Abstract
In order to recover valuable pyrolytic oils, mixed municipal solid waste was pyrolyzed in a fluidized bed reactor. Results showed that liquid products yielded among 38.4–56.5 wt% and separated into water-soluble phases and organic phases. Moisture was concentrated in the water-soluble phases (39.4–57.3 wt%), making them low in carbon content and heating value. On the other hand, the higher carbon content and lower oxygen content of organic phases make their heating value (27.5–32.1 MJ/kg) and quality higher than bio-oils. Water-soluble phases mainly included acids, carboxylics, phenols, and sugars, which could be used as chemical feedstocks and substantial fuel. Organic phases mostly contained aromatics and phenols and could be used as fossil fuels directly or as chemical materials. Heavy metals of Cd and Pb were proved to be poor in both water-soluble phases and organic phases. As for Zn, it was found to be higher in the water-soluble phases at 450 and 550 °C with quartz sand as bed material than that in crude oils. However, Zn content in organic phases was comparable to crude oils. High-aluminum bauxite and attapulgite as bed materials increased heating value of water-soluble phases and organic phases respectively, and both performed well in reducing the Zn content of water-soluble phases. This work proved that it was an operative way to produce valuable pyrolytic oils by pyrolysis of mixed municipal solid waste.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the public report of Ministry of Environmental Protection of the People’s Republic of China (MEP 2014), there were more than 16 million tons of municipal solid waste (MSW) produced in 2013 throughout China. More than three quarters of them had been dealt by landfilling, causing a series of potential environmental problems. However, there were many valuable compounds in MSW which could be used as energy resources, such as plastics, papers, biomass, rubbers, and so on. Their energy could be recovered by thermal treatment. Among all the thermal treatment processes, pyrolysis was considered as an innovative and green alternative to obtain valuable chemicals and fuels (Alper et al. 2015; Chen et al. 2014).
It has been suggested that co-pyrolysis of biomass and synthetic polymers could be an environmentally friendly way for the transformation of commingled lignocellulosic and plastic waste into valuable products such as chemicals or fuels (Brebu et al. 2010). Bhattacharya et al. (2009) investigated the fast pyrolysis of pine wood and waste plastics. The generated liquids exhibited obviously better quality than pine bio-oil. The former were higher in carbon contents, hydrogen contents, and heats of combustion and lower in oxygen contents, waste contents, acid values and viscosities. Paradela et al. (2009) studied the co-pyrolysis of biomass, plastics, and used tires. It was figured out by the authors that the most important parameter that effect the yield and composition of products was plastics content. The increase of plastics content increased liquid yield and also decreased aromatic contents. Co-pyrolysis of lignin and waste plastics was undertaken by Zhang et al. (2015) in a fluidized bed. Increasing PE proportion raised both aromatic and olefin yields. Catalytic co-pyrolysis of black-liquor lignin with PS produced the maximum aromatic yield while co-pyrolysis with PE produced the maximum olefin yield. As for the reason on the upgrading of pyrolytic liquids by co-pyrolysis of biomass and plastics, Zhang et al. (2015) attributed it to that the addition of plastics increased the hydrogen to carbon effective ratio (H/Ceff ratio). Increasing H/Ceff ratio led to the increase of value index of aromatic hydrocarbon products when biomass/HDPE ratio was <1:1. The H/Ceff ratio should be >1.0 so as to achieve high relative content and value index of aromatic hydrocarbons.
Some researchers also drew attention on co-pyrolysis of biomass and other MSW compounds. Martinez et al. (2014) investigated the co-pyrolysis of biomass and waste tires. Experimental results from the auger reactor revealed a remarkable upgrading for some liquid properties such as lower acidic number, lower density, higher pH, higher calorific value, and lower oxygen content. Paradela et al. (2009) researched co-pyrolysis of mixtures of plastics, tires, and forestry biomass wastes. It was figured out that the rise of plastics increased liquids yield, favored the formation of lighter compounds, and promoted the conversion of aromatics into alkanes and alkenes. Miskolczi (2013) studied the co-pyrolysis of HDPE, poly-lactic-acid (PLA) biopolymer, and organic waste. The undesired oxygenated hydrocarbons derived from PLA and organic wastes could be decreased with increasing temperature or adding HDPE into raw material, resulting in the improvement of quality, storage stability or even contaminant level of pyrolysis oil.
From the above discussion, it could be found that most researchers were interested in co-pyrolysis of different MSW compounds rather than real MSW. Besides, the potential environmental influence of pyrolytic products had been rarely reported. In this study, pyrolysis of mixed MSW compounds was applied in a fluidized bed reactor. Yield and properties of pyrolytic products were analyzed comprehensively and compared with bio-oils and crude oils. Organic compositions of pyrolytic oils were detected by gas chromatograph/mass spectrometer (GC/MS). Then the interactions between different compounds in raw material were discussed. Furthermore, heavy metals (Cd, Zn, and Pb) in pyrolytic oils were also measured. In this work, the feasibility of producing highly valuable oil products from pyrolysis of real MSW material was verified. Meanwhile, the potential environmental hazard of the pyrolytic products was also evaluated.
Materials and methods
Raw material
MSW was collected from several communities in Nanjing, China. The incombustible compounds such as glasses, dust, and tiles were removed by manual work. The collected MSW was exposed to sunlight for more than one month to remove surface moisture. Then the air-dried MSW was sorted to several compounds, as shown in Fig. 1. Since most of food wastes were biomass, food waste and biomass were classified as one compound, which made up more than half the compounds. Plastics mainly contained polyethylene (PE), polystyrene (PS), and polyethylene terephthalate (PET), while the main components of papers were newspaper, cardboard, and printing paper.
After the exposure and sorting, compounds in MSW were treated to small pieces. Biomass in MSW was smashed to powder and bulk (with size no more than 1 cm). Plastics and papers in MSW were crashed or chopped to pieces with side length less than 5 mm. Then the treated compounds were mixed together again. The mixed raw material was dried in the oven at 80 °C for more than 48 h before each experiment.
Physical analysis of raw material is shown in Table 1. Higher heating value (HHV) was calculated by the method proposed by Demirbaş (1997). It was demonstrated that the raw material was of high volatile and oxygen content. Heavy metals in MSW are also summarized in Table 1. When comparing with most reported MSW feedstocks, Cd and Zn contents were comparable whereas Pb content was a bit lower (Zhang et al. 2008).
Bed material
Quartz sand (QS), high-aluminum bauxite (HAB), and attapulgite (ATT) with particle size of 0.3–0.4 mm were chosen as bed material. The chemical composition of bed materials was analyzed using an ARL-9800 X-ray fluorescence (XRF, Thermo Fisher Scientific) spectrometer. The specific surface area was measured by a Micromeritics ASAP 2010 apparatus. Results are listed in Table 2.
Fast pyrolysis experimental setup
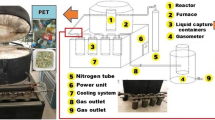
Fast pyrolysis of MSW was carried out in a fluidized bed device, which was shown in Fig. 2. It was consisted by the following systems: feeding system, pyrolysis system, cooling system, gas heating system, and gas purification system.
Raw material was fed by the feeding system. MSW was put into the hopper (1–3), then fed into the reactor (2–1) by a screw feeder (1–1). Feeding rate was controlled by electromotor and gearbox (1–2), and the maximum could reach 10 kg/h. A water cooling jacket (1–4) was used to protect the screw feeder from overheat.
Pyrolysis reactions of MSW were undertaken in the pyrolysis system. Raw material got into the fluidized bed reactor (2–1, with 0.1 m in diameter and 4 m in height) and strongly mixed with the high-temperature bed material, along with the intense pyrolysis reactions undertaken in the reactor. Chars in the pyrolyzed volatiles were separated by two cyclone separators (2–2 and 2–3) and collected by char collectors (2–4 and 2–5). Nitrogen was adopted as fluidizing agent after heated by the gas preheater (2–7), and led into the reactor through the gas compartment (2–6).
The heat volatiles were cooled down in three condensers. The first two condensers (3–1 and 3–2) were designed as direct cooling. Condensable compounds in these condensers were mixed with the cooling medium (ethanol) and cooled by flowing water in the cooling coil at the bottom of the condensers. Cooled liquid in the bottom was sucked up by the circulating pump (3–4 and 3–5) and injected back again to the condenser through four nozzles. Three of the nozzles were arranged in the lid of condenser, distributed evenly along the circumference, while the fourth one was equipped above the gas inlet. The third condenser (3–3) was designed for indirect-cooling. Gas flew through the spiral pipe and cooled by ice-water bath outside the pipe.
Energy required for pyrolysis was provided by hot flue gas in a heating jacket (4–4) outside the fluidized bed reactor. The hot flue gas was supplied by combustion of diesel in the combustor (4–3). Non-condensable gases were discharged after purified by a cyclone separator (5–2) and an activated carbon tank (5–3). An induced draft fan (5–4) was used to keep micro-negative pressure in the pyrolysis reactor (2–1).
Yield calculation methods
Pyrolysis of MSW was undertaken at the temperature of 450, 550, and 650 °C with bed material of QS. For bed material of HAB and ATT, pyrolysis temperature was set as 550 °C. Pyrolysis conditions were represented in the form of “bed material—pyrolytic temperature.” For example, the pyrolysis condition at 450 °C and with QS as bed material was marked as QS-450. Under each pyrolysis condition, raw material was continuously fed into the reactor for 2 h at the feeding rate of 5 kg/h.
In this work, ethanol was used as cooling medium in condenser 1 and condenser 2. Liquid products from three condensers (pyrolytic oils mixed with ethanol) were sampled and evaporated at 30 °C and 15 kPa (absolute pressure) for 4 h in a rotary evaporator. The acquired liquid product was considered as pyrolytic oils. Pyrolytic oil from condenser 1 separated into two layers. The appearance of upper layer was yellow brown and transparent, with good flowability. It was regarded as water-soluble phase, including water-soluble organics and moisture. The lower layer was highly viscous and dark-brown colored. Moreover, pyrolytic oils of condenser 2 and condenser 3 had similar characteristics with the lower layer of condenser 1. Together, the three kinds of oils were considered as organic phases. Afterwards, the yield of water-soluble organics, moisture, and organic phases was determined respectively.
Pyrolytic chars were consisted of the following parts: gathered in two char collectors, remained in the bed material and mixed with liquid products. All of them were collected, dried and weighed separately, and their mass was added up. Thereafter, the yield of chars was obtained.
Non-condensable gases (NCG) were measured online by gas analyzer. Since the flow rate of fluidizing gas was fixed to a certain value, the mass flow of pyrolytic gases could be calculated based on the percentage of each compounds. Consequently, the yield of NCG was acquired.
Physical and chemical analysis methods of liquid and char products
A series of physical properties of pyrolytic oils were tested to evaluate their qualities. Moisture content was measured using a Karl Fischer titrator (ZSD-2, Shanghai Anting Electronic Instrument). Viscosity was tested in 50 °C water bath by the Pinkevitch viscometer (SYD-265B, Shanghai Changji Geological Instrument). Value of pH was determined in an automatic titrator (ZDJ-4A, INESA Scientific Instrument). An elemental analyzer (Elementar vario EL III) was also proposed to analyze the elemental compositions. HHV of pyrolytic oils was calculated theoretically based on their elemental composition.
Chemical composition of pyrolytic oils was detected by GC/MS (Agilent 7890A/5975C). The chromatographic separation was performed using a DB-5 ms capillary column (30 m × 0.25 mm × 0.25 μm). Helium (99.999 %) was used as the carrier gas, with a constant flow rate of 1 mL/min and a split ratio of 30:1. The oven temperature was programmed to increase from 40 °C (kept for 3 min) to 180 °C (kept for 2 min) at a heating rate of 5 °C/min, and continue increasing to 280 °C (kept for 2 min) at a heating rate of 10 °C/min. The GC/MS interface was held at 285 °C and the mass spectrometer was operated in EI mode at 70 eV. Mass spectra were obtained from m/z 35 to 550 amu. Chromatographic peaks were identified with reference to the NIST MS library.
Heavy metals in bio-oils (as well as raw material) were measured by the method proposed by Zhong et al. (2015). Samples were digested with a solution of HNO3, HClO4, and HF (3:2:1 by volume). The Zn and Pb concentrations were determined by flame atomic absorption spectrophotometry (SpectrAA 220FS, Varian, Palo Alto, CA). The Cd concentration was measured by a Varian SpectrAA 220Z spectrophotometer using a graphite furnace.
Elemental analysis of pyrolytic chars was conducted using the same analyzer as pyrolytic oils. HHV of pyrolytic char was also calculated based on elemental analysis. A Belsorp-Mini (MicrotracBEL Crop.) model surface analyzer was used to measure Brunauer-Emmet-Teller (BET) surface area of chars. Microstructure of pyrolytic chars was observed using a ultra Plus (Carl Zeiss Foundation) scanning electron microscope (SEM).
Results and discussion
Products distribution
Figure 3 expresses that the total mass balance of pyrolytic products exceeds around 90 %. Liquid products (including water-soluble organics, moisture, and organic phases) count for 38.4–56.5 wt% in all the products. The highest liquid yield appears at QS-450 condition. However, moisture dominates the content of liquid products (42.1 wt% in liquid products). When QS is used as bed material, yield of liquid products decreases obviously when temperature rises from 450 to 550 °C, and turns to increase slightly at 650 °C. When temperature reaches 650 °C, moisture dictates the liquid phases again (42.9 wt% in liquid products). At the temperature of 550 °C, organic phase (48.4 wt% in liquid products) is most abundant in the liquid product, while moisture and water-soluble organics share a similar ratio.
Both HAB-550 and ATT-550 conditions decrease the yield of liquid products comparing with QS-550 condition. HAB-550 condition increases the water-soluble organics and decreases the other two fractions of liquid products when comparing with QS-550 condition. Accordingly, HAB is proved to be a beneficial additive for producing valuable water-soluble organics. ATT-550 condition decreases all the fractions in liquid products, suggesting that ATT is not favorable for the preparation of liquid products.
Yield of chars decrease gradually from 24.5 to 17.1 wt% when temperature rises from 450 to 650 °C (QS as bed material). On the other hand, yield of NCG keeps ascending along with the increasing temperature. It indicates that the decarbonylation reactions are promoted by the increase of temperature, and the condensable compounds are transformed into NCG products. HAB-550 condition increases yield of both char and NCG slightly comparing with QS-550 condition. ATT-550 condition keeps yield of char unchanged while increases yield of NCG comparing with QS-550 condition, which means ATT accelerates the formation of NCG. A number of acidic sites and large surface area of ATT are responsible for this phenomenon.
Characterization of pyrolytic oils
Characterization of pyrolytic oils is listed in Table 3. Moisture counts for 39.4–57.3 wt% in the water-soluble phases, while only no more than 1.4 wt% in the organic phases. Moisture contents in the water-soluble phases decrease first, followed by an increase when temperature increases from 450 to 650 °C (QS as bed material). Both HAB-550 and ATT-550 conditions decrease the moisture content in the water-soluble phases when comparing with QS-550 condition, especially the former one. Moisture is formed in two ways. First, even dried in the oven, the feedstock absorbs moisture from the air during the feeding process. Second, dehydration reaction occurring during pyrolysis produces moisture. Since moisture from the first way varies little under different conditions, the second way governs the moisture contents in water-soluble phases. High moisture content in water-soluble phases leads to low viscosity and low HHV. Therefore, viscosities of water-soluble phases are more similar to water (0.55 mm2/s at 50 °C) and diesel oil (3–8 mm2/s at 20 °C) than to bio-oils (shown in Table 3). Viscosity reaches the maximum at QS-550 condition, and decreases when temperature increases or decreases. HHV of water-soluble phases is between 8.2 MJ/kg and 13.3 MJ/kg. If the moisture content is reduced to typical moisture content of bio-oils (15–30 wt%), the converted HHV of water-soluble phases will be 13.6–19.3 MJ/kg, which is close to HHV of bio-oils. Carbon content of water-soluble phases is extremely low, while hydrogen and oxygen contents are relatively high. The higher carbon contents in water-soluble phase at QS-550 and HAB-550 conditions are due to the higher content of aromatics and phenols, which is found in Fig. 4. Mole ratio of hydrogen and oxygen of water-soluble phases is between the range of 1.95–2.33, implying that most of hydrogen and oxygen come from moisture.
There is hardly any moisture content in organic phases, which is similar to the properties of crude oils. Viscosities of organic phases are basically in the range of bio-oils, and far less than that of crude oils. When QS is used as bed material, viscosity of organic phase increases as temperature rises from 450 to 550 °C, and decreases significantly at 650 °C. Both HAB-550 and ATT-550 conditions reduce viscosity of organic phases when compared with QS-550 condition, especially the former one. When QS is used as bed material, carbon content of organic phase increases along with the temperature rises. On the contrary, oxygen content decreases when temperature rises. As a result, HHV of organic phase increases as temperature gets higher. HHV of organic phases are far higher than that of bio-oils and reach 60–72 % of that of crude oils. It is proved that the organic phases have a great potential to be used as fuel oils. Both HAB-550 and ATT-550 increase HHV of organic phases compared with QS-550, due to the increase of carbon content and decrease of oxygen content.
The pH values of water-soluble phases and organic phases are within the pH range of bio-oils, and reach the lowest at QS-550 condition, while the highest one lies at QS-650 condition. The pH values are concerned with the contents of acidic and phenolic compounds in the pyrolytic oils, which will be discussed in the next part.
GC/MS analysis of pyrolytic oils
Compounds in the pyrolytic oils are quite complicated. Organics including acids, alcohols, aldehydes, aromatics, esters, ethers, furans, hydrocarbons, ketones, phenols, sugars, and so on have been detected in the water-soluble phases and organic phases by GC/MS. During the GC/MS test, all of the analysis conditions such as sample amount, sample dilution ratio, and chromatographic process are set to the same. Therefore, peak area (PA) of each compound could represent the quantity of products qualitatively. Meanwhile, relative peak area (RPA) expresses the ratio of one product among all the products.
Figures 4 and 5 show results from GC/MS analysis of water-soluble phases and organic phases, respectively. It is interesting to find that total PA of water-soluble phases generally shows an opposite rule to that in organic phases. In other words, if the total PA of water-soluble phase at one pyrolysis condition is higher than another pyrolysis condition, it will show opposite phenomenon for the organic phase. It indicates the delicate balance of products between the water-soluble phases and organic phases.
Main compounds of water-soluble phases include acids, carboxylics (including ketones and aldehydes), phenols, and sugars. These organics together make up more than 80 % among all the compounds. When QS is used as bed material, total PA of the products varies significantly with the raise of temperature. It reaches a maximum at 550 °C, and reduces when temperature rises or decreases. That means the temperature of 550 °C is beneficial for the production of water-soluble organics. Meanwhile, RPA of the main products (except others) keeps ascending slightly as temperature rises. PA of acids in the water-soluble phases becomes highest at 550 °C, which agrees with the lowest pH of water-soluble phases at 550 °C. PA of carboxylics increases apparently as temperature rises from 450 to 550 °C and decreases dramatically when temperature further rises to 650 °C. However, RPA of carboxylics keeps descending along with the increasing temperature. On the other hand, increasing temperature from 450 to 550 °C raises both PA and RPA of sugars, followed by the disappearance of sugars in the products at 650 °C. Carboxylics and sugars have been considered as the degradation products of cellulose fraction in biomass and papers. Their changes in yield indicate the interactions between cellulose fractions and plastics, especially at higher temperature (650 °C). When comparing with QS-550 condition, HAB-550 condition decreases both PA and RPA of acids, carboxylics, and sugars, while increases both PA and RPA of phenols. When bed material changes from QS to ATT, PA of acids decreases while RPA of acids increases. Both PA and RPA of carboxylics and sugars decrease. PA of phenols increases slightly while RPA of phenols increases obviously.
The organic phases are mainly composed of aromatics and phenols. Aromatics consist C8-C18 compounds, with molecular weight of 104–234 and the maximum benzene ring number of 2. They are formed mostly from the decomposition of plastics (Imani Moqadam et al. 2015). Besides, some of the sugars from the degradation of cellulose fraction are also converted to aromatics, especially at relatively high temperature (650 °C). That means the mixed pyrolysis of MSW could be an effective way to produce aromatics. When QS is used as bed material, PA of aromatics decreases as pyrolysis temperature rises from 450 to 550 °C, and increases dramatically as pyrolysis temperature continues rising to 650 °C. Accordingly, RPA of aromatics in the organic phases also shows a similar principle. Many disappeared compounds in the water-soluble phases are converted to aromatics and stratified in the organic phases. Increasing pyrolysis temperature from 450 to 550 °C reduces PA of phenols, and further increasing pyrolysis temperature to 650 °C leads to the increase of phenols. RPA of phenols in the organic phases keeps descending with the rising temperature. The increasing RPA of aromatics and decreasing RPA of phenols at higher temperature make the separation of these two compounds easier, which is beneficial for the further utilization of organic phases. When bed material QS is replaced by HAB, both PA and RPA of aromatics increase. On the other hand, PA of phenols increases while RPA of phenols decreases. It shows that HAB is effective for the production of aromatics, yet not that effective for the production of phenols. Comparing with QS-550 condition, ATT-550 condition increases both PA and RPA of aromatics and phenols. The larger BET surface area of ATT (as shown in Table 2) provides more sites for the reactions including cracking, aromatization, and depolymerization, generating more products like aromatics and phenols.
It is noted from Fig. 4 that most products in water-soluble phases (acids, carboxylics, phenols, sugars, etc.) are derived from the pyrolysis of biomass and papers. It has been widely reported that the main compounds in the MSW, meaning biomass and plastics, will influence each other during the mixed pyrolysis process. Biomass are lower in thermal stability than plastics; therefore, they start decomposing prior to plastics. Radicals from degradation of biomass initiate the depolymerization of plastics. When temperature is lower than 550 °C, the decomposition reactions of plastics, in particular the radical chain reactions and intramolecular hydrogen transfer reactions, are restrained by lignocellulosic chars (Jakab et al. 2001). Consequently, aromatic products in organic phases at QS-450 and QS-550 conditions are much less than that at QS-650 condition. Few works about the mixed pyrolysis of biomass and plastics at temperature higher than 600 °C have been reported, since the mixture has been decomposed thoroughly under 500 °C (Jakab et al. 2001; Onal et al. 2014). When temperature rises to 650 °C, depolymerization and aromatization reactions become active. Part of the aromatics in the organic phases derive from the depolymerization of plastics, while the others are formed by pyrolytic products of biomass through aromatization reactions. For example, sugars as the primary decomposed products of cellulose will be further degraded to acids, carboxylics, furans, and so on. Higher temperature promotes the degradation of sugars, leaving their low content in water-soluble phases at 650 °C. The resulting products react with high H/C ratio radicals (degraded from plastics, such as alkanes, dienes, and so on), generating aromatic compounds at higher temperature (650 °C). From the above discussion, it is indicated that the pyrolytic products of biomass are influenced by that of plastics instead of restrain the later ones at 650 °C. Therefore, PA of products from pyrolysis of biomass (meaning water-soluble phases) at QS-650 condition is much lower than that at QS-450 and QS-550 conditions. Phenols in the organic phases are restrained when temperature rises from 450 to 550 °C. It is indicated that the formation of phenols in organic phases is inhibited due to the increase of compounds in water-soluble phases.
PA of compounds from organic phases at HAB-550 and ATT-550 conditions is obviously higher comparing with QS-550 condition. From Table 2, we can see that the main compound in HAB is Al2O3. It is responsible to the acidity on the surface of HAB. Therefore, the acidity and relatively large surface area on the surface of HAB promote the aromatization reaction. As for ATT, it has both abundant Lewis acid and Brønsted acid sites, as well we the largest surface area among the three bed materials. As a result, the promotion of ATT on the yield of aromatics is proved to be most effective. Besides, both HAB and ATT increase PA of phenols, which means the degradation of lignin is also accelerated by HAB and ATT.
According to Chiaramonti et al. (2007), if moisture content is higher than 30 wt%, the liquid separates into two phases of differing properties. Specifically, the water-soluble phases consist mainly acids, carboxylics, phenols, and sugars, while the organic phases are composed of primarily aromatics and phenols. These features make further utilizing and processing of pyrolytic oils easier. For example, water-soluble organics are regarded as appropriate feedstocks for the production of chemicals and substituted fuel, and have been applied to produce food flavoring, road de-icers, and to generate hydrogen via steam reforming (Li et al. 2012). In addition, when used as substituted fuel, the undesired compounds such as moisture, acids, and carboxylics in the water-soluble phases could be upgraded by extraction, hydrogenation, esterification, etc. On the contrary, the organic phases contain many high HHV compounds, and could be used as substituted fuel for direct combustion or extracting aromatics and phenols.
Heavy metals in pyrolytic oils
The content of heavy metals in pyrolytic oils is listed in Table 4. From which we can see that the content of Cd and Pb in both water-soluble phases and organic phases is very low, which is coincided with the results reported by Raclavska et al. (2015). The low Cd content in raw material is responsible for the low content of Cd in pyrolytic oils. As for Pb, it is mostly concentrated in the solid products, according to Raclavska et al. (2015). Besides, the contents of Cd and Pb are basically higher in the organic phases than in the water-soluble phases under different pyrolytic conditions.
Zn content in pyrolytic oils is much higher than Cd and Pb contents. This is expected since the content of Zn in raw material is much higher than the other two heavy metals. As temperature rises from 450 °C to 650 °C (QS as bed material), Zn content in both water-soluble phases and organic phases decreases obviously. According to Abanades et al. (2002), Zn is mainly existing in the form of ZnAl2O4 (s), Zn2SiO4 (c), ZnCr2O4 (s), and ZnCl2 (g) in the products below 1000 K during the combustion process. When QS is used as bed material, as temperature gets higher from 450 to 650 °C, more Zn has been trapped in the bed material as the zinc silicate pattern. Therefore, less Zn gets into the pyrolytic oils as temperature increases. Compared with QS-550 condition, Zn content in water-soluble phases at HAB-550 and ATT-550 conditions is much lower. It is attributed to the presence of Al2O3 in these bed materials, promoting the formation of zinc aluminate, which fixes more Zn in the bed materials. As for organic phases, Zn content is much lower than water-soluble phases, since Zn prefers to dissolve in the water phases (Stigter et al. 2000).
Stigter et al. (2000) have summarized that Zn content of crude oils is between <0.01 and 62.80 mg/kg. Zn content of water-soluble phases at most conditions is within this range, except QS-450 and QS-550 conditions. However, since Zn prefers to dissolve in the water fraction, a part of Zn in water-soluble phases could be removed in the subsequent water removal process. On the other hand, Zn content in all the organic phases is within the same range of crude oils. Therefore, from the perspective of heavy metal, the organic phases are as safe as crude oils and could be used in a similar way.
Characterization of pyrolytic chars and NCG
Table 5 shows the characteristics of pyrolytic chars. When quartz sand is used as bed material, as temperature rises from 450 to 650 °C, ash content in pyrolytic chars increases while carbon content and hydrogen content decrease. It shows that as temperature gets higher, raw material is pyrolyzed more thoroughly. These changes cause the decrease of HHV along with increasing temperature. Surface area of pyrolytic chars increases monotonically from 2.20 to 5.53 m2/g with the increasing temperature. It coincides with the expectation that high temperature promotes the release of volatiles, and correspondingly enlarges the surface area of chars. Besides, the reaction between C and CO2 producing CO also makes more porous char.
When comparing with QS-550 condition, chars produced at HAB-550 and ATT-550 conditions are higher in ash content and lower in carbon content and hydrogen content. HAB performs more effectively on reducing hydrogen content than QS and ATT. ATT accelerates the formation of gas products, producing more porous char. It explains the highest surface area obtained when ATT is used as bed material.
Figure 6 shows the microstructures of the chars produced from pyrolysis of MSW at 550 °C with different bed materials. These chars have a distinct difference in their microstructures. The surface of char produced at QS-550 condition is covered by small particles, and some of them were even embedded into the surface of the char. These particles are supposed to be pyrolytic products with large molecular weight, such as lignin derived oligomers, oligosaccharides and long-chain hydrocarbons. Their presence increases the carbon content and hydrogen content in chars and therefore increases the HHV. Both chars conducted at HAB-550 and ATT-550 conditions attach less particles than char conducted at QS-550 condition. Correspondingly, their carbon and hydrogen content as well as HHV are lower than that of QS-550 condition. Pores of the char obtained at HAB-550 condition are larger but shallower than the char obtained at QS-550 condition. Surface of the char prepared at ATT-550 condition is rougher and with more small pores than the other two kinds of chars, which result in the highest BET surface area among all the chars.
Main compounds of NCG include CO, CO2, and CH4, with little fraction of H2, as shown in Fig. 7. For all the experimental conditions, CO and CO2 dominate the gas products, which is similar to the report of Xue et al. (2015). When QS is used as bed material, both CO and CO2 increase obviously when temperature rises from 450 to 550 °C, and the rate of increase slows down as temperature continues rising to 650 °C. Higher temperature promotes both decarbonylation and decarboxylation, generating more CO and CO2. CH4 is also promoted by the increasing temperature. Decomposition of both biomass fraction and plastic fraction in raw material contributes to the generation of CH4. Both CO and CO2 vary little as bed material changes from QS to HAB or ATT, whereas CH4 increases remarkably. It is proved that the demethylation reactions are facilitated by HAB and ATT, leading to the decrease of methyl-containing compounds.
Conclusions
Pyrolysis of mixed MSW has been carried out in a fluidized bed reactor. Liquid products count for 38.4–56.5 wt% in all the products and vary with the change of temperature and bed material. The quality of water-soluble phases is inferior to that of bio-oils, due to the high moisture content. However, the organic phases are proved to be with much higher quality than bio-oils and even are comparable to that of crude oils. GC/MS analysis reveals that the water-soluble phases are mainly composed of acids, carboxylics, phenols, and sugars, while organic phases contain mostly aromatics and phenols. In consequence, the pyrolytic oils separating into two layers makes them easier for upgrading and utilizing. Cd and Pb contents of both water-soluble phases and organic phases are quite low. Zn contents of organic phases are no more than that of crude oils. Zn contents in several water-soluble phases are higher than that of crude oils, and could be partially removed along with the moisture.
The increasing temperature decreases the yield of chars as well as increases that of NCG. The pyrolytic chars show the properties of low carbon content and high ash content, leading to the relatively low HHV. The NCG are mainly consisted of CO and CO2, which are difficult to exploit.
In conclusion, pyrolytic oils from pyrolysis of mixed MSW are of high-quality and could be exploited in different ways. For instance, water-soluble phases have the potential to be used as chemical feedstock and substituted fuel. As for organic phases, they are as safe as crude oil and can be used as furnace oil or refined to obtain valuable industrial chemicals including aromatics and phenols. Therefore, this paper proves that it is possible and potential to pyrolyze MSW for producing valuable pyrolytic oils. Moreover, some fundamental references have been provided with regard to value-added and environmentally friendly utilization way of MSW materials.
References
Abanades S, Flamant G, Gagnepain B, Gauthier D (2002) Fate of heavy metals during municipal solid waste incineration. Waste Manage Res 20:55–68
Alper K, Tekin K, Karagöz S (2015) Pyrolysis of agricultural residues for bio-oil production. Clean Technol Environ Policy 17:211–223
Bhattacharya P, Steele PH, Hassan EBM, Mitchell B, Ingram L, Pittman CU Jr (2009) Wood/plastic copyrolysis in an auger reactor: chemical and physical analysis of the products. Fuel 88:1251–1260
Brebu M, Ucar S, Vasile C, Yanik J (2010) Co-pyrolysis of pine cone with synthetic polymers. Fuel 89:1911–1918
Chen DZ, Yin LJ, Wang H, He PJ (2014) Pyrolysis technologies for municipal solid waste: a review. Waste Manage 34:2466–2486
Chiaramonti D, Oasmaa A, Solantausta Y (2007) Power generation using fast pyrolysis liquids from biomass. Renew Sustain Energy Rev 11:1056–1086
Demirbaş A (1997) Calculation of higher heating values of biomass fuels. Fuel 76:431–434
Imani Moqadam S, Mirdrikvand M, Roozbehani B, Kharaghani A, Shishehsaz M (2015) Polystyrene pyrolysis using silica-alumina catalyst in fluidized bed reactor. Clean Technol Environ Policy 17:1847–1860
Jakab E, Blazsó M, Faix O (2001) Thermal decomposition of mixtures of vinyl polymers and lignocellulosic materials. J Anal Appl Pyrolysis 58–59:49–62
Li R, Zhong ZP, Jin BS, Zheng AJ (2012) Application of mineral bed materials during fast pyrolysis of rice husk to improve water-soluble organics production. Bioresour Technol 119:324–330
Martinez JD et al (2014) Co-pyrolysis of biomass with waste tyres: upgrading of liquid bio-fuel. Fuel Process Technol 119:263–271
MEP (2014) The 2014 national large and medium cities annual report on prevention and control of environmental pollution by solid waste. Ministry of Environmental Protection of the People’s Republic of China
Miskolczi N (2013) Co-pyrolysis of petroleum based waste HDPE, poly-lactic-acid biopolymer and organic waste. J Ind Eng Chem 19:1549–1559
Onal E, Uzun BB, Putun AE (2014) Bio-oil production via co-pyrolysis of almond shell as biomass and high density polyethylene. Energy Convers Manag 78:704–710
Paradela F, Pinto F, Gulyurtlu I, Cabrita I, Lapa N (2009a) Study of the co-pyrolysis of biomass and plastic wastes. Clean Technol Environ Policy 11:115–122
Paradela F, Pinto F, Ramos AM, Gulyurtlu I, Cabrita I (2009b) Study of the slow batch pyrolysis of mixtures of plastics, tyres and forestry biomass wastes. J Anal Appl Pyrolysis 85:392–398
Raclavska H, Corsaro A, Hlavsova A, Juchelkova D, Zajonc O (2015) The effect of moisture on the release and enrichment of heavy metals during pyrolysis of municipal solid waste. Waste Manage Res 33:267–274
Stigter JB, de Haan HPM, Guicherit R, Dekkers CPA, Daane ML (2000) Determination of cadmium, zinc, copper, chromium and arsenic in crude oil cargoes. Environ Pollut 107:451–464
Xue Y, Zhou S, Brown RC, Kelkar A, Bai X (2015) Fast pyrolysis of biomass and waste plastic in a fluidized bed reactor. Fuel 156:40–46
Zhang H, He PJ, Shao LM (2008) Implication of heavy metals distribution for a municipal solid waste management system—a case study in Shanghai. Sci Total Environ 402:257–267
Zhang B, Zhong ZP, Ding K, Song ZW (2015a) Production of aromatic hydrocarbons from catalytic co-pyrolysis of biomass and high density polyethylene: analytical Py-GC/MS study. Fuel 139:622–628
Zhang HY, Xiao R, Nie JL, Jin BS, Shao SS, Xiao GM (2015b) Catalytic pyrolysis of black-liquor lignin by co-feeding with different plastics in a fluidized bed reactor. Bioresour Technol 192:68–74
Zhong DX, Zhong ZP, Wu LH, Xue H, Song ZW, Luo YM (2015) Thermal characteristics and fate of heavy metals during thermal treatment of Sedum plumbizincicola, a zinc and cadmium hyperaccumulator. Fuel Process Technol 131:125–132
Acknowledgments
This paper was sponsored by the National Natural Science Fund Program of China (51276040) and the National Key Basic Research Program of China (973 Program, 2013CB228106).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, K., Zhong, Z., Zhong, D. et al. Pyrolysis of municipal solid waste in a fluidized bed for producing valuable pyrolytic oils. Clean Techn Environ Policy 18, 1111–1121 (2016). https://doi.org/10.1007/s10098-016-1102-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-016-1102-6