Abstract
Purpose
Streptococcus dysgalactiae subsp. equisimilis (SDSE) has increasingly been recognised as a significant pathogen that causes a myriad of infections, ranging from cellulitis to invasive infections, including bacteraemia and even toxic shock syndrome. The aim of this study was to examine the epidemiology and disease manifestations of bacteraemia caused by SDSE.
Methods
We retrospectively reviewed cases of SDSE bacteraemia in adults aged ≥ 18 years admitted to four public hospitals in Western Sydney, Australia, between January 2015 and December 2020. We reviewed demographics, comorbidities, disease manifestations, management, and outcomes.
Results
There were 108 patients with SDSE bacteraemia over a six-year period. The median age of individuals with SDSE bacteraemia was 70 years (interquartile range, IQR, 58–85 years). Cardiovascular disease (46%), chronic skin conditions (44%) and diabetes (37%) were the most common comorbidities. Ten patients (9%) with SDSE bacteraemia had healthcare-acquired infections. Skin and skin structure infections (SSTIs) were the most common presentations (59%), while bone and joint infections (BJIs) represented 13% of the cases. Twenty patients (19%) had septic shock on presentation. Fifteen patients (14%) were prescribed clindamycin, while one patient received intravenous immunoglobulin (IVIg). Infective endocarditis (IE) was present in 3% of patients; however, only 44% of the total patients had an echocardiogram. The 30-day mortality rate was 13%, but it was greater in those aged > 75 years (21%). The average length of hospital stay for patients who survived was 15 days, and the average duration of intravenous therapy was 12 days.
Conclusion
SDSE bacteraemia is typically a community-onset infection with a fifth of patients in our cohort presenting with septic shock. Though complications such as BJI (13%) and IE (3%) are infrequent, 30-day mortality is high at 21% in those aged > 75 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Group C and G Streptococcus (GCGS) are catalase-negative, gram-positive cocci that form large colonies with clear haemolysis on blood agar, similar to Streptococcus pyogenes (group A Streptococcus, GAS) [1,2,3]. GCGS human isolates are now grouped together as Streptococcus dysgalactiae subsp. equisimilis (SDSE) [1, 4]. They are normal commensal flora of the oral cavity and known colonisers of the skin, the gastrointestinal tract, and the female genitourinary tract [5, 6]. SDSEs are now widely acknowledged as significant pathogens that cause a myriad of infections, ranging from cellulitis to invasive infections, including bacteraemia and even toxic shock syndrome (TSS) [7, 8]. Large epidemiological studies have been hindered by taxonomic obscurity [1]. Several studies have reported the incidence of group C Streptococcus and group G Streptococcus individually, while others have reported it as another β-hemolytic Streptococcus (distinct from Streptococcus pyogenes and Streptococcus agalactiae) [9]. SDSEs express M proteins, which are highly genetically related to S. pyogenes, sharing several virulence factors and causing a similar range of clinical infections [2, 3, 8, 10]. The incidence of bacteraemia due to SDSE has been on the rise worldwide, but the reason for this is unclear; this may be related to the clonal expansion of a few strains carrying certain virulence factors [9, 11,12,13].
Several clinical case series have shown that invasive forms of this infection are more common in elderly polymorbid individuals, with reported mortality rates ranging from 15 to 18% [14]. They have also been reported to cause infective endocarditis (IE), although they were initially excluded as typical pathogens in the modified Duke criteria published in 2000 for IE [15]. However, the updated 2023 Duke-International Society for Cardiovascular Infectious Diseases (Duke-ISCVID) criteria now include SDSE as a typical microorganism causing IE [16]. Data on IE due to SDSE have been scarce, with quite a few studies dating back to the 1990s. In a few case series from the 1980s, the incidence of IE has been reported to be as high as 25 to 50% [6]. The incidence of IE may be underreported due to the relative infrequency of investigating SDSE bacteraemia for endocarditis. Lother et al. (2017) reported that transthoracic echocardiogram (TTE) was only performed in one-third of patients with SDSE infection, of whom 6% had endocarditis, with over half having embolic complications [11]. In a Danish study analysing 901 cases of SDSE bacteraemia over a 10-year period (2008–2017), IE was found in 6.4% of cases [17].
The aim of this study was to examine the epidemiology and disease manifestations of bacteraemia due to SDSE in Western Sydney over a 6-year period (2015–2020) by examining common presentations.
Materials and methods
Case definition
The criteria for inclusion of patients in the study were based on the isolation of group C, group G Streptococcus (GCGS) or Streptococcus dysgalactiae subsp. equisimilis (SDSE) via blood culture. The identification was as reported by the laboratory based on MALDI results, β -haemolysis, and large colony sizes (≥ 0.5 mm). Hence, the Streptococcus anginosus group, which can be grouped with Lancefield C and/or G on latex testing, was excluded.
Inclusion criteria
-
Age ≥ 18 years.
-
Presenting to four Western Sydney hospitals (Westmead, Blacktown, Mt Druitt, and Auburn) during the study period (January 2015 to December 2020 inclusive).
Exclusion criteria
-
Polymicrobial bacteraemia.
-
Mismatches between patient records in the initial database and electronic medical records (eMRs).
The Institute of Clinical Pathology and Medical Research (ICPMR) at Westmead Hospital is a public health reference laboratory providing pathology services to a large population in Western Sydney. The microbiology department at Westmead Hospital (Sydney, Australia) maintains a database of all positive blood cultures. We extracted samples from patients with GCGS or SDSE bacteraemia. The database included blood culture collection date, principal manifestation, antimicrobial therapy, and mortality date. Missing and additional data were collected by accessing eMRs, which have been in use in the study hospitals since 2015. These data included demographic information, admission details, comorbidities, number of positive blood cultures, and additional investigations, including echocardiography, use of antibiotics, their route, and duration. The 7-day and 30-day mortality rates were also reviewed. Chronic diseases were defined as per the treating physician and were grouped per organ system affected as recorded in the eMR. Obesity was only recorded as a chronic medical condition if it was documented on eMR and not based on individual BMI calculations. Healthcare-associated infections (HAIs) were defined as bacteraemia occurring at least 48 h after hospital admission. Postoperative skin and soft tissue infections within 30 days of surgery were included in HAI.
The data were entered into Microsoft Excel. Statistical analyses, including statistical tests (chi-square test or Fisher’s exact test) and descriptive analysis (mean/percentage to characterise the data), were performed. A p value < 0.05 was regarded as statistically significant. The study was approved by the Western Sydney Local Health District Research Ethics Committee (HREA 2021/ETH00080).
Results
A total of 114 cases of SDSE bacteraemia were recorded in adults aged ≥ 18 years during the study period (2015–2020). We excluded four patients with polymicrobial bacteraemia (two with Staphylococcus aureus, one with Acinetobacter spp., one with Clostridium septicum) and two patients with no matching electronic records. The data of the remaining 108 patients were reviewed, of which 20 were reported as GCS (group C Streptococcus), 63 were GGS (group G Streptococcus) and 24 were reported to be SDSE. The baseline characteristics are described in Table 1.
60% of the patients were aged over 65 years, and over half were male (56%). The median age was 70 years (interquartile range [IQR] 58–85 years). Nearly a quarter of the patients were from a residential aged care facility (23%). The majority of the patients had at least one comorbidity (96%), with cardiovascular disease being the most common (46%), followed by chronic skin conditions (44%) and diabetes (37%). Almost one-third of patients (32%) had a history of minor skin trauma prior to hospital admission. The majority of infections were community acquired (91%), with only ten HAIs (p < 0.001).
Seven patients (6%) were documented as Persons Who Inject Drugs (PWID). Four patients were on dialysis. No blood cultures were secondary to complications of a central or peripheral cannula. Five patients had recently undergone surgery within seven days prior to hospital admission. All these patients underwent skin surgery (biopsy, debridement, or amputation).
Most patients had clearance blood cultures collected (93%), with only nine patients (8%) having more than one positive blood culture. Of the 9 patients with more than one positive blood cultures, two had infective endocarditis, one had septic arthritis, and three had skin and skin structure infections. The remaining patients had no identifiable focus. Less than half, 44% (48/108) of the patients had a TTE, with three patients having IE, 6.3% (3/48), using modified Dukes criteria. Eight out of these 48 patients had trans-oesophageal echocardiogram (TOE), while one patient refused TOE. Three patients with cardiac implantable electric devices (CIEDs) did not have TTE/TOE. Forty-six out of 108 patients underwent a computerised tomography (CT) scan to determine the source of infection or a deeper collection. Nuclear medicine scans were infrequently performed, with less than 6% of patients investigated with either of the available modalities (bone scan/gallium scan/white cell scan/positron-emission tomography scan).
Foci/site of infection
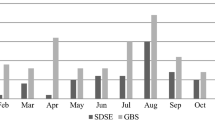
The most common sites of infection with SDSE bacteraemia were the skin and skin structure (59%), followed by bone and joint infection (13%). 11% of patients had no attributable source, while 3% had left-sided endocarditis. All patients with IE had prosthetic valves and cardiac comorbidities. The distribution is illustrated in Fig. 1.
Mortality rate
During the first 30 days, the all-cause mortality rate was 13% (14/108). Patients with no clear focus had a high mortality rate, 40% (5/14), while one patient with IE died due to a perforated mitral valve. 16% of the total patients (17/108) required admission to the intensive care unit, of whom five (29%) died.
We reviewed mortality based on age (age ≥ 75 or < 75 years), with nine deaths in the ≥ 75 years age group and five in the < 75 years age group. There was no significant relationship between age and mortality (p = 0.055). The results are summarised in Table 2.
Selection and duration of antimicrobial treatment
Of the 108 patients, six were allergic to penicillin. Of the patients who survived on day 30, details regarding choice and duration were not available for two patients, while one patient with IE was scheduled for lifelong suppressive therapy with suspected endovascular graft infection. These three patients were not included. Ninety-one of the remaining 108 patients were analysed. β-lactams were the preferred principal therapy in the majority of patients (85/91, 93%). Benzylpenicillin was the most common β-lactam and was used in 47 patients (52%), followed by cefazolin (15%) and ceftriaxone (12%). Only twelve patients (13%) were empirically treated with benzylpenicillin. 15% of patients (14/91) were also prescribed clindamycin, although the indications were not clearly documented. Penicillin allergy had no association with 30-day mortality.
Of the 14 patients who died, two patients had no antimicrobial therapy, as they were transitioned to end-of-life care early in their admission due to age, comorbidities and prior advanced care directives. Rest of the patients had empirical β-lactams.
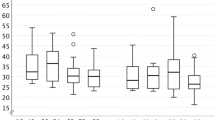
The average length of hospital stay for patients who survived was 15 days, and the average duration of IV therapy was 12 days. We analysed the duration of antibiotic treatment for SSTIs, which represented close to 60% of our sample. The mean duration of IV therapy was 9 days, while the total duration of therapy was 16 days. The box plot in Fig. 2 shows the spread and skewness, with a total duration between 7 and 28 days.
Discussion
This study provides an overview of SDSE blood stream infections over a 6-year period (2015–2020). SDSE bacteraemia is typically a community-onset infection in older individuals with underlying chronic skin conditions or diabetes. The median age of individuals with SDSE bacteraemia was 70 years (interquartile range IQR, 58–85 years). SSTIs were the most common presentations (59%), while bone and joint infections (BJIs) represented 13% and IE represented 3% of our patients. The 30-day mortality rate was 13%, but it was greater in those aged > 75 years (21%). Our results are consistent with previous findings that, unlike patients with group A Streptococcus, patients with SDSE bacteraemia are relatively older with comorbidities [18,19,20,21]. Wright et al. (2022) examined epidemiologic data on invasive group C and G Streptococcus in Western Australia and reported similar findings [21]. Age, male sex, chronic skin conditions, intravenous drug use, cardiovascular disease, alcohol consumption, diabetes, obesity, and immunosuppression have been found to be risk factors for sepsis and bacteraemia caused by pyogenic streptococci [5, 10, 22, 23].
The clinical manifestations of SDSE infections and affected risk groups in our study are similar to those described in previous studies [18,19,20, 22,23,24]. Over 96% of the patients had one or more chronic medical conditions. SSTIs represented 59% of the patients in our study, while cardiovascular disease (46%), followed by chronic skin conditions (44%) and diabetes (37%) were the most common comorbidities. Cardiovascular disease and diabetes have been previously implicated as potential risk factors for infection with pyogenic streptococci strains, including SDSE [24, 25]. Any medical comorbidity leading to skin breakdown increases the risk of invasive infection due to SDSE, and chronic skin conditions have been commonly reported in patients with invasive streptococcal infections [9, 24]. A hyperglycaemic environment and immune dysfunction as well as microvascular and macrovascular complications lead to an increase in the frequency of infections among patients with diabetes [26].
In a population-based study from Finland by Rantala et al. (2009), the case fatality rates due to group C and G Streptococcus were reported to be 22% and 15%, respectively [27]. The reported mortality rate due to invasive infections ranges from 15 to 18% [14]. From an Australian perspective, Harris et al. (2011) from Queensland reported a 28-day all-cause death rate of 5.5% in a younger cohort with a mean age of 43 years, while more recently, Wright et al. (2022) reported a 90-day all-cause death rate of 9% in Western Australia for invasive SDSE infections, not limited to bacteraemia. [12, 21].
An association between obesity and an increased risk of SSTIs has been reported in previous studies [24, 28]. A Danish population-based study by Nevanlinna et al. (2023) reported obesity to be a common comorbidity and a risk factor for SDSE infection (53% of patients with a BMI > 30) [24]. Obesity was documented as a comorbidity in less than a quarter (23%) of our patients. This is lower than in previously reported studies, but individual BMIs were not calculated for our patients.
Some investigators have suggested that bacteraemia with SDSE may be associated with underlying malignancy, but this has not been established [23]. In our cohort, approximately one in five patients (18%) had malignancies listed as a comorbidity.
In a recent study from Manitoba, Canada, Lother et al. (2017) reported a 6% incidence of IE with a mortality of 17%, despite only 33% of patients undergoing TTE [29]. Similar rates were observed in a large prospective cohort study by Murdoch et al. (2009) and a retrospective review by Loubinoux et al. (2013) [30, 31]. Our cohort had a lower incidence of IE than did those in previous studies (3%). In our population, fewer than half of the patients had a TTE in the setting of SDSE bacteraemia despite the potential risk of IE. Overall, 44% of patients underwent any form of investigation for IE (TTE/TOE). In our cohort, all 3 patients with IE had prosthetic valves and cardiac comorbidities The true incidence of IE due to SDSE is unclear, with reported rates < 1.5% to > 6%, depending on risk factors [16, 29,30,31,32]. Patients with multiple positive blood cultures and cardiac risk factors like prosthetic valve or CIED should be investigated with an echocardiogram to rule out IE. The recently updated 2023 Duke-ISCVID criteria will improve sensitivity for diagnosis of IE, although it is crucial that clinical judgement be the cornerstone for considering echocardiogram in patients with SDSE bacteraemia.
The treating physician guided the duration and choice of antimicrobial therapy. A formal Infectious Diseases consult was not requested for all patients. The data, except for bacteraemia secondary to skin and skin structure, were limited due to the small sample size. For SSTIs, the mean durations of IV and total therapy were 9 and 16 days, respectively. For many infections, including SDSE bacteraemia, no data have established the superiority of IV therapy or that a longer duration is better. Unnecessary hospital stays increase the risk of nosocomial infections, while shorter courses also help reduce the antibiotic footprint. Many modern RCTs have shown that short-course regimens are effective, and early transition to oral antibiotics is at least as effective as IV for a select group of patients [33, 34]. Prospective studies could examine the benefits of an Infectious Diseases consult and potentially shorter duration of therapy for uncomplicated bacteraemia and early transition to orals.
The strengths of our study include robust demographic and comorbidity data from four major public hospitals in the district. This is one of the largest reported series on SDSE bacteraemia. This study provides an overview of common presentations, including a good sample size for SSTIs. The same clinician reviewed all records. Clinical information was available regarding the need for hospital admission, length of stay, admission to the ICU, antimicrobial therapy, and mortality.
Limitations of this review include its retrospective study design and the use of eMRs for data collection. For example, obesity rates in our population were far lower than expected, as this was based on eMR documentation. Individual BMI calculations were not feasible due to incomplete or inaccurate data. Our sample size for infections other than skin/soft tissue infection was limited. Most other manifestations represented < 10% of the total cases. We did not review surgical interventions, and hence, we cannot infer whether they played any role in the duration or choice of antimicrobial therapy. Our review did not include blood cultures submitted to private laboratories, although the ICPMR laboratory at Westmead Hospital receives almost all blood culture specimens from hospitalised patients in the area. Alcohol use has been reported in previous studies as a risk factor, but we did not collect this information because it was not clearly documented on eMR. Antimicrobial susceptibility testing is not routinely reported given predictable susceptibility, so we cannot comment on any antimicrobial resistance.
In conclusion, this review describes the epidemiology and clinical features of SDSE bacteraemia. It is typically a community-onset infection in older individuals with underlying chronic skin conditions or diabetes. Skin and skin structure infections are common, with a low rate of complications such as BJI (13%) and IE (3%). The rates of invasive infections are expected to increase, and this review highlights areas that will benefit from future studies addressing current gaps in knowledge. A prospective study looking at the incidence and risk factors for IE for SDSE bacteraemia, including its incidence in PWID, would help address some of these gaps. Future studies could also address the impact of an Infectious Diseases review and its effect on the choice and duration of antimicrobial agents, hospital stay, and mortality in invasive SDSE bacteraemia patients.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
References
Facklam R (2002) What happened to the Streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev 15:613–630. https://doi.org/10.1128/CMR.15.4.613-630.2002
Cunningham MW (2000) Pathogenesis of Group A Streptococcal infections. Clin Microbiol Rev 13:470–511. https://doi.org/10.1128/CMR.13.3.470
Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A et al (2014) Disease manifestations and Pathogenic Mechanisms of Group A Streptococcus. Clin Microbiol Rev 27:264–301. https://doi.org/10.1128/CMR.00101-13
Haslam DB, St. Geme JW, Groups C (2018) and G Streptococci. Principles and Practice of Pediatric Infectious Diseases :736–737.e1. https://doi.org/10.1016/B978-0-323-40181-4.00122-5
Baracco GJ (2019) Infections caused by Group C and G Streptococcus (Streptococcus dysgalactiae subsp. Equisimilis and others. Epidemiological and clinical aspects. Gram-positive pathogens. ASM, Washington, DC, USA, pp 275–283. https://doi.org/10.1128/9781683670131.ch17.
Wessels MR, Sexton DJ, Hall KH (2023) Group C and group G streptococcal infection. UpToDate. https://www.uptodate.com.acs.hcn.com.au/contents/group-c-and-group-g-streptococcal-infection?search=group%20c%20and%20g&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed March 11, 2023)
Baracco GJ (n.d.) Infections caused by Group C and G Streptococcus (Streptococcus dysgalactiae subsp. Equisimilis and others): epidemiological and clinical aspects. https://doi.org/10.1128/microbiolspec.GPP3
Xie O, Davies MR, Tong SYC (2024) Streptococcus dysgalactiae subsp. equisimilis infection and its intersection with Streptococcus pyogenes. Clin Microbiol Rev. https://doi.org/10.1128/cmr.00175-23
Oppegaard O, Glambek M, Skutlaberg DH, Skrede S, Sivertsen A, Kittang BR (2023) Streptococcus dysgalactiae Bloodstream infections, Norway, 1999–2021. Emerg Infect Dis 29:260–267. https://doi.org/10.3201/eid2902.221218
Barros RR Antimicrobial resistance among beta-hemolytic streptococcus in Brazil: an overview. Antibiotics 2021;10. https://doi.org/10.3390/antibiotics10080973
Lother SA, Demczuk W, Martin I, Mulvey M, Dufault B, Lagacé-Wiens P et al (2017) Clonal clusters and virulence factors of group C and G Streptococcus causing severe infections, Manitoba, Canada, 2012–2014. Emerg Infect Dis 23:1092–1101. https://doi.org/10.3201/eid2307.161259
Harris P, Siew DA, Proud M, Buettner P, Norton R (2011) Bacteraemia caused by beta-haemolytic streptococci in North Queensland: changing trends over a 14-year period. Clin Microbiol Infect 17:1216–1222. https://doi.org/10.1111/J.1469-0691.2010.03427.X
Oppegaard O, Mylvaganam H, Skrede S, Lindemann PC, Kittang BR Emergence of a Streptococcus dysgalactiae subspecies equisimilis stG62647-lineage associated with severe clinical manifestations. Sci Rep 2017;7. https://doi.org/10.1038/s41598-017-08162-z
Rantala S (2014) Streptococcus dysgalactiae subsp. equisimilis bacteremia: an emerging infection. Eur J Clin Microbiol Infect Dis 33:1303–1310
Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr., Ryan T et al (2000) Proposed modifications to the Duke Criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633–638. https://doi.org/10.1086/313753
Fowler VG Jr, Durack DT, Selton-Suty C, Athan E, Bayer AS, Chamis AL et al (2023) The 2023 Duke-International Society for Cardiovascular Infectious diseases Criteria for Infective endocarditis: updating the modified Duke Criteria. Clin Infect Dis 77:518–526. https://doi.org/10.1093/cid/ciad271
Chamat-Hedemand S, Dahl A, Østergaard L, Arpi M, Fosbøl E, Boel J et al (2020) Prevalence of infective endocarditis in Streptococcal Bloodstream infections is dependent on streptococcal species. Circulation 142:720–730. https://doi.org/10.1161/CIRCULATIONAHA.120.046723
Ekelund K, Skinhøj P, Madsen J, Konradsen HB (2005) Invasive group A, B, C and G streptococcal infections in Denmark 1999–2002: epidemiological and clinical aspects. Clin Microbiol Infect 11:569–576. https://doi.org/10.1111/j.1469-0691.2005.01169.x
Broyles LN, Van Beneden C, Beall B, Facklam R, Lynn Shewmaker P, Malpiedi P et al (2009) Population-based study of invasive disease due to β-Hemolytic streptococci of groups other than a and B. Clin Infect Dis 48:706–712. https://doi.org/10.1086/597035
Ruppen C, Rasmussen M, Casanova C, Sendi P (2017) A 10-year observational study of Streptococcus dysgalactiae bacteraemia in adults: frequent occurrence among female intravenous drug users. Swiss Med Wkly 147:w14469. https://doi.org/10.4414/smw.2017.14469
Wright CM, Moorin R, Pearson G, Dyer J, Carapetis J, Manning L (2022) Invasive infections caused by Lancefield Groups C/G and A Streptococcus, Western Australia, Australia, 2000–2018. Emerg Infect Dis 28:2190–2197. https://doi.org/10.3201/eid2811.220029
Roujeau J-C, Sigurgeirsson B, Korting H-C, Kerl H, Paul C (2004) Chronic dermatomycoses of the foot as risk factors for Acute Bacterial Cellulitis of the Leg: a case-control study. Dermatology 209:301–307. https://doi.org/10.1159/000080853
Laupland KB, Ross T, Church DL, Gregson DB (2006) Population-based surveillance of invasive pyogenic streptococcal infection in a large Canadian region. Clin Microbiol Infect 12:224–230. https://doi.org/10.1111/j.1469-0691.2005.01345.x
Nevanlinna V, Huttunen R, Aittoniemi J, Luukkaala T, Rantala S Major risk factors for Streptococcus dysgalactiae subsp. equisimilis bacteremia: a population-based study. BMC Infect Dis 2023;23. https://doi.org/10.1186/s12879-023-07992-9
Factor SH, Levine OS, Harrison LH, Farley MM, Mcgeer A, Skoff T et al (2005) Risk Factors for Pediatric Invasive Group A Streptococcal Disease
Casqueiro J, Casqueiro J, Alves C (2012) Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab 16:27. https://doi.org/10.4103/2230-8210.94253
Rantala S, Vuopio-Varkila J, Vuento R, Huhtala H, Syrjänen J (2009) Predictors of mortality in beta-hemolytic streptococcal bacteremia: a population-based study. J Infect 58 4:266–272
Kaspersen KA, Pedersen OB, Petersen MS, Hjalgrim H, Rostgaard K, Møller BK et al (2015) Obesity and risk of infection: results from the Danish blood Donor Study. Epidemiology ;26
Lother SA, Jassal DS, Lagacé-Wiens P, Keynan Y (2017) Emerging group C and group G streptococcal endocarditis: a Canadian perspective. Int J Infect Dis 65:128–132. https://doi.org/10.1016/j.ijid.2017.10.018
Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG Jr, Bayer AS et al (2009) Clinical presentation, etiology, and Outcome of Infective endocarditis in the 21st Century: the international collaboration on endocarditis–prospective cohort study. Arch Intern Med 169:463–473. https://doi.org/10.1001/archinternmed.2008.603
Loubinoux J, Plainvert C, Collobert G, Touak G, Bouvet A, Poyart C et al (2013) Adult invasive and noninvasive infections due to Streptococcus dysgalactiae subsp. equisimilis in France from 2006 to 2010. J Clin Microbiol 51:2724–2727. https://doi.org/10.1128/jcm.01262-13
Xie O, Tong SYC (2023) Heterogeneity in risk of newly classified typical Streptococci as causes of Infective Endocarditis. Clin Infect Dis 77:1219–1220. https://doi.org/10.1093/cid/ciad392
Wald-Dickler N, Holtom PD, Phillips MC, Centor RM, Lee RA, Baden R et al (2022) Oral is the New IV. Challenging decades of blood and bone infection dogma: a systematic review. Am J Med 135:369–379e1. https://doi.org/10.1016/j.amjmed.2021.10.007
Davar K, Clark D, Centor RM, Dominguez F, Ghanem B, Lee R et al (2023) Can the future of ID escape the Inertial Dogma of its past? The exemplars of shorter is better and oral is the New IV. Open Forum Infect Dis 10:ofac706. https://doi.org/10.1093/ofid/ofac706
Acknowledgements
N/A.
Funding
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design, with C.C. and R.D. submitting the ethics for this analysis. Material preparation, data collection and analysis were performed by P.S. The first draft of the manuscript was written by P.S., and all the authors commented on previous versions of the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The retrospective study was reviewed and approved by the Western Sydney Local Health District Research Ethics Committee (HREA 2021/ETH00080).
Ethics
We certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Clinical trial number
Not Applicable, please see ethics approval above.
Human Ethics and consent to participate
Not Applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Solanki, P., Colaco, C. & Dotel, R. Analysis of bacteraemia caused by group C and G Streptococcus (Streptococcus dysgalactiae subsp. equisimilis) in Western Sydney over a 6-year period (2015–2020). Eur J Clin Microbiol Infect Dis 43, 1807–1814 (2024). https://doi.org/10.1007/s10096-024-04903-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-024-04903-x