Abstract
Purpose
Cancer patients are at heightened risk for invasive aspergillosis (IA), a condition associated with elevated mortality risk. The JF5-based Aspergillus Galactomannoprotein Lateral Flow Device (AspLFD) offers rapid point-of-care testing (POCT) for IA. This study evaluated the diagnostic performance of AspLFD in cancer populations.
Methods
This retrospective study examined cancer patient bronchoalveolar lavage fluid (BALF) and serum samples collected between September 2021 and January 2023. Both AspLFD and galactomannan (GM) assays were conducted, and the results were analysed by two independent researchers.
Results
This study included 242 samples from 218 cancer patients, with 58 BALF and 184 serum samples. The overall agreement between AspLFD and GM assay results was 92.1%, with a kappa value of 0.552. AspLFD diagnosed proven/probable IA with a sensitivity and specificity of 91.7% and 95.3%, respectively, whereas GM exhibited sensitivity and specificity values of 83.3% and 93.7%, respectively. There were no statistical differences in the sensitivity and specificity between the two methods (P > 0.05). For serum analyses, AspLFD and GM exhibited similar sensitivity (66.7% vs. 66.7%, P > 0.05) and specificity (98.6% vs. 96.6%, P > 0.05) values. However, the sensitivity of the AspLFD was superior to the GM assay (100% vs. 88.9%) in BALF analyses but the difference was not statistically significant (P > 0.05), with no difference in specificity (83.7% vs. 83.7%, P > 0.05). In the solid-tumour cohort, both the AspLFD and GM assay exhibited high sensitivity (100% for both) and specificity (94.2% vs. 92.8%, P > 0.05).
Conclusion
The AspLFD demonstrated good performance in diagnosing IA in cancer patients, especially those with solid tumours. The AspLFD is thus an alternative POCT, particularly when GM evaluations are not readily available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive aspergillosis (IA) is a life-threatening infectious disease that affects individuals with compromised immune function [1]. Cancer patients, including those with hematologic malignancies and solid tumours, are often in a state of prolonged immunosuppression due to their underlying disease and/or cancer treatment, placing them at increased risk for infection by Aspergillus and other invasive fungi [2,3,4]. Once IA develops, the mortality rate among cancer patients increases significantly [5].
Early diagnosis and prompt antifungal treatment are critical for enhancing the survival rate of patients with IA [6, 7]. Nevertheless, early diagnosis of IA remains challenging for several reasons. First, IA lacks specific clinical characteristics and features in the early stages [6]. Second, blood cultures are typically negative, and cancer patients are often precluded from undergoing invasive procedures to obtain sterile site specimens, making it difficult to confirm a diagnosis of IA [8]. Third, due to limitations in current mycological testing techniques, methods currently available to obtain microbiological evidence of IA are sub-optimal [9]. For example, fungal cultures from respiratory samples are insufficient for effectively distinguishing between colonization and infection. The positive predictive value of fungal cultures for IA diagnosis is low, especially in nonhematologic cancer patients and those who have received antifungal therapy [6, 10, 11].
Galactomannan antigen (GM) testing is an additional important microbiological method for assisting in the diagnosis of IA [12]. GM testing utilizes a rat monoclonal antibody (EB-A2) to detect the galactomannan antigen, which is a polysaccharide component of the Aspergillus cell wall [13]. However, the diagnostic performance of GM varies highly across different studies and can be affected by interpretational criteria and the patient’s baseline disease status [6, 14, 15]. Moreover, most commercial GM testing kits are based on enzyme-linked immunosorbent assays (ELISAs). Due to cost-related factors, testing institutions or hospitals must accumulate multiple samples for batch testing, which does not allow for immediate testing of individual samples. This can delay the early diagnosis and initiation of treatment for IA patients, preventing them from fully benefiting [8, 16].
Galactomannoprotein, released during the growth of Aspergillus, represents another novel antigenic target for IA-specific detection beyond galactomannan [17]. In 2008, Thornton developed an Aspergillus-specific lateral-flow device (AspLFD) that uses a mouse monoclonal antibody (JF5) to detect the galactomannoprotein antigen, which is an extracellular glycoprotein secreted constitutively during the active growth of Aspergillus [17, 18]. The AspLFD enables rapid and convenient point-of-care testing (POCT) that addresses the problem of extended sample turnaround time, and it is now commercially available [19]. Multiple studies have demonstrated that the AspLFD exhibits excellent performance in the diagnosis of IA in patients with hematologic diseases, patients who have undergone organ transplantation or have respiratory illnesses, and patients in the intensive care unit (ICU) [20,21,22,23]. However, there is currently a lack of data regarding the diagnostic performance of the AspLFD in cancer populations, particularly in patients with solid tumours. Therefore, this study compared the performance of the AspLFD and GM assay in diagnosing IA within a cancer cohort and evaluated whether the AspLFD could serve as an alternative biomarker for diagnosing IA in high-risk cancer patients.
Methods
Study design and ethics
This study was conducted in Anhui Provincial Cancer Center at the First Affiliated Hospital of USTC, China. We performed a retrospective analysis of serum and bronchoalveolar lavage fluid (BALF) samples from cancer patients who were tested for IA. The samples included in this study were collected between September 2021 and January 2023. As per the standard of care, all specimens were screened using an Aspergillus GM ELISA kit. Residual samples were stored at − 80℃ for subsequent AspLFD analysis.
Patient information was extracted from electronic medical records, and relevant data regarding host factors, clinical features, and microbiological parameters were collected according to the established consensus on IA from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) [12]. Cases of proven, probable, and possible IA were classified based on EORTC/MSGERC definitions [12]. Excluding the established categories of proven, probable, and possible cases, any case lacking evidence of Aspergillus infection was designated as non-IA. The study protocol was reviewed and approved by the Institutional Ethics Committee (IEC) of the First Affiliated Hospital of USTC (IEC reference code: 2024-013).
Aspergillus GM ELISA
GM testing was performed using a Dynamiker Aspergillus GM ELISA kit (Dynamiker, Tianjin, China) in accordance with the manufacturer’s instructions. In brief, 300 µL of serum sample was mixed with 100 µL of sample processing solution, heated at 100℃ for 3 min, and then centrifuged at 10,000 × g for 10 min, after which the supernatant was utilized for GM analysis. BALF samples were centrifuged at 1,000 × g for 5 min, and the supernatant was subjected to GM analysis. A total of 50 µL of pretreated serum or BALF sample was added to each well, followed by the addition of 50 µL of anti-GM antibody. The samples were then incubated at 37℃ for 90 min, and after washing, 100 µL of enzyme-conjugated antibody was added, and the samples were incubated at 37℃ for 30 min. Following another wash, chromogenic substrate was added to each well and incubated for 15 min. The reaction was then terminated, and the absorbance was measured within 5 min at an optical density of 450 nm. The GM concentration was calculated based on a standard curve, and the results were interpreted according to the EORTC/MSGERC 2020 criteria [12].
Aspergillus AspLFD
The AspLFD test was administered using an OLM Aspergillus LFD kit (Richardson Guangzhou Centre for Fungal Diagnostics and Research, Guangzhou, China) following the manufacturer’s guidelines. Briefly, serum or blood-mixed BALF samples were centrifuged at 14,000 × g for 1 min. Next, 150 µL of supernatant was mixed with 300 µL of sample processing solution, heated at 100℃ for 3 min, and then centrifuged again at 14,000 × g for 5 min. A total of 70 µL of the resulting supernatant was applied to the test strip. Similarly, non–blood-mixed BALF samples were processed by centrifugation at 14,000 × g for 1 min, and then 70 µL of the supernatant was directly applied to the test strip. The results were read 30 min post-application. Test results were interpreted as follows: the simultaneous presence of red lines at the test (T) and control (C) positions indicated a positive result; the appearance of the C-line alone denoted a negative result; and the absence of the C-line signified an invalid result. The results for each sample were independently evaluated by two anonymous researchers who were blinded to the patient’s diagnosis.
Statistical analysis
The results of the GM and AspLFD tests were analysed statistically to determine the positive percent agreement (PPA) and negative percent agreement (NPA) between the results for serum, BALF, and all samples for both methods. The sensitivity and specificity of the GM and AspLFD tests for the diagnosis of proven/probable IA were computed. P values and statistical significance were assessed using the chi-square test or Fisher’s exact test, with a two-sided P value of < 0.05 considered to indicate statistical significance. The Kruskal-Wallis test were used to compare multiple median values for different groups. Confidence intervals (CIs) were estimated using the Clopper-Person method. Data were analysed using GraphPad Prism software, version 8.4.2.
Results
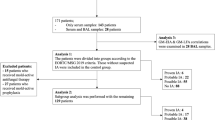
This study examined 254 samples from 230 cancer patients, comprising 193 serum samples and 61 BALF samples. Twelve patients were excluded due to incomplete data (Fig. 1). The remaining 218 patients were diagnosed according to the EORTC/MSGERC criteria [12], as follows: 39 IA patients—1 proven, 11 probable, and 27 possible IA patients—and 179 non-IA patients. The diagnostic basis of proven and probable IA is listed in Supplemental Table 1. As shown in Fig. 1, there was one patient with proven IA, who was a solid tumour patient. The type of the proven IA sample was BALF. There were 11 patients with probable IA, including 2 with hematologic malignancies and 9 with solid tumours. There were 11 samples of probable IA, of which 8 were BALF samples and 3 were serum samples. There were 27 patients with possible IA, including 15 with hematologic malignancies and 12 with solid tumours. There were 39 samples of possible IA, of which 6 were BALF samples and 33 were serum samples. There were 179 non-IA patients, of which 51 had hematologic malignancies and 128 had solid tumours. Finally, there were 191 non-IA samples, of which 43 were BALF samples and 148 were serum samples.
The demographic and clinical characteristics of the included patients are presented in Table 1. The majority of patients were male (157/218, 72.0%). The median age was 60 years (range 3–89 years), and the median body mass index (BMI) was 22.25 kg/m2 (range 14.2–31.2 kg/m2; BMI information was missing for 36 individuals). Hematologic malignancies were present in 68 of 218 patients (31.2%), whereas solid tumours were present in 150 of 218 patients (68.8%). A total of 25 of 218 patients (11.5%) had received treatment in an ICU within the 6 months prior to testing. Among all 218 patients, 72 (33.0%) experienced leukopenia, and 40 (18.3%) had severe neutropenia. Prophylactic antifungal treatment was administered to 88 of 218 patients (40.4%), and 4 patients (1.8%) died within 90 days.
Overall, no significant differences were observed in terms of age, gender, BMI, and leukopenia across the four patient groups: Proven IA, Probable IA, Possible IA, and non-IA (P > 0.05). Although patients with Probable IA exhibited higher ICU admission rates and 90-day mortality compared to the other three groups, these differences were not statistically significant (P > 0.05). However, significant differences were noted among the groups concerning underlying diseases, neutropenia, and antifungal prophylaxis (P < 0.05). Specifically, the proportion of patients with hematologic malignancies was higher in the Possible IA group compared to the other three groups. A statistically significant difference was found between Possible IA and non-IA groups (P < 0.05), but not between Possible IA and either Proven IA or Probable IA groups (P > 0.05). The proportion of patients with solid tumors in the Proven IA group (100%) was higher than in the other three groups, but this difference was not statistically significant (P > 0.05). The proportion of solid tumor patients in the Probable IA group (81.8%) was higher than that in the Possible IA and non-IA groups, but again, this difference was not statistically significant (P > 0.05). The proportion of solid tumor patients in the non-IA group was significantly higher than in the Possible IA group (71.5% vs. 44.4%), with statistical significance (P < 0.05). Furthermore, the incidence of neutropenia in the Proven IA group (100%) was higher than in the other three groups, but this difference was not statistically significant (P > 0.05). The incidence of neutropenia in the Possible IA group was significantly higher than in the non-IA group (37% vs. 15.1%), with statistical significance (P < 0.05). The use of antifungal prophylaxis in the Proven IA group (100%) was higher than in the other three groups, but this difference did not reach statistical significance (P > 0.05). The proportion of antifungal prophylaxis usage in the Possible IA group was significantly higher than in the non-IA group (70.4% vs. 35.2%), achieving statistical significance (P < 0.05).
Details regarding the analysis and comparison of GM and AspLFD test are shown in Table 2. Of 242 results, there were 14 concordant positives and 209 concordant negatives for both the GM and AspLFD tests, with an overall agreement rate of 92.1% (95% CI, 88-95.2%) and kappa value of 0.552 (95% CI, 0.374–0.730). Besides, 11 samples tested positive with the AspLFD but negative with the GM test, and another 8 samples tested negative with the AspLFD but positive with the GM test. The total PPA was 63.6% (95% CI, 40.7–82.8%), whereas the total NPA was 95% (95% CI, 91.2–97.5%). Further analysis of different sample types revealed that 11 of 58 BALF samples were concordantly positive and 36 of 58 were concordantly negative in the GM and AspLFD tests, yielding a PPA of 73.3% (95% CI, 44.9–92.2%) and NPA of 83.7% (95% CI, 69.3–93.2%). The detection results for the AspLFD and GM assay in the 11 BALF samples were inconsistent. 7 samples tested positive for the AspLFD but negative for GM, while the remaining 4 samples exhibited negative results for the AspLFD but positive results for the GM assay. Among serum samples, 3 of 184 were concordantly positive, and 173 of 184 were concordantly negative, providing a PPA of 42.9% (95% CI, 9.9–81.6%) and NPA of 97.7% (95% CI, 94.3–99.4%). The detection results for AspLFD and GM testing in 8 serum samples were inconsistent. 4 samples tested positive for the AspLFD but negative for GM assay, while the remaining 4 samples exhibited negative results for the AspLFD but positive for GM assay. The comparative analysis revealed that the positive rates for AspLFD and GM test in BALF samples did not exhibit significant differences (P > 0.05). This finding was mirrored in the serum samples, where the positive rates for AspLFD and GM test were similarly nonsignificant (P > 0.05).
Of the 25 AspLFD-positive cases, 1 case had proven IA, 10 had probable IA, 5 had possible IA, and the remaining 9 had non-IA. Among the 22 GM-positive cases, 1 case had proven IA, 9 had probable IA, and the remaining 12 had non-IA. The performance of the GM and AspLFD in diagnosing IA was analysed and compared, as shown in Table 3. Given the stringent criteria for proven IA, there was only one proven case of IA in this study, consistent with previous reports [15, 16, 23, 24]. Following the approach used in previous studies, we considered both probable and proven IAs as true positives and used the non-IA cases as a control group in assessing the diagnostic performance of GM and AspLFD tests. Overall, the AspLFD demonstrated greater sensitivity than did the GM test (91.7% vs. 83.3%), but the difference is not statistically significant (P > 0.05). Both the GM test and the AspLFD exhibited commendable performance in specificity (95.3% vs. 93.7%, P > 0.05) and Negative Predictive Value (NPV), with rates of 99.5% and 98.9% (P > 0.05). However, the AspLFD demonstrated a superior Positive Predictive Value (PPV) compared with the GM test (55% vs. 45.5%), although the difference was not statistically significant (P > 0.05). In addition, the Youden’s statistic for the AspLFD was also higher than that for the GM test (0.87 vs. 0.77), indicating that its diagnostic performance is not inferior to that of GM test.
In the analysis of BALF samples, the AspLFD and the GM test showed similar performance in terms of specificity (83.7% vs. 83.7%, P > 0.05), PPV (56.25% vs. 53.33%, P > 0.05), and NPV (100% vs. 97.3%, P > 0.05). AspLFD showed a higher sensitivity (100% vs. 88.9%, P > 0.05) and a higher Youden’s statistic (0.84 vs. 0.73) compared to GM test. In the analysis of serum samples, both methods exhibited comparable sensitivity (66.7% vs. 66.7%, P > 0.05), specificity (98.6% vs. 96.6%, P > 0.05), NPV (99.3% vs. 99.3%, P > 0.05), and Youden’s statistic (0.65 vs. 0.63), but the AspLFD showed a higher PPV (50% vs. 28.6%, P > 0.05). Although the benefits of AspLFD do not reach statistical significance, this at least suggests that the performance of AspLFD is comparable to, if not better than, that of GM test.
Upon further analysis of the two subgroups in solid tumours, both the AspLFD and GM tests exhibited satisfactory sensitivity (100% for both), specificity (94.2% vs. 92.8%, P > 0.05), NPV (100% for both) and Youden’s statistic (0.94 vs. 0.93). AspLFD demonstrated a superior PPV in comparison to GM (55.6% vs. 50%), but the difference was not statistically significant. The hematologic malignancy subgroup included 2 cases diagnosed with proven/probable IA and 53 non-IA cases. Both the proven and probable IA patients tested negative for GM, whereas 2 of the 53 non-IA cases tested positive. AspLFD results were positive in 1 of the 2 proven/probable IA cases and in 1 of the 53 non-IA cases, yielding a sensitivity of 50.0% (95% CI, 1.3–98.7%) and specificity of 98.1% (95% CI, 89.9–100.0%).
In this study, 19 samples from 19 different patients demonstrated discordant AspLFD and GM test results, and detailed information is presented in Table 4. Of these samples, 8 (4 serum and 4 BALF samples) were GM-positive but AspLFD-negative, and 1 of these 8 patients (12.5%) presented with IA-related radiologic findings on CT. Conversely, samples from 11 patients were AspLFD-positive but GM-negative, including 4 serum and 7 BALF samples, and 1 of these 11 patients had Aspergillus cultured from sputum, whereas 6 (54.5%) exhibited IA-related radiologic findings on CT.
Discussion
This study represents the first retrospective evaluation of the application and performance of the AspLFD for the diagnosis of IA in a cancer cohort. Overall, the AspLFD demonstrated superior sensitivity to the GM test with comparable specificity, especially in the analysis of BALF samples. Although the advantages of AspLFD do not reach statistical significance, this at least suggests that its performance is comparable to, if not better than, that of GM test.
The AspLFD is a POCT for the rapid detection of Aspergillus galactomannoprotein antigen. The utility of the AspLFD for diagnosing IA has been reported in several studies [17,18,19,20,21, 25,26,27]. Pan et al. conducted a meta-analysis of the diagnostic performance of the AspLFD, which exhibited a sensitivity and specificity of 86% and 93%, respectively, for BALF samples and 68% and 87%, respectively, for serum samples [28]. Heldt and Hoenigl also summarised past research and reported overall sensitivity and specificity values of 73% and 90%, respectively, for AspLFD analysis of BALF samples [29]. In our study, the sensitivity of the AspLFD in analyses of serum samples (66.7%) closely aligned with the results reported by Pan et al., but the specificity was higher (97.2%). Notably, the sensitivity of our method for assessing BALF samples (100%) significantly exceeded that of Pan and Heldt (86% and 73%, respectively) [28, 29]. This discrepancy is likely attributable to differences in the populations included in the studies. Our study exclusively examined cancer patients, comprising individuals with solid tumours (150/218) and hematologic malignancies (68/218).
The performance of the AspLFD in diagnosing IA among patients with hematologic malignancies as reported in the literature varies widely, with sensitivity values ranging from 26 to 82% [19, 23, 24, 27]. Heldt and Hoenigl evaluated the diagnostic efficacy of the AspLFD across patient groups, including those with solid organ transplants, ICU patients, and those with respiratory diseases. Their findings suggest that the sensitivity is lowest in patients with hematologic malignancies, with values below the overall population average (67% vs. 73%) [29]. This finding is in line with our results, in which the sensitivity of the AspLFD in diagnosing IA in the hematologic malignancy subgroup (50%) was lower than that in both the solid tumour subgroup (100%) and the overall study population (91.7%). The results of our study enhance understanding of the diagnostic performance of the AspLFD within a cancer patient cohort, indicating its potential sensitivity and specificity, particularly among patients with solid tumours.
In clinical practice, patients with suspected IA often use antifungal medications pre-emptively, which may significantly reduce the detection sensitivity of analyses of serum biomarkers but not the sensitivity of analyses of biomarkers in BALF [30, 31]. This tendency was also observed in our study, in which 88 of 218 patients (40.4%) had used antifungal drugs pre-emptively before testing. The sensitivities of the AspLFD and GM tests in analyses of BALF samples were notably greater than those observed with serum samples, possibly due to the pre-emptive use of antifungal drugs.
This study has several limitations. First, this was a single-centre retrospective study involving a limited number of patients. Second, invasive biopsies could not be performed for many of the cancer patients, making it difficult to obtain pathological tissue. Therefore, confirmation of proven IA cases through histopathology is rare. Additionally, this study used the results of GM testing as microbiological evidence according to the EORTC/MSG criteria, which could have introduced bias and consequently overestimation of GM test performance [23].
Conclusion
In conclusion, the AspLFD performed well in diagnosing IA in cancer patients and could serve as an alternative when GM testing is not readily available. Under such circumstances, the AspLFD enables rapid diagnosis of IA and early initiation of antifungal therapy, which is beneficial for patient outcomes. Future research efforts should focus on enhancing the sensitivity of the AspLFD for analyses of serum samples to achieve rapid, early, and accurate diagnosis of IA.
Data availability
The data that support the findings of this study are available upon reasonable request from the corresponding author.
References
Dagenais TR, Keller NP (2009) Pathogenesis of aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev 22(3):447–465. https://doi.org/10.1128/cmr.00055-08
Douglas AP, Smibert OC, Bajel A et al (2021) Consensus guidelines for the diagnosis and management of invasive aspergillosis. Intern Med J 51(Suppl 7):143–176. https://doi.org/10.1111/imj.15591
Liu B, Totten M, Nematollahi S et al (2020) Development and evaluation of a fully automated molecular assay targeting the mitochondrial small subunit rRNA gene for the detection of Pneumocystis Jirovecii in bronchoalveolar lavage fluid specimens. J Mol Diagn 22(12):1482–1493. https://doi.org/10.1016/j.jmoldx.2020.10.003[Erratum in J Mol Diagn 2021
Xue T, Kong X, Ma L (2023) Trends in the epidemiology of Pneumocystis pneumonia in immunocompromised patients without HIV infection. J Fungi (Basel) 9(8):812. https://doi.org/10.3390/jof9080812
Chen CA, Ho CH, Wu YC, Chen YC, Wang JJ, Liao KM (2022) Epidemiology of aspergillosis in cancer patients in Taiwan. Infect Drug Resist 15:3757–3766. https://doi.org/10.2147/IDR.S370967
Arvanitis M, Mylonakis E (2015) Diagnosis of invasive aspergillosis: recent developments and ongoing challenges. Eur J Clin Invest 45(6):646–652. https://doi.org/10.1111/eci.12448
Inoue K, Muramatsu K, Nishimura T et al (2022) Association between early diagnosis of and inpatient mortality from invasive pulmonary aspergillosis among patients without immunocompromised host factors: a nationwide observational study. Int J Infect Dis 122:279–284. https://doi.org/10.1016/j.ijid.2022.05.048
Calero AL, Alonso R, Gadea I et al (2022) Comparison of the performance of two galactomannan detection tests: Platelia Aspergillus Ag and Aspergillus Galactomannan Ag Virclia Monotest. Microbiol Spectr 10(2):e0262621. https://doi.org/10.1128/spectrum.02626-21
Kanaujia R, Singh S, Rudramurthy SM (2023) Aspergillosis: an update on clinical spectrum, diagnostic schemes, and management. Curr Fungal Infect Rep 4:1–12. https://doi.org/10.1007/s12281-023-00461-5
Horvath JA, Dummer S (1996) The use of respiratory-tract cultures in the diagnosis of invasive pulmonary aspergillosis. Am J Med 100(2):171–178. https://doi.org/10.1016/S0002-9343(97)89455-7
Perfect JR, Cox GM, Lee JY et al (2001) The impact of culture isolation of aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis 33(11):1824–1833. https://doi.org/10.1086/323900
Donnelly JP, Chen SC, Kauffman CA et al (2020) Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 71(6):1367–1376. https://doi.org/10.1093/cid/ciz1008
Stynen D, Sarfati J, Goris A et al (1992) Rat monoclonal antibodies against aspergillus galactomannan. Infect Immun 60(6):2237–2245. https://doi.org/10.1128/iai.60.6.2237-2245.1992
Ku NS, Han SH, Choi JY et al (2012) Diagnostic value of the serum galactomannan assay for invasive aspergillosis: it is less useful in non-haematological patients. Scand J Infect Dis 44(8):600–604. https://doi.org/10.3109/00365548.2012.657672
Egger M, Penziner S, Dichtl K et al (2022) Performance of the Euroimmun aspergillus antigen ELISA for the diagnosis of invasive pulmonary aspergillosis in bronchoalveolar lavage fluid. J Clin Microbiol 60(4):e0021522. https://doi.org/10.1128/jcm.00215-22
Buil JB, Huygens S, Dunbar A et al (2023) Retrospective multicenter evaluation of the VirClia galactomannan antigen assay for the diagnosis of pulmonary aspergillosis with bronchoalveolar lavage fluid samples from patients with hematological disease. J Clin Microbiol 61(5):e0004423. https://doi.org/10.1128/jcm.00044-23
Thornton CR (2008) Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin Vaccine Immunol 15(7):1095–1105. https://doi.org/10.1128/CVI.00068-08
Roiz-Mesones MP, Pintos-Fonseca AM, Ahedo-García N, Alegría-Puig CR (2023) Evaluation of the EUROIMMUN aspergillus antigen immunoenzyme assay in serum and bronchoalveolar lavage fluid samples. Enferm Infecc Microbiol Clin (Engl Ed) 41(3):176–180. https://doi.org/10.1016/j.eimce.2021.08.018
White PL, Parr C, Thornton C, Barnes RA (2013) Evaluation of real-time PCR, galactomannan enzyme-linked immunosorbent assay (ELISA), and a novel lateral-flow device for diagnosis of invasive aspergillosis. J Clin Microbiol 51(5):1510–1516. https://doi.org/10.1128/jcm.03189-12
Willinger B, Lackner M, Lass-Flörl C et al (2014) Bronchoalveolar lavage lateral-flow device test for invasive pulmonary aspergillosis in solid organ transplant patients: a semiprospective multicenter study. Transplantation 98(8):898–902. https://doi.org/10.1097/TP.0000000000000153
Prattes J, Flick H, Prüller F et al (2014) Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am J Respir Crit Care Med 190(8):922–929. https://doi.org/10.1164/rccm.201407-1275OC
Eigl S, Prattes J, Lackner M et al (2015) Multicenter evaluation of a lateral-flow device test for diagnosing invasive pulmonary aspergillosis in ICU patients. Crit Care 19(1):178. https://doi.org/10.1186/s13054-015-0905-x
Mercier T, Schauwvlieghe A, de Kort E et al (2019) Diagnosing invasive pulmonary aspergillosis in hematology patients: a retrospective multicenter evaluation of a novel lateral flow device. J Clin Microbiol 57(4):e01913–e01918. https://doi.org/10.1128/jcm.01913-18
Aerts R, Mercier T, Houben E, Schauwvlieghe A, Lagrou K, Maertens J (2022) Performance of the JF5-based galactomannoprotein EIA compared to the lateral flow device and the galactomannan EIA in serum and bronchoalveolar lavage fluid. J Clin Microbiol 60(11):e0094822. https://doi.org/10.1128/jcm.00948-22
Hoenigl M, Koidl C, Duettmann W et al (2012) Bronchoalveolar lavage lateral-flow device test for invasive pulmonary aspergillosis diagnosis in haematological malignancy and solid organ transplant patients. J Infect 65(6):588–591. https://doi.org/10.1016/j.jinf.2012.10.003
Hoenigl M, Prattes J, Spiess B et al (2014) Performance of galactomannan, beta-D-glucan, aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J Clin Microbiol 52(6):2039–2045. https://doi.org/10.1128/jcm.00467-14
Held J, Schmidt T, Thornton CR, Kotter E, Bertz H (2013) Comparison of a novel aspergillus lateral-flow device and the Platelia® Galactomannan assay for the diagnosis of invasive aspergillosis following haematopoietic stem cell transplantation. Infection 41(6):1163–1169. https://doi.org/10.1007/s15010-013-0472-5
Pan Z, Fu M, Zhang J, Zhou H, Fu Y, Zhou J (2015) Diagnostic accuracy of a novel lateral-flow device in invasive aspergillosis: a meta-analysis. J Med Microbiol 64(7):702–707. https://doi.org/10.1099/jmm.0.000092
Heldt S, Hoenigl M (2017) Lateral Flow assays for the diagnosis of invasive aspergillosis: current status. Curr Fungal Infect Rep 11(2):45–51. https://doi.org/10.1007/s12281-017-0275-8
Marr KA, Laverdiere M, Gugel A, Leisenring W (2005) Antifungal therapy decreases sensitivity of the aspergillus galactomannan enzyme immunoassay. Clin Infect Dis 40(12):1762–1769. https://doi.org/10.1086/429921
Acet-Öztürk NA, Ömer-Topçu D, Vurat Acar K et al (2024) Impact of posaconazole prophylaxis and antifungal treatment on BAL GM performance in hematology malignancy patients with febrile neutropenia: a real life experience. Eur J Clin Microbiol Infect Dis 43(1):33–43. https://doi.org/10.1007/s10096-023-04686-7
Acknowledgements
The authors thank the Richardson Guangzhou Centre for Fungal Diagnostics and Research for providing the AspLFD kit for research. This centre had no role in the study design, data collection and analysis, publish decision, or manuscript preparation.
Funding
This study was supported in part by the Natural Science Foundation of Anhui Province (grant number 2208085MH253), People’s Republic of China.
Author information
Authors and Affiliations
Contributions
Lijuan Wan: Conceptualization, Data Curation, Formal Analysis, Writing–Original Draft Preparation; Xueqin Cai: Data Curation, Methodology; Meng Ling: Formal Analysis, Writing–Review & Editing; Jinsong Kan: Validation; Meiling Yin: Visualization; Huiyan Wang: Conceptualization, Investigation, Writing–Review & Editing, Funding Acquisition.
Corresponding author
Ethics declarations
Ethical approval
This study involving human participants was reviewed and approved by Institutional Ethics Committee (IEC) of the First Affiliated Hospital of USTC (code 2024-013). This study was conducted in line with the Declaration of Helsinki.
Consent to participate
Informed consent was waived by the committee because of the retrospective use of remnant and de-identified samples. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wan, L., Cai, X., Ling, M. et al. Evaluation of the JF5-based Aspergillus galactomannoprotein lateral flow device for diagnosing invasive aspergillosis in cancer patients. Eur J Clin Microbiol Infect Dis 43, 1221–1229 (2024). https://doi.org/10.1007/s10096-024-04830-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-024-04830-x