
- K G :
-
Total mass transfer coefficient (kmol/(m2 h kPa))
- k G :
-
Mass transfer coefficient of the gas phase (kmol/(m2 h kPa))
- k L :
-
Mass transfer coefficient of the liquid phase (m/h)
- L :
-
Mass velocity of the liquid (kg/(m2 s))
- M :
-
Relative molecular mass (kg/kmol)
- N i :
-
Mass transfer flux (m3/h)
- P :
-
Total pressure (kPa)
- V I :
-
The inert gas velocity in the gas phase (kmol/(m2 h))
- x i :
-
The mole fraction of the component i in the liquid phase
- x i,int :
-
The mole fraction of the component i in the liquid film at gas–liquid interface
- y i :
-
The mole fraction of the component i in the gas phase
- y * i :
-
Equilibrium mole fraction of the component i in the gas phase
- y i,int :
-
The mole fraction of the component i in the gas film at gas–liquid interface
- y j :
-
The mole fraction of the component j in the gas phase
- z :
-
Packing height (m)
- σ :
-
Liquid surface tension (mN/m)
- σ c :
-
Critical surface tension of the packing material (mN/m)
- μ G :
-
Gas viscosity (Pa s)
- μ L :
-
Liquid viscosity (Pa s)
- ρ G :
-
Gas density (kg/m3)
- ρ L :
-
Liquid density (kg/m3)
- ϕ ij :
-
Correlation coefficient between component i and component j
References
Aleboyeh A, Daneshvar N, Kasiri MB (2008) Optimization of C.I. Acid Red 14 azo dye removal by electrocoagulation batch process with response surface methodology. Chem Eng Process 47:827–832. https://doi.org/10.1016/j.cep.2007.01.033
Bao GH, Guo SQ, Zhang YF et al (2008) Experimental study on desulfurization performance of hot gas at medium and high temperature. J Shanghai Univ 14:299–305. http://www.journal.shu.edu.cn//EN/Y2008/V14/I3/299
Bontozoglou V, Karabelas AJ (1991) Numerical-calculation of simultaneous absorption of H2S and CO2 in aqueous hydroxide solutions. Ind Eng Chem Res 30:2598–2603. https://doi.org/10.1021/ie00060a016
Dou B, Pan W, Jin Q et al (2009) Prediction of SO2 removal efficiency for wet flue gas desulfurization. Energy Convers Manag 50:2547–2553. https://doi.org/10.1016/j.enconman.2009.06.012
Garner FH, Long R, Pennell A (1958) The selective absorption of hydrogen sulphide in carbonate solutions. J Appl Chem 8:325–336. https://doi.org/10.1002/jctb.5010080509
Kawase M, Otaka M (2013) Removal of H2S using molten carbonate at high temperature. Waste Manag 33:2706–2712. https://doi.org/10.1016/j.wasman.2013.08.002
Kiil S, Michelsen ML, Damjohansen K (1998) Experimental investigation and modeling of a wet flue gas desulfurization pilot plant. Ind Eng Chem Res 37(2792–28):06. https://doi.org/10.1021/ie9709446
Luo D, Liu J (2006) Analysis of blockage in the tannin extract desulfurization tower and discussion on preventive measures. Coal Chem Ind 34:24–26 (in Chinese)
Meng X, Li Y (2010) Study on the effect of by-products in desulfurization solution of tannin extract on desulfurization solution. Coal Convers 33:22–25 (in Chinese)
Occupational exposure limits for hazardous agents in the workplace. National standards for occupational health in People’s Republic of China (GBZ 2.1-2007)
Onda K, Takeuchi H, Okumoto Y (1968) Mass transfer coefficients between gas and liquid phases in packed columns. Chem Eng Jpn 1:56–62. https://doi.org/10.1252/jcej.1.56
Pang J, Lin PS, Yan WJ et al (2013) Application of MSQ-3 catalyst in the desulphurization of semi water gas. Gas Purif 4:3–4 (in Chinese)
Park KY, Kang TW (1995) Computer-simulation of H2S and CO2 absorption processes. Korean J Chem Eng 12:29–35. https://doi.org/10.1007/BF02697703
Qi S, Yin K, Yang Q (2006) Application of 888 desulfurization catalyst for desulfurization of coke oven gas. Coal Chem Ind 34:58–59 (in Chinese)
Rotenberg Y, Boruvka L, Neumann AW (1983) Determination of surface tension and contact angle from the shapes of axisymmetric fluid interfaces. J Colloid Interface Sci 93:169–183. https://doi.org/10.1016/0021-9797(83)90396-X
Shang F (2012) Industrial technology of preparing sodium sulfide from absorption of hydrogen sulfide with sodium hydroxide solution. Inorg Chem Ind 44:42–43 (in Chinese)
Shi J, Wang JD, Chen MH et al (1996) Handbook of chemical engineering. Chemical Industry Press, Beijing (in Chinese)
Shimizu T, Ohishi T, Iwashita K et al (1997) Wet type flue gas desulfurization apparatus: EP, US5605552[P]
Tao S, Fang C, Zhou Y et al (2012) Density and viscosity of sodium borohydride in aqueous sodium hydroxide solution. Inorg Chem Ind. https://doi.org/10.3969/j.issn.1006-4990.2012.02.006
Treybal RE (1980) Mass-transfer operations, 3rd edn. McGraw-Hill Book Company, Singapore
Wallin M, Olausson S (1993) Simultaneous absorption of H2S and CO2 into a solution of sodium carbonate. Chem Eng Commun 123(1):43–59. https://doi.org/10.1080/009864493089361
Wang H, Liu YZ, Diao JY et al (2007) Discussion on the blocking problem of PDS method for desulphurization tower. Small Nitrogenous Fertil Plant 35:11–13 (in Chinese)
Wang X (2001) Progress of hydrogen sulfide waste gas treatment. Tech Equip Environ Pollut Control 24(4):77–84 (in Chinese)
Wang YL, Niu YX, Ling KC (2008) Study on the effect of by-products in desulfurization solution of tannin extract on desulfurization solution viscosity. Appl Chem Ind 11:6 (in Chinese)
Yang S, Shao Y, Pei D et al (1991) Desulfurization technology and catalysis mechanism of PDS catalyst. Petrochem Technol (in Chinese)
Yang LH, Liu SQ, Jie L et al (2003) Study on the method of absorbing CO2 and H2S simultaneously via waste alkali liquor and the mass transfer model. J China Coal Soc 28:64–68 (in Chinese)
Yang HY, Yong-Hong QI, Liu YT (2012) The research of treatment efficiency on acid gas with alkali absorption method. Shanxi Chem Ind 4:13 (in Chinese)
Yang C, Huangfu L, Su H et al (2014) Determination of pH value and acid-base property of ionic liquid aqueous solutions. Acta Chim Sin 72:495. https://doi.org/10.6023/A13091014
Yi Z, Xiang G, Wang H et al (2008) A model for performance optimization of wet flue gas desulfurization systems of power plants. Fuel Process Technol 11:1025–1032. https://doi.org/10.1016/j.fuproc.2008.04.004
Yue JC, Zhang W, Cheng HN et al (2017) Effects of secondary salts on wet desulfuration. Chem Eng. https://doi.org/10.3969/j.issn.1005-9954.2017.08.005
Zhang XL (2005) H2S removal from the coal gas of coke oven plant by PDS+ vegetable tannin extract method. Shanxi Chem Ind 25:76–77 (in Chinese)
Zhang J (2006) Study on catalytic wet oxidation of H2S into sulfur on Fe/Cu catalyst. Energy Chem 15:63–69. https://doi.org/10.1016/S1003-9953(06)60009-1
Zhang G, Chen Y (2008) Superficial analysis of Na2CO3 absorption mechanism in PDS catalytic desulfurization technology. Chem Fertil Ind 3:016 (in Chinese)
Zhang YL, Yu XJ, Wang QL et al (2015) Adsorption of zinc onto anionic ion-exchange resin from cyanide barren solution. Chin J Chem Eng 23:646–651. https://doi.org/10.1016/j.cjche.2014.01.003
Zhao CJ, Huang J, Chen JX et al (2006) Influence factors of gas desulfurization in water gas and semi-water gas system. Gas Purif 4:13–16 (in Chinese)
Zhao DL, Wang XM, Han Q (2009) The atomization characteristics of wet flue gas desulfurization spray tower under single layer spraying. Electr Power Environ Prot 5:003 (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yue, Jc., Chu, Cl., Zhang, W. et al. Influence of by-product salts and Na2CO3 contents on gas–liquid mass transfer process in wet desulfurization of water gas. Clean Techn Environ Policy 20, 1367–1375 (2018). https://doi.org/10.1007/s10098-018-1541-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-018-1541-3