Abstract
Introduction
Burkholderia cepacia complex (BCC) are non-fermenting Gram-negative bacteria that can chronically colonize the lungs of people with cystic fibrosis (pwCF), causing a severe and progressive respiratory failure, post-transplant complications and epidemic outbreaks. Therefore, rapid and accurate identification of these bacteria is relevant for pwCF, in order to facilitate early eradication and prevent chronic colonization. However, BCCs are often quite difficult to detect on culture media as they have a slow growth rate and can be hidden by other fast-growing microorganisms, including Pseudomonas aeruginosa and filamentous fungi.
Material and methods
We evaluated the sensitivity of CHROMagar™ B. cepacia agar using 11 isolates from a well-characterized BCC collection, using BCA agar (Oxoid, UK) as a gold standard. We also studied 180 clinical sputum samples to calculate positive (PPV) and negative (NPV) predictive values. Furthermore, we used three of the well-characterized BCC isolates to determine the limit of detection (LOD).
Results
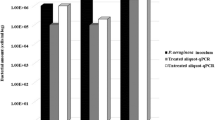
Eleven isolates grew on CHROMagar™ B. cepacia at 37ºC after 48 h. The NPV and PPV of CHROMagar™ B. cepacia were 100% and 87.5%, respectively. The LOD of CHROMagar™ B. cepacia was around 1 × 103 CFU/ml, requiring a ten-fold dilution lower bacterial load than BCA for BCC detection.
Conclusion
CHROMagar™ B. cepacia agar proved to have a very good sensitivity and specificity for the detection of clinical BCCs. Moreover, the chromogenic nature of the medium allowed us to clearly differentiate BCC from other Gram-negative species, filamentous fungi and yeasts, thereby facilitating the identification of contaminants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In people with cystic fibrosis (pwCF), chronic bacterial colonization of the respiratory tract by opportunistic pathogens is a leading cause of morbidity and mortality [1]. Pathogenic bacteria such as Staphylococcus aureus or Pseudomonas aeruginosa mainly cause infections, but nonfermenting Gram-negative bacilli such as Stenotrophomonas maltophilia, Achromobacter xylosoxidans or Burkholderia cepacia complex (BCC) are increasingly found [2]. BCC is a group that includes more than 22 species that are closely related to each other phenotypically, but genetically distinct [3]. Until not many years ago, BCC included nine species: B. cepacia, B. multivorans, B. cenocepacia, B. stabilis, B. vietnamiensis, B. dolosa, B. ambifaria, B. anthina and B. pyrrocinia. However, this number has increased in recent years, including in the complex other species such as B. ubonensis, B. latens, B. diffusa, B. arboris, B. seminalis, B. metallica, B. contaminans and B. lata [4,5,6]. These bacteria are ubiquitous in the environment and can survive long periods in hostile environments such as disinfectants, distilled water, and nebulizers [1, 7]. In Spain, the two most clinically prevalent species in pwCF were traditionally B. cenocepacia and B. multivorans but their prevalence has declined in recent years and there has been an increase in B. contaminans infections [8, 9]. BCC infections are generally opportunistic and are treated with ceftazidime and other extended spectrum cephalosporins due to their intrinsic resistance to other antimicrobials [10]. In pwCF, however, this complex is among one of the most important pathogens since it can cause the so-called "B. cepacia syndrome", a severe and progressive respiratory failure with bacteremia, very difficult to eradicate, that causes a decrease in life expectancy of patients [11]. Some studies have shown that BCC bacteria are the pathogens that have the most negative impact on the patient's lung function, only surpassed by Mycobacterium abscessus in severity [12]. Therefore, a rapid and accurate identification of these bacteria is relevant for pwCF.
Isolates from BBC grow slowly in normal culture media and could go unnoticed in pwCF due to the massive growth of other organisms in respiratory samples unless selective media are used [13]. Different commercial culture media have been marketed for the detection and identification of BCC isolates. CHROMagar™ B. cepacia (CHROMagar Paris, France) is a new, not yet commercialized, highly selective chromogenic medium for the detection of most BCC bacteria. This selective medium limit or inhibits the growth of yeasts, filamentous fungi, unwanted Gram-negative bacteria and completely inhibits the growth of Gram-positive bacteria. BCC strains should grow as blue colonies with a surrounding halo, mostly at 36 h, but growth may be delayed until 48 h (Fig. 1).
In this article we evaluate the sensitivity of the CHROMagar™ B. cepacia medium using: i) pure isolates from a well-characterized BCC collection (March 2013 – November 2013) [9] and ii) clinical samples to determine the positive and negative predictive values iii) three isolates to determine the limit of detection (LOD) [9].
Materials and methods
To assess the sensitivity of the medium, 11 BCC isolates previously characterized in our laboratory were used. These isolates were obtained from pwCF during a multicenter study (2013) conducted at national level in Spain [9]. A 0.5 McFarland suspension of these strains was prepared using fresh cultures grown overnight on Columbia blood agar at 37℃. Fifty microliters of these suspensions were seeded on CHROMagar™ B. cepacia and incubated at 37℃ for 48 h.
For the second part of the study, a total of 180 sputum samples were analyzed. Seventy-three pwCF who attended at our center (Ramón y Cajal University Hospital) provided fresh samples. The rest of them (n = 107) came from pwCF included in a national multicenter study (January 2021 – November 2021; 6 CF Units from 6 Spanish Hospitals) and were kept frozen at -80℃ until processing at our center. The samples were slowly thawed at 4 °C (when necessary) and cultured following standardized protocols (see Supplementary material) [9]. For BCC isolation, samples were cultured on CHROMagar™ B. cepacia and, in parallel, on Burkholderia cepacia medium (BCA, OXOID, UK; product ref: PO0938A). BCA is a pre-poured plate which is routinely used in our clinical microbiology laboratory to detect BCC. CHROMagar™ B. cepacia is a dehydrated culture medium that was prepared following manufacturer’s instructions. Both media were incubated for 48 h at 37 °C, following an extended incubation at room temperature for a further 7 days [9]. All isolates that grew on both media were identified using MALDI-TOF MS (Bruker-Daltonics, Germany) [14]. The growth of other microorganisms than BCC on both media was also recorded.
To carry out the third part of the study, LOD was assessed using CHROMagar™ B. cepacia medium and BCA as well as 3 clinical isolates (2 B. cepacia, 1 B. vietnamiensis) [15]. The isolates were suspended in NaCl 0.9% to a density equivalent to 0.5 McFarland (ca. 2 × 108 CFU/mL), serial tenfold dilutions were performed and 100 µL were plated on CHROMagar™ B.cepacia medium and on BCA medium and incubated at 37ºC for 48 h to count viable colonies. The experiments were performed in triplicate.
Results
For the first target, we seeded the 11 well-characterized BCC strains from a previous multicenter study and found that 11/11 (100%) isolates grew after 48 h at 37 °C. Four of the 11 isolates grew well at 36 h, but most needed up to 48 h for good growth.
Regarding the second objective, the growth data on both media used to calculate the negative and positive predictive values are shown in Table 1. Twelve out of the 180 sputum samples were positive for BCC isolates on BCA medium and 14 samples were positive on CHROMagar™ B. cepacia medium (two additional isolates). It should be noted that the number of colonies growing on CHROMagar™ B. cepacia plates from these two samples was very small, being 18 CFU/ml for one and 27 CFU/ml for the other.
Different filamentous fungi, yeasts and Gram-negative bacilli that were not BCC grew in both media, whereas all Gram-positives were inhibited. Among Gram-negative bacteria, Achromobacter xylosoxidans was the most frequently not inhibited on either medium (42.2% and 73.7% of inhibition on CHROMagar™ B. cepacia and BCA, respectively). We also observed in two occasions the growth of a multiresistant Serratia marcescens in both media. Thirty-one samples were positive for Pseudomonas aeruginosa, but this microorganism grew only in one case on BCA medium and not on CHROMagar™ B. cepacia. To note that a relatively high percentage of yeasts were able to growth in both media and, regarding filamentous fungi, only half of them were able to grew on CHROMagar™ B. cepacia medium.
Considering as positive any kind of growth on both media, negative predictive values (NPV) and positive predictive values (PPV) of CHROMagar™ B. cepacia medium were 100% and 20.9%, respectively. The corresponding values for BCA were 98% and 19%, respectively. The PPVs were low in both media because there was growth of other microorganisms. Nevertheless, in the CHROMagar™ B. cepacia medium colonies that were not BCC could be easily identified by their white (not blue) color except for two A. xylosoxidans, whose colonies had a blue halo. In fact, we must consider that both media are chromogenic so we reanalyzed the PPV and the NPV considering the bluish (CHROMagar™ B. cepacia) or the pinkish (BCA) color of the colonies. The new NPV and PPV obtained for CHROMagar™ B. cepacia were 100% and 87.5%, respectively, and the corresponding ones for BCA were 95.9% and 75%, respectively.
The limit of detection (LOD) of BCC on CHROMagar™ B. cepacia and BCA media were determined using 3 isolates. The media LOD varied. In the case of CHROMagar™ B. cepacia, the LOD were around 1 × 103 CFU/ml, but in the case of BCA medium it was 1 × 104 CFU/ml. Therefore, to detect BCCs on BCA medium, we need a ten-fold higher bacterial load of the organism in a sputum sample (see Table 2).
Discussion
This is the first study to test the accuracy of CHROMagar™ B. cepacia medium in the detection of BCC isolates and its ability to inhibit other microorganisms. There are few studies comparing different culture media to detect BCC from clinical sputum samples from pwCF. Marrs et al. (2021) compared 3 culture media: Burkholderia cepacia selective agar (BCSA, bioMérieux), BD Cepacia medium (Becton–Dickinson) and MAST Cepacia medium (MAST Diagnostics), the sensitivity of BCSA being the best of all media (100%) [16]. In our study, CHROMagar™ B. cepacia medium had also 100% sensitivity, being comparable to that of the BCSA. It should be noted that we did not obtain any false negative results. Among false positive growths, most of them were easily discriminable since it did not present the characteristic blue halo. Only 2 A. xylosoxidans grew with a blue halo, reducing the PPV to 87.5%. The concordance that we obtained with both media in the PPV and NPV values was very similar. The main advantage of the evaluated medium lies in its specific selective properties, which facilitate the direct detection of BCC isolates due to the blue halo they present in the medium with the absence of growth of non-BCC isolates. This advantage has been observed for other different chromogenic media that have shown similar good sensitivity and specificity results in detecting pathogenic or multiresistant bacteria [17, 18]. We should point out that, despite the positive results in the evaluation of CHROMagar™ B. cepacia, additional MALDI-TOF screening should be still needed to confirm results.
Sputum samples also contain bacteria commonly found in hospitals, such as different Enterobacterales or Staphylococcus aureus. Interestingly, the CHROMagar™ B. cepacia medium was able to inhibit the growth of all Gram-positives and most Enterobacterales. This is important since other fast-growing bacteria can delay the detection of BCC by overgrowth when using standard culture media. Therefore, having a high specificity can be an advantage in the context of CF, where slow-growing pathogens can easily be obscured by other bacteria or fungi [19]. Regarding Enterobacterales, only 2 isolates of multiresistant S. marcescens were able to grow on CHROMagar™ B. cepacia, probably due to its intrinsic resistance to colistin. With regard to A. xylosoxidans, it grew more often on CHROMagar™ B. cepacia medium than on BCA medium. However, the absence of a characteristic blue halo allowed us to differentiate it properly from BCC in all but two cases. Moreover, A. xylosoxidans is an emerging CF pathogen which has also a fastidious growth, so the possibility to isolate it on CHROMagar™ B. cepacia agar could be considered an advantage.
Concerning the molecular methods, specific PCR on direct sample has demonstrated higher sensitivity and specificity than culture media in some studies [19, 20]. This is probably due to a low bacterial load in sputum samples. In our experience, we saw a relatively low LOD of CHROMagar™ B. cepacia medium (1 × 103 CFU/mL), so maybe it should be advisable to seed a larger sample volume to enhance the sensitivity.
A drawback of CHROMagar™ B. cepacia medium is that it must be manually prepared, and this can be somewhat laborious and time-consuming to be performed in clinical laboratories. Another limitation of our work is the low number of BCC positive samples studied, which can be explained by the low prevalence of these microorganisms in our pwCF [21].
In summary, we have evaluated the efficacy of CHROMagar™ B. cepacia medium and has shown a very good sensitivity and specificity for the detection of BCC. In addition, this chromogenic medium allows to differentiate the growth of other species in a way that is easy to interpret. This feature may allow its incorporation into clinical microbiology laboratories.
References
Cullen L, Mcclean S (2015) Bacterial adaptation during chronic respiratory infections. Pathogens 4:66–89. https://doi.org/10.3390/pathogens4010066
LiPuma JJ (2010) The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev 23:299–323. https://doi.org/10.1128/CMR.00068-09
Rojas-Rojas FU, López-Sánchez D, Meza-Radilla G, Méndez-Canarios A, Ibarra JA, Estrada-de los Santos P (2019) The controversial Burkholderia cepacia complex, a group of plant growth promoting species and plant, animals and human pathogens. Rev Argent Microbiol 51:84–92. https://doi.org/10.1016/j.ram.2018.01.002
Medina-Pascual MJ, Valdezate S, Villalón P, Garrido N, Rubio V, Saéz-Nieto JA (2012) Identification, molecular characterization and antimicrobial susceptibility of genomovars of the Burkholderia cepacia complex in Spain. Eur J Clin Microbiol Infect Dis 31:3385–3396. https://doi.org/10.1007/s10096-012-1707-6
Mahenthiralingam E, Bischof J, Byrne SK, Radomski C, Davies JE, Av-Gay Y et al (2000) DNA-Based diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia Genomovars I and III. J Clin Microbiol 38:3165–3173. https://doi.org/10.1128/JCM.38.9.3165-3173.2000
Vanlaere E, LiPuma JJ, Baldwin A, Henry D, de Brandt E, Mahenthiralingam E et al (2008) Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov. and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. Int J Syst Evol Microbiol 58:1580–1590. https://doi.org/10.1099/ijs.0.65634-0
Gonçalves PJR de O, Hume CCD, Ferreira AJ, Tsui S, Brocchi M, Wren BW, et al (2019) Environmental interactions are regulated by temperature in Burkholderia seminalis TC3.4.2R3. Sci Rep 9:5486. https://doi.org/10.1038/s41598-019-41778-x
Medina-Pascual MJ, Valdezate S, Carrasco G, Villalón P, Garrido N, Saéz-Nieto JA (2015) Increase in isolation of Burkholderia contaminans from Spanish patients with cystic fibrosis. Clin Microbiol Infect 21:150–156. https://doi.org/10.1016/j.cmi.2014.07.014
de Dios CJ, del Campo R, Royuela A, Solé A, Máiz L, Olveira C et al (2016) Bronchopulmonary infection–colonization patterns in Spanish cystic fibrosis patients: Results from a national multicenter study. J Cyst Fibros 15:357–365. https://doi.org/10.1016/j.jcf.2015.09.004
Rhodes KA, Schweizer HP (2016) Antibiotic resistance in Burkholderia Species. Drugs Resist Updat 28:82–90. https://doi.org/10.1016/j.drup.2016.07.003
Mahenthiralingam E, Urban TA, Goldberg JB (2005) The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol 3:144–156. https://doi.org/10.1038/nrmicro1085
Qvist T, Taylor-Robinson D, Waldmann E, Olesen HV, Hansen CR, Mathiesen IH et al (2016) Comparing the harmful effects of nontuberculous mycobacteria and Gram negative bacteria on lung function in patients with cystic fibrosis. J Cyst Fibros 15:380–385. https://doi.org/10.1016/j.jcf.2015.09.007
Gilligan PH (2014) Infections in patients with cystic fibrosis. Clin Lab Med 34:197–217. https://doi.org/10.1016/j.cll.2014.02.001
Dingle TC, Butler-Wu SM (2013) MALDI-TOF mass spectrometry for microorganism identification. Clin Lab Med 33:589–609. https://doi.org/10.1016/j.cll.2013.03.001
Vrioni G, Daniil I, Voulgari E, Ranellou K, Koumaki V, Ghirardi S et al (2012) Comparative evaluation of a prototype chromogenic medium (ChromID CARBA) for detecting carbapenemase-producing Enterobacteriaceae in surveillance rectal swabs. J Clin Microbiol 50:1841–1846. https://doi.org/10.1128/JCM.06848-11
Marrs ECL, Perry A, Perry JD (2021) Evaluation of three culture media for isolation of Burkholderia cepacia complex from respiratory samples of patients with cystic fibrosis. Microorganisms 9(12):2604. https://doi.org/10.3390/microorganisms9122604
Pérez-Viso B, Aracil-Gisbert S, Coque TM, Del Campo R, Ruiz-Garbajosa P, Cantón R (2021) Evaluation of CHROMagar™-Serratia agar, a new chromogenic medium for the detection and isolation of Serratia marcescens. Eur J Clin Microbiol Infect Dis 40(12):2593–2596. https://doi.org/10.1007/s10096-021-04328-w
García-Fernández S, Hernández-García M, Valverde A, Ruiz-Garbajosa P, Morosini MI, Cantón R (2017) CHROMagar mSuperCARBA performance in carbapenem-resistant Enterobacteriaceae isolates characterized at molecular level and routine surveillance rectal swab specimens. Diagn Microbiol Infect Dis 87:207–209. https://doi.org/10.1016/j.diagmicrobio.2016.11.014
Whitby PW, Dick HLN, Campbell Iii PW, Tullis DE, Matlow A, Stull TL (1998) Comparison of culture and PCR for detection of Burkholderia cepacia in sputum samples of patients with cystic fibrosis. J Clin Microbiol 36:1642–1645. https://doi.org/10.1128/jcm.36.6.1642-1645.1998
Drevinek P, Vosahlikova S, Dedeckova K, Cinek O, Mahenthiralingam E (2010) Direct culture-independent strain typing of Burkholderia cepacia complex in sputum samples from patients with cystic fibrosis. J Clin Microbiol 48:1888–1891. https://doi.org/10.1128/JCM.02359-09
Barrio Gómez De Agüero MI, García Hernández G, Gartner S, de La Cruz ÓA, Barroso NC, Montaner AE, et al (2009) Protocol for the diagnosis and follow up of patients with cystic fibrosis. An Pediatr 71:250–264. https://doi.org/10.1016/j.anpedi.2009.06.020
Acknowledgements
CHROMagar™ B. cepacia media was kindly provided by CHROMagar. We also thank the members of the GEIFQ group (Grupos Español para el Estudio de la Colonización/Infección Broncopulmonar en Fibrosis Quística). GEIFQ members include the following: Ainhize Maruri-Aransolo, Juan de Dios-Caballero, Rafael Cantón, Malkoa Michelena-González, Luis Máiz, Saioa Vicente (Hospital Ramón y Cajal, Madrid); Esther Quintana (Hospital Virgen del Rocío, Sevilla); María Dolores Pastor-Vivero (Hospital de Cruces, Barakaldo); Antonio Álvarez (Hospital Vall d'Hebrón, Barcelona); Rosa Girón, Teresa Alarcón (Hospital de la Princesa, Madrid); Carmen Luna-Paredes (Hospital 12 de Octubre, Madrid); Marta Ruiz de Valbuena, María Concepción Prados (Hospital de la Paz, Madrid); Silvia Castillo-Corullón (Hospital Clinic de Valencia, Valencia); María José Selma, Amparo Solé (Hospital la Fe, Valencia); Maria Cols-Roig (Hospital Sant Joan de Deu, Barcelona); Pedro Mondéjar-López (Hospital Virgen de la Arrixaca, Murcia); Estela Pérez Ruiz, Casilda Olveira, Pilar Caro Aguilera, Pilar Bermúdez Ruiz (Hospital General de Málaga, Málaga); Carla López Causapé, Joan Figuerola, Antonio Oliver (Hospital Son Espases, Palma de Mallorca); Oscar Asensio (Consorci Corporació Sanitària Parc Taulí, Sabadell).
Funding
JdDC has a Juan Rodes contract (Ref. JR18/00034) and AMA a predoctoral contract (Project PI19/01043) from the Instituto de Salud Carlos III and the European Regional Development Fund (ERDF, “A way to achieve Europe”).
This study was funded by Plan Estatal de I + D + I 2017–2020 (PI19/01043 and PI22/1045) and CIBER de Enfermedades Infecciosas (CIBERINFEC) (CB21/13/00084), Instituto de Salud Carlos III. Madrid, Spain.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maruri-Aransolo, A., de Dios Caballero, J., Michelena, M. et al. Evaluation of CHROMagar™ B. cepacia agar for the detection of Burkholderia cepacia complex species from sputum samples of patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis 43, 1349–1353 (2024). https://doi.org/10.1007/s10096-024-04845-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-024-04845-4