Abstract
Plastics have become indispensable materials in the world. They are non-biodegradable polymers of mostly containing carbon, hydrogen. Due to their non-biodegradability and low life, HDPE, LDPE, and PP contribute significantly to the problem of Municipal Waste Management. Thermal and Catalytic converting of these materials into the valuable liquids like gasoline and diesel would be a promising method of waste management. Current paper focuses on catalytic cracking of the mixed polyethylene and Polypropylene in the presence of silica alumina catalyst in a semi batch reactor operating isothermally at ambient pressure with a statistical approach. The parameters affecting degradation of polymer mixture studied in this paper include the temperature (410–450 °C), catalyst (10–50 wt%), and feed composition (1–5). The statistical Taguchi experimental design method has been used to optimize the reaction condition in degradation process in order to maximize the gasoline production. The liquid and gas products were analyzed by GC/FID to find out their composition. Using more catalyst leads the reaction to produce more aromatic components. The result of experiments discussed in this work compared with empirical data shows that the use of Taguchi as a DOE method has an appropriate approach to the optimum condition. Arrhenius law as a kinetic model at optimum reaction condition has been developed and the activation energy determined. The model gives a suitable representation of the experimental results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Waste plastics cause a very serious environmental problem due to their non-biodegradability and disposal problems. Landfill is a way to disposal of the plastics, which is not recommended due to its extra economic and environmental problems. Incineration is the second way for waste polymers disposal in which the release of toxic gases into the atmosphere poses serious air pollution problems (Seddegi et al. 2002). Tertiary recycling, in which waste plastic is converted into useful chemicals, is recognized as the most promising recycling method. The most commonly used conservative chemical methods for waste polymer recycling are pyrolysis and catalytic degradation. Thermal and catalytic degradation of waste plastics are two types of chemical recycling processes. In these methods carbon–carbon chains at high temperatures (about 400–900 °C) crack into low molecular weight hydrocarbons. Thermal degradation of polymers into light components has a significant problem in which chain structures would break at high temperatures and more coke and gaseous are produced (Jozef and Miriam 2008). These problems reduce the effectiveness of the process due to high cost and low quality of the products. In addition, these processes require high temperatures typically more than 500 °C and even up to 900 °C. However, catalytic degradation occurs at low temperatures rather than the thermal process (Kumar et al. 2011; Akpanudoh et al. 2005). Moreover, adequate catalysts form hydrocarbons in the range of automobile fuel (Wei et al. 2010). Some researchers have done studies in order to find out the effects of various catalysts on the degradation of polymers (López et al. 2011; Zhou et al. 2004). Lin et al. found that FCC catalyst has a significant effect in the pyrolysis of thermoplastics (Lin and Yang 2007). Mordi et al. pyrolyzed a number of plastic types over a zeolite ZSM-5 catalyst and showed that the using more catalyst leads the reaction to produce more aromatic components (Mordi et al. 1994). Research results that expressed by Seddegi et al. show that the conversion of plastic waste into liquid fuel occurs at about 400–450 °C on a laboratory scale (Lin and Yang 2007). Elordi et al. studied several zeolite catalysts for cracking of HDPE. They indicated that the type of catalysts has a significant influence on yield and also the component distribution of products (Elordi et al. 2009). Marcilla et al. showed that Zeolitic catalysts promote the degradation process and are suitable to overcome the difficulties of thermal process (Marcilla et al. 2005). Appropriate catalysts are anticipated to control the product yield, hydrocarbon distribution, and reduce significantly the reaction temperature from polymer degradation (Lin and Yang 2007).
Moreover, Reaction kinetic has usually been calculated using the Arrhenius method. Encinar et al. worked on kinetics of the degradation of various polymers such as PS LDPE, ABS, PET, and PP (Encinar and González 2008). They have investigated different heating rates in order to find out reaction constant and activation energy under first order reaction approximation (Elordi et al. 2009). Salem et al. investigated the kinetics of degradation of HDPE at different temperatures. As a method of kinetic study, he suggested a first order calculation model based on the experiment. In his model, the kinetic was studied based on liquids, gaseous mixtures, waxes, aromatics, and char products under first order reaction approach (Al-Salem and Lettieri 2010).
The literature reveals that in the earlier studies no efforts have been given to evaluate the catalytic degradation of the polymers by performing experimental design method. However, the above review clearly shows that nearly all investigators have so far investigated various catalysts, kinetics, and the components of the products based on the experimental studies and GC analysis of the products. In particular, to the best of author’s knowledge, design of experiment as statistical methods seems to be non-existent. The primary purpose of the current work is to fill this gap. There are some DOE investigations on automotive and other chemical processes which could be suitable benchmarks for current study (Hossein et al. 2013).
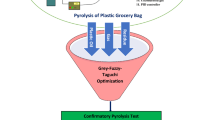
As a method of optimization, Taguchi proposed a statistical method which assists the researchers to achieve their target in a precise and short way. Taguchi method for product and process development and optimization focuses on three basic design consideration as system design, parameter design, and tolerance design. System design step refers to the consideration of the various materials, processes, and methods to achieve a goal. Mostly, researchers use scientific and engineering principles to determine the basic system configuration. In the parameter design step, specific values in a system will be determined. This step would help the scientists to archive nominal condition and parameters for specific targets. Usually, the objectives do specify these nominal parameter values such that the variability transmitted from uncontrollable or noise variables are minimized. Tolerance design uses to determine the best tolerances for the parameters of a process. Taguchi recommends that statistical experimental design methods to be employed to assist optimizations, particularly during parameter design. The key component of Taguchi’s philosophy is reduction of the variability around the target value. This paper focuses on parameter design to achieve the target of the study where it is to study the production of gasoline and diesel products from catalytic degradation of the mixed polymers (HDPE/LDPE/PP) by design of experiment method. A systematic plan of experiments has been performed on the basis of Taguchi experimental design. It is a simple, effective approach to investigate optimum condition to produce maximum yield of gasoline.
Materials and methods
Materials
Polymers
Polyethylene (HDPE, LDPE) and polypropylene (PP) plastic granule were obtained from Bandar imam petrochemical Ltd and maroon petrochemical Ltd, respectively. The properties of the polymers showed that LDPE and HDPE had 0.43 and 2.2 g/10 min and PP had 16 g/10 min melt flow index, respectively.
Catalyst
The catalyst was supplied at Abadan Petro Frayande Polymer Ltd. It is generally made up of five separate main components like, Zeolite (molecular sieve), Platinum, Active matrix component, Inactive matrix component, and Binder.
The inactive matrix component and binder control the overall activity of catalyst by diluting the high active components to the proper activity level and providing the proper particle strength and morphology.
The main components of the catalyst are shown in Table 1.
The catalyst consists of Lewis and Bronsted acid sites, which are significant factor in determining the catalytic activity and product selectivity. Because Bronsted acid sites play a proton addition role and Lewis acid sites involve hydride abstraction, cracking of the polymers occurs as easy as non-catalytic pyrolysis. The provided Lewis and Brønsted (proton donor) inside catalyst controlled acidity from its crystalline structure. Al content per unit cell or Si/Al ratio of catalyst has also a remarkable impact on the cracking reaction. The acid sites are prepared by Al species in the catalyst which consists of silica and alumina. High acid site density helps the cracking reaction of hydrocarbons.
GC analysis
The liquid and gas products derived from the degradation of the plastics were analyzed to determine the main consisting components. Each product was analyzed mainly by a capillary column gas chromatography with flame ionization detection (GC/FID). The GC/FID equipment used was an AGILENT, US 10420012, GC equipped with an HP- 5, capillary column (50 m × 25 μm ID, 0. 5 μm film of silica gel film). Helium was the carrier gas at a flow rate of 15.8 ml/min, and the temperature of the injector was 250 °C, at 10 psi constant pressure.
That was possible as the employed capillary column separated the components of the fractions according to their volatility/boiling point. A calibration mixture containing normal alkanes and alkenes, Methane to undecane (C1 C11+) was prepared and used to assign each retention time observed from the chromatogram to a boiling point. This enabled the whole analysis of a sample to be divided into intervals between the boiling points of the normal alkanes of the calibration mixture.
Quantification of compounds on the GC/FID was done by an external standard method and relative retention times once the component has been reliably identified on a gas chromatography/mass spectrometry (GC/MS) instrument.
Reaction Process
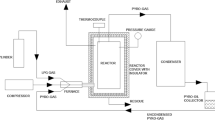
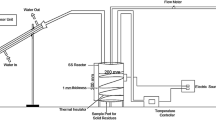
The reactor utilized in this work was a batch autoclave reactor; a schematic diagram of the experimental set-up is shown in Fig. 1. A 1,000 mL Pyrex reactor heated by an external electrical mantle with a heating rate of 20 °C/min. Nitrogen gas was used as an inert gas to purging the reactor at the beginning of experiments to ensure an inert pyrolysis condition.
100 g of sample was used in each experiment. Residence time was set to zero while the set cracking temperature was attained, and the experiment maintained at that temperature for the required residence time of each run.
For plastic samples, a set of experiments was carried out between 410 and 450 °C, to investigate the effect of temperature and catalyst on the pyrolysis of the polyethylene and polypropylene mixture.
Products have been liquefied by the condenser and collected in Pyrex bottles to get ready for analysis. The mass balances written with the weight of gas obtained from the Ideal Gas Law at room temperature and the combined weights of oil and residue in the reactor, after discharging the gas product. Once the weight of residue was determined after filtration, the difference was ascribed to be the weight of oil produced. Each experiment was carried out several times to check for reproducibility.
Finally, liquid samples analyzed by GC (FID) set in order to determine the quality of the components. Liquids distilled by fractional method and the physical properties of the samples investigated by Grabner Co instruments. The conversion for volatile products was calculated as the fraction of volatile products to the initial mass of reactant polymers mixture.
Taguchi experimental design Method
The first step before using the design of experiment (DOE) technique is to know how many variables (factor) and levels are available. To obtain the optimum point, many parameters are influential, each of which should be considered in certain conditions. The parameters affecting the degradation of the polymer mixture studied in this paper include temperature, catalyst wt%, and feed composition.
The following levels, which are determined in the light of literature and preliminary tests, were investigated in our study:
-
Temperatures: (1) 410, (2) 420, (3) 430, (4) 440, and (5) 450 °C.
-
Catalyst: (1) 10, (2) 20, (3) 30, (4) 40, and (5) 50 wt%.
-
Feed composition: 1, 2, 3, 4, and 5.
-
Feed compositions are shown in Table 2.
Table 2 Feed composition
Orthogonal array of methods used to find the number of experiments, while the number of factors and levels were identified. Considering all the mentioned conditions, one has to run eighty-one (53 = 125) experiments to cover all possible scenarios. Taguchi experimental design method was adopted to reduce the number of the experiments. Although the experiments are less through Taguchi, the ultimate effect is the same and the same conclusion may be achieved. Therefore, using Taguchi technique, the number of experiments was reduced down to 25, which are shown in Table 3.
As mentioned above, the Taguchi method was used to design the experiments via selection of optimum number of parameters. As seen in Table 3, the orthogonal array consists of 3 columns and 25 rows indicating 25 experiments need to be carried out. The Taguchi design was selected according to three working parameters (controllable factors) with five levels of each.
Table 4 illustrates L25 array, being filled with parameters and levels of the experiments. Each experiment was repeated three times to obtain the accuracy of the data.
Results and Discussions
Optimization of the process
In order to determine the effect of each variable on the output, the signal-to-noise ratio is required. Signal-to-noise ratio could be calculated by Eq. (1), where ŷi is the mean value, si is the variance, and yi is the value of the performance characteristic for a given experiment.
where
i = experiment number
u = trial number
Ni = Number of Trials for the experiment i
The most indispensable principle in the Taguchi method for analyzing experimental data is signal/noise ratio. In order to optimize the operating parameters, the S/N ratio calculation was performed. In this study, the S/N ratio should have a maximum value to obtain optimum gasoline components. Also, liquid production should be maximized following the Taguchi method. Based on the Taguchi model, for maximum desired liquid, calculation was chosen based on “the larger is better.” In this case, the S/N ratio is calculated from Eq. (1).
The maximum S/N ratio calculated for each parameter shows the optimum condition for liquid production. Thus, the optimum degradation condition for maximum liquid production was found as 450 °C, 30 % catalyst, and feed 3, respectively, in L25 orthogonal array Taguchi investigation method. Level values of the factors obtained, according to the Taguchi design, are given in Table 5 and Fig. 2.
The different values of S/N ratio between maximum and minimum are also shown in Table 5 as Delta. The Temperature and the catalyst are two factors that have the highest difference between values, 6.54 and 3.96, respectively. Based on the Taguchi prediction, the maximum difference between values of S/N ratio will have the most important effect on surface roughness (Ra).
According to Fig. 2, it seems that increasing of the temperature will increase the liquid production. Consequently, the optimum liquid production determined from the experiments and Taguchi method will be 450 °C for the Temperature, 30 % catalyst, and feed 3.
Although maximum liquid production is one of the most significant aims of this study, liquid production is not the main purpose. The main purpose of this study is to find the optimum condition to produce maximum gasoline quantity which directly depends on the liquid production. Due to the maximum desired yield of gasoline, calculation was chosen based on “the larger is better” as well. The maximum S/N ratio calculated for each parameter shows the optimum condition for gasoline production. The optimum degradation condition for maximum gasoline production was found, 420 °C, 50 %, and 1, as temperature, catalyst portion, and feed number, respectively, in L25 orthogonal array Taguchi investigation method. Regarding the Taguchi design, the obtained level values are given in Table 6 and Fig. 3. According to Delta (the difference between maximum and minimum values of S/N ration) from Table 6, Temperature, Catalyst, and Feed are the most significant variables in this process, respectively. It reveals that the process is utterly sensitive to the temperature alteration. Increasing of the temperature weakened the polymer chain structure. As a result of this fact, more light hydrocarbons produced. The temperature and the catalyst are two factors that have the greatest influences in gasoline production.
Liquid and gas products were qualified with gas chromatography analysis. While comparing the oil products from mixed polymers degradation over various amounts of catalyst at different temperatures, the results reflect the differing cracking effect of the reaction condition because of the catalyst, temperature, and feed composition interactions. The individual volatile hydrocarbon products of feed (1) degradation at 420 °C and over various catalyst portions are demonstrated in Table 7 (Sakaki et al. 2013). GC analysis of the liquid products illustrates that Ethyl benzene is the main component of the liquid products. Product distributions with current catalyst portion consist more Ethyl benzene with over 30 wt% in the range of C5–C10. Nevertheless, high temperature reaction condition resulted in more heavier streams with potentially large amounts of C11+ (12 wt%). The liquid hydrocarbons were in the range of C5–C11+. As known, the hydrocarbons in the range of C5–C10 belong to gasoline cut. The components peak before 112 min retention time belongs to gasoline cut.
In order to obtain maximum production of the gasoline quantity from the polymers, another analysis was done. Taguchi also can predict the results of the experiments. In this investigation, Taguchi method was used to predict the levels and factors. In order to approach close predictions with the real results, Taguchi needs series of the practical results. Levels and factors required for Taguchi method for prediction of the maxim gasoline quantity in the liquid products are presented in Table 8. Both experimental and predicted results are shown in Fig. 4. The prediction also enables the researchers to investigate the influence of each factor on the Gasoline quantity production. Figure 4 clearly shows the impact of temperature, catalyst, and feed as effective factors on the quantity of gasoline production. From both figures, it is evident that a close approach to the experimental results has been obtained by prediction from Taguchi method. The graphic mean of S/N ratios versus factor levels for maximum Gasoline Quantity production is shown in Fig. 5. It could be concluded from Fig. 4 that the maximum gasoline production has been obtained from degradation of feed 1 at 420 °C, 50 % catalyst. According to the effect of the catalyst and the temperature in the previous studies, these results from Taguchi are expectable. The addition of catalyst is anticipated to moderate the decomposition temperature, to raise the decomposition rate, and to modify the components.
Gas products were also captured in a cylinder in order to be qualified and quantified with gas chromatography analysis. Table 9 indicates the composition of the gas products at optimum reaction condition. Regarding Table 9, maximum components of the gaseous are Ethylene and Propylene, which are by-products of the PE&PP cracking. It seems that these hydrocarbons are broken tails of the long chain structures because of the weakness of the tails in comparison with the other parts of the chain structures. The important thing is that these hydrocarbons could be a great source to perform required energy of the reaction.
Kinetic Study
The Arrhenius model is proposed in this study for calculating the kinetics of the reaction; a method in which the rate constant of the reaction is expressed and defined as
Isothermal investigation is one of the trusted procedures for calculating the kinetic parameters of the reaction. In this procedure, the below formulae for rate constant and reaction order shall be used to calculate the activation energy.
where W is the mass of polymer, K can be expressed by the constant of Arrhenius law, and n represents the order of the reaction.
To calculate the order of reaction, F is assumed as a function of W with the order of n (Encinar and González 2008). Equation (8) is obtained via integrating Eq. (6).
A plot of left side of Eq. (7) versus Ln (W) should draw a straight line whose slope is n.
The kinetic parameters of the reaction at optimum reaction condition are presented below in Table 10.
Conclusion
Although plastic wastes cause significant environmental problems, they also could be regarded as an economical and valuable source of chemical feed stocks and energy. The Taguchi experimental design was used to obtain optimum condition on catalytic degradation of the polymers. The purpose of the current study was to determine the maximum quantity of gasoline production from the degradation of the mixed Polyethylene and polypropylene using Taguchi as a DOE method. Our findings suggest that Taguchi is the most promising DOE method in order to investigate the optimum condition to produce a maximized factor such as gasoline. The second major finding was that about 51 % of the polymers could be degraded to gasoline components which can be used as automobile fuel. Finally, Taguchi suggested that 420 °C and 50 % catalyst is the most promising condition for maximum production of the gasoline from 40, 20, and 40 % HDPE, PP, and LDPE as mixed polymers, respectively.
Abbreviations
- ABS:
-
Acrylonitrile butadiene styrene
- DOE:
-
Design of experiment
- GC/FID:
-
Gas chromatography/flame ionization detector
- GC/MS:
-
Gas chromatography/mass spectrometry
- HDPE:
-
High density polyethylene
- LDPE:
-
Low density polyethylene
- PP:
-
Polypropylene
References
Akpanudoh NS, Gobin K, Manos G (2005) Catalytic degradation of plastic waste to liquid fuel over commercial cracking catalysts: effect of polymer to catalyst ratio/acidity content. J Mol Catal A 235(1–2):67–73. doi:10.1016/j.molcata.2005.03.009
Al-Salem SM, Lettieri P (2010) Kinetic study of high density polyethylene (HDPE) pyrolysis. Chem Eng Res Des 88(12):1599–1606. doi:10.1016/j.cherd.2010.03.012
Elordi G, Olazar M, Lopez G, Amutio M, Artetxe M, Aguado R, Bilbao J (2009) Catalytic pyrolysis of HDPE in continuous mode over zeolite catalysts in a conical spouted bed reactor. J Anal Appl Pyrolysis 85(1–2):345–351
Encinar JM, González JF (2008) Pyrolysis of synthetic polymers and plastic wastes. Kinetic study. Fuel Process Technol 89(7):678–686. doi:10.1016/j.fuproc.2007.12.011
Hossein M, Behrooz R, Mohammad RS, Mojtaba M, Saeedeh IM (2013) Using Taguchi method to determine optimum process conditions for flue gas desulfurization through an amine scrubber. Clean Technol Environ Policy. doi:10.1007/s10098-013-0593-7
Jozef M, Miriam V (2008) Catalytic and thermal cracking of selected polyolefins. Clean Technol Environ Policy 10:121–130. doi:10.1007/s10098-007-0132-5
Kumar S, Panda AK, Singh RK (2011) A review on tertiary recycling of high-density polyethylene to fuel. Resour Conserv Recycl 55(11):893–910. doi:10.1016/j.resconrec.2011.05.005
Lin YH, Yang MH (2007) Catalytic conversion of commingled polymer waste into chemicals and fuels over spent FCC commercial catalyst in a fluidised-bed reactor. Appl Catal B 69(3–4):145–153. doi:10.1016/j.apcatb.2006.07.005
López A, de Marco I, Caballero BM, Laresgoiti MF, Adrados A, Aranzabal A (2011) Catalytic pyrolysis of plastic wastes with two different types of catalysts: ZSM-5 zeolite and Red Mud. Appl Catal B 104(3–4):211–219. doi:10.1016/j.apcatb.2011.03.030
Marcilla A, García-Quesada JC, Sánchez S, Ruiz R (2005) Study of the catalytic pyrolysis behaviour of polyethylene–polypropylene mixtures. J Anal Appl Pyrolysis 74(1–2):387–392. doi:10.1016/j.jaap.2004.10.005
Mordi RC, Dwyer J, Fields R (1994) H-ZSM-5 catalysed degradation of low density polyethylene, polypropylene, polyisobutylene and squalane: influence of polymer structure on aromatic product distribution. Polyme Degrad Stabil 46(1):57–62. doi:10.1016/0141-3910(94)90109-0
Sakaki SA, Roozbhani B, Shishesaz M, Abdollahkhani N (2013) Catalytic degradation of the mixed polyethylene and polypropylene into middle distillate products. Clean Technol Environ Policy. doi:10.1007/s10098-013-0688-1
Seddegi ZS, Budrthumal U, Al-Arfaj AA, Al-Amer AM, Barri SAI (2002) Catalytic cracking of polyethylene over all-silica MCM-41 molecular sieve. Appl Catal A 225(1–2):167–176. doi:10.1016/S0926-860X(01)00872-9
Wei TT, Wu KJ, Lee SL, Lin YH (2010) Chemical recycling of post-consumer polymer waste over fluidizing cracking catalysts for producing chemicals and hydrocarbon. Fuels Resour Conserv Recycl 54:952–961. doi:10.1016/j.resconrec.2010.02.002
Zhou Q, Zheng L, Wang Y-Z, Zhao G-M, Wang B (2004) Catalytic degradation of low-density polyethylene and polypropylene using modified ZSM-5 zeolites. Polym Degrad Stabil 84(3):493–497. doi:10.1016/j.polymdegradstab.2004.01.007
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roozbehani, B., Sakaki, S.A., Shishesaz, M. et al. Taguchi method approach on catalytic degradation of polyethylene and polypropylene into gasoline. Clean Techn Environ Policy 17, 1873–1882 (2015). https://doi.org/10.1007/s10098-015-0901-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-015-0901-5