Abstract
The article describes the research and subsequent application of the electric coagulating agents and galvanic coagulating agents. These may be used in order to raise the standard of the treatment of wastewater containing oil substances. A method of desalination of natural waters and wastewater by means of a dialyzer with a multilayer liquid membrane was designed. Our research obtained by the new way of filtration of oil substances and suspended substances has been designed for an ultra-gentle treatment of water. In comparison with already familiar methods like ultra-filtration and reverse osmosis, this new way has certain advantages. The function of materials will be examined in this article. Based on the research, we have found that the main advantages of the method using the coagulating agent are treatment efficiency, simple installation, etc. Agents can be used to increase the level of treatment of wastewater containing crude oil substances. We found and have demonstrated that, compared to usual methods used in the industry, e.g. heating (evaporation), ion exchange and other methods of demineralization (desalination) of wastewater, the presented way has several advantages like decreasing energy losses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The main sources of water pollution are the extractive industry, piping systems for oil transportation, petrochemical plants, tankers, petrol stations, etc. It is well known that the most common sources of wastewater pollution are crude oil, mazut, petroleum, oils, etc (Kamenšcikov and Bogomolskij 2006; Achmedžanov et al. 2011).
Oil substances can be emulsified, soluble or creating a surface layer in the water. The environmental protection from oil is one of the present day priorities. Measurements aimed at the removal of oil and oil substances from water can save a certain number of natural resources and keep water areas and reservoirs clean. Researchers have shown that wastewater can contain various chemical substances (tetraethyllead, phenols, etc.). It has been found that the amount of oil that gets into the water increases with the time of its exposure. For example, if the time of exposure increases from 2 to 120 h, the concentration of oil in the water increases from 0.2 to 0.8 mg/l for crude oil, and from 1.4 to 11.9 mg/l for petrol A76 (Stachov 1983).
Treatment of wastewater containing oil substances has to provide
-
maximum extraction of oil substances from water,
-
further utilization of the treated water in industrial processes and
-
minimum discharge of wastewater into watercourses.
There are three types of plants available for wastewater treatment: general, local and urban ones. Treatment by means of a general plant is designed for an overall wastewater treatment in a factory/company (grit chambers, oil traps, settling tanks, flotation units and filtration units, settling ponds) (Commission Directive 2009/90/EC and 2000/60/EC).
Local treatment plants are designed for wastewater disposal directly in technological works. Coagulation, flocculation, sedimentation, filtration, ultra-filtration, flotation, ion exchange and reverse osmosis are used for this.
Filtration is a process of removing particulate matter from water by forcing the water through a porous media. This porous media can be natural, in the case of sand, gravel and clay, or it can be a membrane wall made of various materials. Sometimes, large particles are settled before filtration; this is called sedimentation.
Ultra-filtration is a type of membrane filtration in which hydrostatic pressure forces a liquid against a semi permeable membrane. A semipermeable membrane is a thin layer of material capable of separating substances when a driving force is applied across the membrane. An ultra-filtration filter has a pore size around 0.01 μ. Once considered a viable technology only for desalination, membrane processes are increasingly employed for removal of bacteria and other microorganisms, particulate material, and natural organic material, which can impart colour, tastes, and odours to the water and react with disinfectants to form disinfection by products. As advancements are made in membrane production and module design, capital and operating costs continue to decline (Zeman and Zydney 1996).
Osmosis is the spontaneous net movement of solvent molecules through a semipermeable membrane into a region of higher solute concentration, in the direction that tends to equalize the solute concentrations on the two sides.
Reverse osmosis is a highly efficient technique for dewatering process streams, concentrating/separating low-molecular-weight substances in solution, or cleaning wastewater. It has the ability to concentrate all dissolved and suspended solids. The permeate contains a very low concentration of dissolved solids. Reverse osmosis is typically used for the desalination of seawater. Reverse osmosis filters have a pore size around 0.0001 μ. After water passes through a reverse osmosis filter, it is essentially pure water. In addition to removing all organic molecules and viruses, reverse osmosis also removes most minerals that are present in the water. Reverse osmosis removes monovalent ions, which means that it desalinates the water (Staff 2011).
Mechanical, physical–chemical, chemical and biological methods are used for wastewater treatment and the removal of oil substances from the wastewater. The mechanical methods, which can be often used in practice, include sedimentation, centrifugation and filtration, the physical–chemical ones include flotation, coalescence and sorption, and the chemical ones include chlorination, ozonization, etc. Nowadays, the most frequent methods are those of the physical–chemical treatment of wastewater, based on the change of the physical condition of the impurities, which usually require application of reagents. This also includes occurrence of problems with utilization of waste materials, dregs, and it is also required to replace the production of non-renewable resources (lime, mineral salts) and the production of reagents (range of acids, alkali, ferrous salts, and aluminium). Theoretical calculations have shown that the potential possibility of electrochemical treatment of water is hundred times greater than sorption, as far the economy, speed and quality are concerned (Kamenšcikov and Bogomolskij 2006).
A collecting system represents a form of containment of urban waste water—that is, a means of separating it from the natural environment until it reaches the connection point to the treatment plant. It could therefore be argued that to qualify as appropriate, a system must ensure that urban waste water is similarly contained and separated from the surrounding environment. The Directive regulates the main conventional pollutants in treated wastewater discharges from treated plants. These are total suspended solids, chemical oxygen demand, biochemical oxygen demand, total nitrogen and total phosphorus. However, other parameters shall also be considered especially when making the assessment of receiving waters to designate sensitive areas and to achieve water quality objectives of water bodies. Treated urban wastewater is reused to ensure that there will be no adverse effect to the environment (Quevauviller et al. 2006).
Research objective and methods
Electrical coagulation
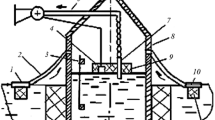
The objective of this paper is the elaboration of new technologies and plants for the treatment of wastewater containing oil substances. The essence of wastewater treatment by electro coagulation lies in its processing by electric current, using metal electrodes exposed to electrolytic solution. Under the direct current, the anode material gets into the treated water in the form of ions which hydrolyse quickly, creating poorly soluble aluminium and ferric hydroxides. Hydroxides are to be found in the water at a dispersed state which has a considerable sorption activity. Disperse hydroxides, coagulated into flakes, catch ions and molecules of soluble impurities, as well as various microparticles which are found in the water in a suspended or emulsified state. The main fields of the utilization of electrocoagulation are removal of ions of non-ferrous metals, especially ions of hexavalent chromium, iron, silicon, fluorine, arsenic, as well as oils, oil products, etc., from the wastewater. By the traditional scheme of wastewater treatment, electric coagulating agents are built into an existing treatment system and contribute to a higher standard of the treatment of wastewater containing oil substances. Figure 1 shows a scheme of an electric coagulating agent (Staršich, No. 2013125187/03).
Scheme of an electric coagulating agent: 1 case, 2 tank of plan parallel electrodes, 3 positive and negative current supply, 4 input connector, 5 fluid output connector, 6 upper attachment, 7 lower attachment, 8 separator, 9 ejector, 10 centrifugal pump, 11 upper pipe socket, 12 piping, 13 absorption pump socket, 14 pump discharge outlet, 15 ejector nozzle, 16 absorption ejector socket, 17 separator socket, 18 ejector diffuser, 19 piping, 20 piping, 21 lower pipe socket of the separator
The electric coagulating agent works as follows (Fig. 1 ) The initial fluid enters the inlet 4 through the input connector into the case, and proceeds from top between the plan parallel electrodes, which are concentrated into the tank 2. The electric circuit is switched on and the direct current enters the plan parallel electrodes through the positive and negative current supplies 3. The wastewater treatment takes place, flowing out from the tank through the fluid output connector 5. At the same time, also fouling of the inter-electrode space by particles of mechanical impurities takes place.
The input connector no. 4 and fluid output connector no. 5 overlap to clean the inter-electrode space; valves on pipes 19 and 20 are opened and the dregs from the silica sand with particles sized 2–5 mm, which present 25 % ratio of the separator’s volume 8. The remaining volume of the separator is filled with water. When the electromotor is switched on, the centrifugal pump 10 starts to operate and dregs occur. The dregs are directed through the piping 19 at the speed of 7–8 m/s into the lower attachment 7 and then into the plan parallel electrode tank, cleaning the surface of the electrode intensively at the same time. Then the dregs from mechanical impurities continue into the upper attachment 6, and through the piping 20 into the lower pipe socket of the separator 21. The process of the cleaning of the inter-electrode space is cyclical.
Separation of the sand from the water takes place in the separator: sand in the form of dregs is slowly absorbed by the ejector and water which is purified from the sand is sucked in by the pump. The pump stops after the electromotor is switched off, the sand in the separator settles to its bottom and the water will rise above it. The inter-electrode spaces are cleaned every 8 h and it lasts about 20–30 min.
Power consumption by the current intensity of 15 A and voltage of 10 V equals 0.15 kW for 1 m3 of water. The lifespan of the tank of the two plan parallel electrodes is about two months without cleaning.
The wastewater of the Yaya Oil Refinery (Kemerovskaya region) contains (data collected before applying the agents treatment) oil products 25–100 mg/l, suspended substances 200 mg/l, sulphates 500 mg/l, chlorates 300 mg/l and water hardness 5 mg/l. Table 1 shows the indicators after applying agents of wastewater treatment by means of flocculation agents and electric coagulating agents of the Yaya Oil Refinery.
Analysis of the data presented in Table 1 has shown that wastewater treatment by means of electric coagulating agents is the most effective. The main advantages of the method using electric coagulating agents are efficiency of the treatment, simple installation, etc. A disadvantage of the electric coagulating agents is an increased consumption of power and metal for the electrodes.
The advantages of electric coagulating agent are as follows:
-
Mechanical filtration addresses only two issues in wash rack wash water: suspended solids larger than 30 µm, and free oil and grease. Emulsified oil and grease cause damage to the media filters, resulting in very high maintenance costs. Electrocoagulation addresses any size of suspended solids (including destructive >30 µm particles and heavy metals that can wear and tear pressure washers and pose an environmental and employee hazard).
-
Chemical treatment addresses suspended solids, oil and grease, and some heavy metals—but may require up to three polymers and multiple pH adjustments for proper treatment. This technology requires the addition of chemicals resulting in expensive, messy and labour-intensive treatment. This process also requires addition of compressed air for floatation of coagulated contaminants. Generally filtration is also required as a post-treatment phase for polishing. Electrocoagulation requires no filters, no daily maintenance and no additives and removes any size of suspended solids, oil, grease and heavy metals.
Galvanic coagulation
An alternative to electrocoagulation is the galvanic coagulation. The process of the treatment of industrial wastewater and purification from oils and oil substances by galvanic coagulation lies in the activity of a mixture of components of a galvanic couple connected for a short time with various electrochemical potentials of the anode–cathode (iron–coke, iron–copper) type in an aqueous environment of wastewater. At the same time, an electrochemical reaction takes place, a result of which is the formation of ions of the two-valence Fe2+ and the three-valence Fe3+. Sediment is created in the process of sedimentation of iron hydroxide Fe3+. As for the ions of the three-valence Fe3+, they are reduced into a compound of a three-valence iron of FeCl3 type by the contact with a component of the iron mixture. The compound of FeCl3 ferric chloride is a coagulating agent, helping the coagulation of oils, suspended particles into compact aggregates, which float to the surface of wastewater or fall down like sediment.
The surface regeneration of the components mixture of the galvanic couple connected for a short time is affected by ultrasound waves with the frequency of 1–5 kHz and its anode component is filled with small, flat or cylindrical steel springs. Due to the ultrasound waves affecting the mixture components of the galvanic couple connected for a short time, a continuous regeneration of the diffusion boundary layer on the surface of the components of the galvanic couple mixture occurs, intensifying the process of iron ions regeneration.
By the activity of the ultrasound waves on the anode component of the mixture, which is created by small flat or cylindrical springs, the last ones in the row are pressed and expanded and thus dispose of impurities caught on their surface. This is how the process of regeneration of components’ surface is accelerated.
Wastewater containing oil emulsion and cooling liquid has been subject to the treatment. Initially, the wastewater contains oil 1580 mg/l and suspended substances 1350 mg/l. It has been treated by a tower-like mechanism, which has been filled with a galvanic mixture of iron and copper chips with the dose rate 4:1. The pH of the wastewater was 4–4.5. Copper chips were only filled in a single application; the iron ones have been refilled repeatedly as needed. The terminal velocity of the wastewater flow through the mechanism was 5–7 m/h. The research has shown that the level of the treatment/purifying the water from oils is 96.0–98.0 % when the water is processed within 10–20 min.
The galvanic mixture in the mechanism has been processed with ultrasound waves of 1–5 kHz, produced by four radial emitters. A generator UZG-10-22 with the power output of 8 kW and a magnetostrictive converter PMS-6-22 with a radiation plane of 300 mm2 and intensity of about 0.15 W/mm2 has been used for ultrasound waves.
The second alternative includes an anode component of the mixture of the galvanic couple (iron) put into the mechanism in the form of flat springs with the spacing between the maxima 3–6 mm, as well as cylindrical springs with the diameter of 5 mm with the spacing between the threads from 1 to 5 mm. Figure 2 shows a scheme of the galvanic coagulating agent (Maximov, No. 20131203541/05).
The galvanic coagulating agent works as follows (Fig. 2 ) Wastewater containing emulsified oils and suspended substances enters the input socket and gets into the cylindrical case of the galvanic coagulating agent. Wastewater interacts with the mixture of components of the galvanic couple connected for a short time, secured between two cross partitions, and an electrochemical reaction takes place resulting into occurrence of ions of iron as well as oxide compounds which are a strong coagulant of impurities in the form of oil, mazut or oil products.
Floating oils and suspended substances rise into the upper part of the case and emerge through a socket which can be found between the case and the sediment emerges from the case through a socket in the lower part of the case.
Regeneration of the galvanic couple takes place by the start-up of the generator, waves of which get into the galvanic couple by means of radially distributed magnetostrictive converter, waveguides and the emitter.
The advantages of galvanic coagulating agent are as follows:
-
the current does not flow through the wastewater but instead the mechanical modulator is used with which the disadvantages of passing electric current through the wastewater are not present,
-
the main advantages of mechanical acting upon the wastewater rise from the fact that undesired gases are not created due to the impact of passing electric current (production of various unwanted chemical consistencies is absent),
-
the power of a magnetostrictive element can be regulated as necessary to achieve the required quality of water at the outlet.
Due to the ultrasound waves affecting the components of the mixture of the galvanic couple connected for a short time, a continuous renewal of the diffusion boundary layer on the surface of the mixture components takes place, which prevents them from being covered in oil and suspended substances, continuous renewing of the surface of the components of the galvanic couple, thus intensifying the process of generation of ions of iron. Table 2 shows the indicators of wastewater treatment.
Table 2 shows that the treatment efficiency is 86 % for oils and oil products, and 93 % for suspensions. Moreover, a decrease in salt content and water hardness as well as the option of extraction of heavy metal ions has been found. The mechanism shown in Fig. 3 can be used for the treatment of wastewater removing organic and inorganic substances in utility lines of companies in the petrochemical industry. By the flotation of particles of impurities or mineral particles, the process of formation of the complex bubble-particle takes place in three stages by the conventional way: an air bubble approaching particles in the liquid stage, contact of the bubble with the particle, after which they cling together or adsorption with the particle. Better conditions, so that they can cling together, have been observed by hydrophilic mineral particles or impurities with the dimensions of 0.1–1.0 mm. Small particles of impurities with the diameter below 5 µm will not cling to the air bubbles.
In the presented way, hydrated ions are put into certain volumes of treated wastewater with air bubbles with the formation of the complex hydrated ions–air bubble–particle of impurities, which float on the water surface (Maximov, No. 20121099957/05). The foam product is then removed from the de-sludged water.
A hydrated complex ion-bubble-particle with a positive charge, used by water treatment, is formed in two stages.
In the first stage, due to Coulomb forces of attraction between positively and negatively charged particles of the hydrated ions, an aggregate (hydrated ions-particle) is formed. This interaction between the negatively charged colloidal particles of impurities and positively charged hydrated ions leads to the neutralization of their charges and to the formation of bigger aggregates hydrated ions–particle. In the second stage, the aggregate (hydrated ions-particle) clings to the air bubble due to adhesion and a complex (hydrated ion-bubble-particle) is formed which floats on the water surface and forms a foam product.
Wastewater treatment takes place as follows (presented in Fig. 3 ) Wastewater containing impurities gets to case 1 through the input socket 2. At the same time, an air flow in the form of bubbles comes through the socket of the input 4. These bubbles float on water surface later. Ions then proceed to the tube 9 through the horizontal piping and later to the treated water in the bottom part of the case. As the hydrated ions have both a positive and a negative charge, neutral particles acquire their charge when coming into contact with them, i.e. they are ionized. In the lower part of the case separated from the upper part of the case by a membrane 7, a complex ions–bubble–particle of impurities is formed. Through the central hole 8, the complex rises to the upper part of the case on the membrane, where the foam product is formed. The treated water is let out through the socket 3, the foam product through the socket 5 and dregs through the socket 6.
The level of treatment—purification from suspended substances is up to 20 mg/l and from oil products up to 0.3 mg/l by this mechanism. The main volume of wastewater in oil factories comprises formation water, usually separated from oil by drainage mechanisms. Moreover, impure wastewater occurs by the washing of surfaces of oil wells and platforms by the loading of oil into tank wagons and also by the drilling of oil wells in the form of used solutions. Wastewater of oil companies is dirty from oil and has a high level of mineralization. The composition of the wastewater in oil companies can vary, depending on oil and formation water, as well on the technology of oil extraction. The main pollutants of this wastewater are crude oil products 40–45 g/l, suspended substances 5–20 g/l, iron salts up to 200 mg/l, and hydrogen sulphide up to 300 mg/l. The general salt content usually ranges from 10 to 200 g/l and more (Commission Directive 2009/90/EC and 2000/60/EC).
Wastewater in companies for crude oil extraction is usually used for irrigation of crude oil seams. Wastewater treatment is hereby reduced to their preparation. In this case, the following conditions are required:
-
suspended substances size below 0.05 mm (not over 10 mg/l),
-
oil substances (not over 25 mg/l),
-
three-valence iron (not over 0.50–2 mg/l).
In cases when one part of the wastewater of crude oil factories is released into water tanks, their deep purification—demineralization (desalination)—has to be set. Compared to usual methods used in the industry, e.g. heating (evaporation), ion exchange and other methods of demineralization (desalination) of wastewater, the presented way has an advantage that heating up and overheating of water is not required and other advantages.
Electrolytic desalination
Electrolysis is a process of separation of salt ions, carried out in a multi-chamber device—electrodialyzer under continuous electric current, flowing through membranes. The electrolytic method is based on the movement of the charged particles in the continuous electric field and it is used for the demineralization of wastewater of industrial companies.
The electrolytic process of desalination takes place as follows We will examine the desalination of wastewater on the example of NaCl water solution. If the chamber with the initial water of the case of the electrodialyzer is filled with the water solution of NaCl, which is an electrolyte and dissociates into ions when electric current passes through it, salt molecules will dissociate into positively charged Na+ ions and negatively charged Cl− ions. When the continuous electric current passes through the given solution, cations, i.e. positively charged Na+ ions, will move towards the cathode, and anions, i.e. negatively charged Cl− ions, towards the anode. By electrolysis of water solutions, hydrogen ions and hydroxide ions (OH) are formed besides salt ions due to dissociation of water. The formed ions of salts and water move towards relevant electrodes. Cations of salts and hydrogen approach the cathode and anions of salts and hydroxide ions approach the anode.
Water desalination in the electrodialyzer takes place as follows (presented in Fig. 4 ) Layers of the liquid multilayer membrane—glycerine, nitrobenzene, cleansing water, toluene and ethyl alcohol—are poured one after another into the case 1. As the densities of the layers of the anionic, cationic membrane and the initial water differ, they do not mix. The nominal thickness of membrane layers should be 40–45 mm by an average concentration of layers in the treated water of 20–30 mg/l.
Electrodialyzer: 1 case, 2 cathode, 3 anode, 4 anionic membrane made from layers of ethanol and toluene (no 1, no 2), 5 cationic membrane made from layers of nitrobenzene and glycerol (no 4, no 5), 6 initial feed channel, 7 dialysed outlet channel, 8 discharge channel of the anionic chamber, 9 discharge channel of the cationic chamber, 10 anionic chamber, 11 cationic chamber, 12 tank, 13 piping, 14 circulation pump
By a higher concentration of salts in the treated water than the average concentration, it is necessary to increase the thickness of all layers of the liquid membrane by about 10–15 %, directly proportionally to their concentration in the aqueous solution. If the concentration of salts in the treated water is below average, the thickness of all layers of the liquid membrane has to be decreased by about 10–15 % directly proportionally to their concentration in the aqueous solution.
Under the continuous electric current, cations in the chamber with the initial water approach to the negatively charged cathode, diffuse through the cationic membrane and get into the chamber 11 and anions, which move towards the positively charged anode, diffuse through the anionic membrane and get into the chamber 10. The concentrate from the chamber 10 is drained through the channel of the concentrate outlet 8 and from the chamber 11 through the outlet channel 9.
Desalinated water (dialysed) is drained from the case through the channel of the dialysed outlet 7. The change of the thickness of layers of the multilayer liquid membrane is achieved by the pumps 14. For example, if it is required to decrease the thickness of the first layer No 1 of the membrane, the first circulation pump 14 is started up, which pumps the liquid of the membrane of the piping 13 from case 1 over to one of the tanks 12. Analogically, the thickness of other membrane layers decreases.
If it is required to increase the thickness of the first membrane layer, the second circulation pump 14 is started up, which pumps the membrane liquid in the piping 13 from the tank 12 into the case. Analogically, the thickness of other layers decreases. On their way to the cathode, Na+ cations, kept in the chamber of the treated water, get through a layer of the cationic membrane of nitrobenzene and glyceride, which are to be found lower from the layer of the treated water. On their way to the anode, Cl− anions, kept in the chamber of the treated water, get through a layer of the anionic membrane of toluene and ethyl alcohol which are to be found above the layer of the treated water. The level of desalination by using the electrodialyzer by the final treatment of potable water is 98–99 %.
Electrical separation
The electric separator is one of mechanical methods of separation of liquids, especially of crude oil products and it can be used to separate non-polar liquids in the electric field in industrial companies processing crude oil. It is known that it is very time-consuming and difficult to acidify crude oil due to its constant structure. Crude oil contains impurities of mineral and organic origin. The mineral impurities include sand, clay particles, solutions of salts, as well as products of sulphur and solutions of waste alkaline cellulose by its technical processing. The organic impurities include phenols, fatty acids, etc.
In the electric separator (Maximov, RU 137475 U1), by the rotation of the anode in the form of a hollow tube with through holes, particles of mineral impurities are affected by the centripetal force, under which the particles get out of the mechanical impurities from the tube through the holes, and they are hit against the case walls and settle in the bottom part. Figure 5 shows a scheme of an electric separator.
The division of crude oil products in the electric separator takes place as follows (presented in Fig. 5 ) Crude oil products which contain impurities of mineral and organic origin get into the case 1 from the main pipe 8 through the input socket 2 and the hollow tube 7 and they fill the space between the anode and the cathode. By the start-up of the system under the current between the electrodes 5 and 6, the permanent electric field is generated. Under the electric field forces, the particles of impurities settle on electrodes 5 and 6. Particles of organic impurities settled on the electrodes fall to the bottom part of the case and are let out through the outlet socket for impurities 4. At the same time, the electromotor 9 is started up and rotation is transferred to the tube 7 through the toothed couple 10 and the tube also functions as an anode.
There are various filtrating materials known and used for the treatment of wastewaters containing crude oil products: rice hulls, wood shavings and ash (Vasiljev et al., RU 2311220 C1). Filtrating material for industrial wastewater treatment and purification from crude oil products contains a component of vegetable origin and material containing coal in the form of ash from combusting this component, soaked with crude products. At the same time, a provisionally processed 5–15 % solution of ferric chloride by the ratio of components and ash equalling 1: (0.1–0.2) is put into this vegetable component. By using this vegetable component, provisionally processed by a 5–15 % solution of ferric chloride, iron hydroxides are formed in the filtrating material, which induce a coagulating activity on emulgated crude oil products, which increases the sorption capacity of the filtrating material. Moreover, iron ions disinfect the microflora, which reduces the colouring of the treated water (Vasiljev et al., RU 2311220 C1).
The treatment of waters containing crude oil products takes place as follows Four glass filters in the form of columns with the diameter of 30 mm and height of 850 mm were filled with the filtrating material to the height of 730 mm, and at the same time, wastewater was supplied to all columns, containing 30 mg/l of emulsified crude oil products with the speed of filtration of 3 mg/l (Table 3).
Table 3 shows that the efficiency of the treatment/purification of wastewater from crude oil substances by means of the filtrating material represents 99.1–99.3 %, 130–145 grades. For the treatment of wastewater under industrial conditions, the filtrating material is to be found in a net-like container with the dimensions of 1200 × 1500 × 800 mm and meshes of 150–150 mm. The density under which the container was filled with the filtrating material was 100–120 kg/m3. Modified filtrating material can be not only used for the purification of wastewater from crude oil substances, but also for the extraction of ions of heavy metals from water (iron, copper, zinc, nickel, cobalt, etc.). Other materials of vegetable origin can be used instead of straw: weed, reed, hay, needles, leaves, as well as their organic component.
It is recommended to use a mixture of wood shavings, sawdust and sulphur coal in the ratio of 2:1 in the filtrating material (Vasiljev et al. SU 1421373 A1) for wastewater treatment. In comparison with the traditional filtrating material (mixture of straw and ash), the new material allows to increase the duration of the filtrating cycle 1.75–2.1 times, to increase the efficiency of water purification from crude oil products by 3.9–5.0 %.
The comparison of electrical coagulation with other methods based on the principle of mechanical filtration is as follows:
-
higher efficiency in terms of temporal dependency on the volume of filtered water,
-
mechanical water purification is much simpler,
-
secured cleaning without defects caused by clogging,
-
better management and control of cleaning in terms of electronic security and management,
-
substantially lower level of concentrations of toxic substances (up to 23 %),
-
better resistance of treatment system for the distribution of the impurities in the wastewater.
These results are based on the work of experimental research conducted in real treatment plant. Major improvements were detected particularly in the cleaning process, in terms of quality of treated water, technical manipulation in the cleaning process, technical equipment of the treatment system, manipulation of wastewater transportation to the treatment plant and the outlet of treated water from the treatment plant. Also the higher treatment system components’ service life was observed which lead even to a multiple increase in the volume of the output flow at some of the treatment plants. Reasons for the increased service life of components are based on the principle of cleaning and are also the result of a smaller concentration of aggressive substances at the plant output.
Conclusions
-
(1)
It is pointed out that electric coagulating agents and galvanic coagulating agents can be used to increase the level of treatment of wastewater containing crude oil substances, having certain advantages in comparison with traditional flocculants.
-
(2)
Researchers have shown that electric energy consumption by the current of 15 A and voltage of 10 V equals 0.15 kW on 1 m3 of water. The lifespan of the case of two plan parallels electrodes is 2 months. The wastewater of the Yaya Oil Refinery (Kemerovskaya region) contains oil products 25–100 mg/l, suspended substances 200 mg/l, sulphates 500 mg/l, chlorates 300 mg/l and water hardness 5 mg/l. Wastewater treatment with the help of Yaya Oil Refinery by the electric coagulating agent shows that the level of treatment/purification from crude oil products is 80 % (15–20 mg/l). The main advantages of the method using the electric coagulating agent are treatment efficiency, higher efficiency in terms of temporal dependency on the volume of filtered water, simple installation and better resistance of treatment system for the distribution of the impurities in the wastewater. A disadvantage of the coagulating agent is the increased consumption of electric energy and metal for electrodes.
-
(3)
A way of desalination of natural waters and wastewaters using an electrodialyzer with a multilayer liquid membrane has been presented. Compared to usual methods used in the industry, e.g. heating (evaporation), ion exchange and other methods of demineralization (desalination) of wastewater, the presented way has an advantage in decreasing energy losses and others as in stated study (Voutchkov 2011).
-
(4)
Filtrating material for the purification of industrial wastewaters from crude oil products contains a component of vegetable origin and material containing coal in the form of ash from combustion of this component, soaked up with crude oil products. By using this vegetable component, provisionally processed by a 5–15 % solution of ferric chloride, iron hydroxides are formed in the filtrating material, which induces the coagulating activity on emulsified crude oil products, which increases the sorption capacity of the filtrating material. Moreover, iron ions disinfect the microflora, which reduces the colouring of the treated water (Vasiljev et al. RU 2311220 C1).
-
(5)
It is recommended to use (Vasiljev et al. SU 1421373 A1) a mixture of wood shavings, sawdust and sulphur coal in the ratio of 2:1 in the filtrating material for wastewater treatment. In comparison to traditional filtrating material (mixture of straw and ash), the new material allows increasing the time of the filtrating cycle 1.75–2.1 times and increasing efficiency of the purification of water from crude oil products by 3.9–5.0 %.
References
Achmedžanov TK, Achtanov EK, Nubajeva BM (2011) Ways of improvement of pipelines//materials from the International scientific-technical conference. In: Oil and gas in the West Siberia dedicated to the 55. Anniversary of the Tyumen State Oil and Gas University, Tyumen, p 244
Borges ME, Hernández T, Esparza P (2014) Photocatalysis as a potential tertiary treatment of urban wastewater: new photocatalytic materials. Clean Technol Environ Policy 16:431
Commission Directive 2000/60/EC of 23 October 2000 of the European Parliament and of the Council, framework for Community action in the field of water policy
Commission Directive 2009/90/EC of 31 July 2009 laying down, pursuant to Directive 2000/60/EC of the European Parliament and of the Council, technical specifications for chemical analysis and monitoring of water status
Crispim A, Sampaio A, Ramalho E, Ramos L, Caetano NS, Silva PC, Fernandes R (2010) Biodiesel from fleshings. J Soc Leather Technol Chem 94:39
Doraisamy P, Nandakumar NB, Maheswari M, Selvamurugan M (2013) Comparative performance of anaerobic reactors for treatment of sago industry wastewater. Clean Technol Environ Policy 15:391
Fathima NN, Aravindhan R, Rao JR, Nair BU (2011) Stabilized protein waste as a source for removal of color from wastewaters. J Appl Polym Sci 120:1397
Gaidau C, Niculescu M, Stepan E, Taloi D, Filipescu L (2009) Additives and advanced biomaterials obtained from leather industry by-products. Rev Chim 60:501
Kalyanaraman C, Sri Bala Kameswari K, Sudharsan Varma V, Tagra S, Raghava Rao J (2013) Studies on biodegradation of vegetable-based fat liquor-containing wastewater from tanneries. Clean Technol Environ Policy 15:633
Kamenščikov FA, Bogomoľskij EI (2006) Removal of crude oil substances from the surface of water and ground. Moscow, p 521
Kameswari KSB, Kalyanaraman C, Thanasekaran K (2014) Evaluation of various pre-treatment processes on tannery sludge for enhancement of soluble chemical oxygen demand. Clean Technol Environ Policy 16:369
Kanimozhi R, Vasudevan N (2014) Effect of organic loading rate on the performance of aerobic SBR treating anaerobically digested distillery wastewater. Clean Technol Environ Policy 16:467
Maximov EA Electric separator, RU 137475 U1
Maximov EA Electrodialyzer with a multilayer liquid membrane (positive solution No. 2012139933/05)
Maximov EA Mechanism for filtration of suspension solutions (positive solution No. 2013131606)
Maximov, E.A. Ways and mechanism of industrial wastewater treatment (positive solution No. 20131203541/05
Maximov EA Ways of purification of natural waters and wastewater (positive solution No 20121099957/05)
Mukherjee R, Sengupta D, Sikdar SK (2013) Parsimonious use of indicators for evaluating sustainability systems with multivariate statistical analyses. Clean Technol Environ Policy 15:699
Pintor AMA, Vilar VJP, Botelho CMS, Boaventura RAR (2014) Optimization of a primary gravity separation treatment for vegetable oil refinery wastewaters. Clean Technol Environ Policy 16:1725
Quevauviller P, Thomas O, Beken AV (2006) Wastewater quality monitoring and treatment., Water quality measurements seriesWiley, Hoboken 7
Sekaran G, Karthikeyan S, Evvie C, Boopathy R, Maharaja P (2013) Oxidation of refractory organics by heterogeneous fenton to reduce organic load in tannery wastewater. Clean Technol Environ Policy 15:245
Shaffei KA, Moustafa AB, Mohamed WS (2008) Grafting emulsion polymerization of glycidyl methacrylate onto leather by chemical initiation systems. J Appl Polym Sci 109:3923
Sikdar SK (2012) Measuring sustainability. Clean Technol Environ Policy 14:153
Sikdar SK (2013) Resurgence of fossil fuels? Clean Technol Environ Policy 15:203
Sikdar SK, Sengupta D, Harten P (2012) More on aggregating multiple indicators into a single index for sustainability analyses. Clean Technol Environ Policy 14:765
Song J, Tao WY, Chen WY (2008) Ultrasound-accelerated enzymatic hydrolysis of solid leather waste. J Clean Prod 16:591
Stachov EA (1983) Purification of wastewater containing oil substances; protection and transport of oil substances. Leningrad, p 234
Staff A (2011) Reverse osmosis and nanofiltration. American Water Works Association, Denver, p 226
Staršich VV, Maximov EA Positive solution No. 2013125187/03
Vasiljev VI, Dolotov AI, Kazilov PV, Cipačeva MA Filtrating material, SU 1421373 A1
Vasiljev VI, Kazilov PV, Vološčuk EA Filtrating material for industrial wastewater treatment, RU 2311220 C1
Voutchkov N (2011) Desalination plant concentrate management. Water Treatment Academy Publications, Bangkok, p 48
Wang XC, Ren LF, Qiang TT (2009) Novel way of transformation of tannery waste to environmentally friendly formaldehyde scavenger. Environ Prog Sustain Energy 28:285
Zeman LJ, Zydney AL (1996) Microfiltration and ultrafiltration: principles and applications. CRC Press, Boca Raton, p 642
Acknowledgments

Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maksimov, E.A., Kreheľ, R. & Pollák, M. Prospective systems and technologies for the treatment of wastewater containing oil substances. Clean Techn Environ Policy 18, 161–170 (2016). https://doi.org/10.1007/s10098-015-1003-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-015-1003-0