Abstract
Background
A clear cutoff value of galactomannan (GM) has not been established for chronic pulmonary aspergillosis (CPA) and is frequently extrapolated from invasive pulmonary aspergillosis. We performed a systematic review and meta-analysis to evaluate the diagnostic performance of serum and bronchoalveolar lavage (BAL) GM, and to propose a cutoff.
Methods
We extracted from the studies the cutoff of serum or/and BAL GM associated with true positives, false positives, true negatives, and false negatives. We performed a multi-cutoff model and a non-parametric random effect model. We estimated the optimal cutoff and the area under the curve (AUC) for GM in serum and BAL samples.
Results
Nine studies from 1999 to 2021 were included. Overall, the optimal cutoff of serum GM was 0.96 with a sensitivity of 0.29 (95%CI: 0.14–0.51); specificity of 0.88 (95%CI: 0.73–0.95); and AUC of 0.529 (with a CI: [0.415–0.682] [0.307–0.713]). The AUC for the non-parametric ROC model was 0.631. For BAL GM the cutoff was 0.67 with a sensitivity of 0.68 (95%CI: 0.51–0.82), specificity of 0.84 (95%CI: 0.70–0.92), and AUC of 0.814 (with a CI: [0.696–0.895] [0.733–0.881]). The AUC for the non-parametric model was 0.789.
Conclusion
The diagnosis of CPA requires the assessment of a combination of mycological and serological factors, as no single serum and/or BAL GM antigen test is adequate. BAL GM performed better than serum, with better sensitivity and excellent accuracy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspergillosis is a disease caused by filamentous fungi of the genus Aspergillus, which are easily inhaled and deposited deep in the lungs leading to a variety of pulmonary syndromes, based on host immune response and the presence of lung comorbidities [1]. One of its forms is a progressive chronic disease of the lung, called chronic pulmonary aspergillosis (CPA) [2, 3].
CPA is a severe fungal infection usually seen in immunocompetent or mildly immunosuppressed patients with underlying structural lung diseases [4, 5]. According to European guidelines, CPA is divided into 5 different subtypes: chronic cavitary pulmonary aspergillosis, chronic fibrosing pulmonary aspergillosis, simple aspergilloma, Aspergillus nodule, and subacute invasive aspergillosis [6]. CPA affects 3 million people worldwide and consequently has been recognized as a global burden [7, 8]. Furthermore, contemporary series suggest a 50–85% 5-year mortality [9]. In spite of its high mortality, CPA is considered a neglected fungal disease when compared to invasive pulmonary aspergillosis (IA) [5]. Thus, the consolidated knowledge of IA is extrapolated to CPA in several situations, such as the diagnostic cutoff of galactomannan (GM).
GM is a polysaccharide component of the cell walls of Aspergillus spp., which is released during tissue invasion by the fungal hyphae [10, 11]. A meta-analysis of GM in bronchoalveolar lavage (BAL) showed a pooled sensitivity of 86-97% and pooled specificity of 83-95% with a cutoff of 1.0 for IA; [12]. while serum GM had a pooled sensitivity of 0.48–0.92, and a pooled specificity of 0.85–0.95 in meta-analyses [13,14,15,16].
However, observational studies have shown that the sensitivity and specificity of GM are lower in CPA [10], thus the usefulness of GM for CPA remains to be clarified [17]. A clear cutoff value has not been established for CPA. [11] We performed a systematic review and meta-analysis of published studies to evaluate the diagnostic performance of serum and BAL GM in CPA, and to propose a cutoff.
Methods
We performed a systematic review strictly following the Preferred Reporting Items for a Systematic Review and Meta-analysis (PRISMA) and PRISMA for Diagnostic Test Accuracy (PRISMA-DTA) checklist in all phases of this study: implementation, analysis, and reporting [18, 19].
Eligibility criteria
We included studies that included patients with CPA, compared with other non-CPA lung diseases or healthy individuals, regarding the serum and/or BAL value of galactomannan. We excluded case reports, unpublished articles, animal studies, and studies without a detailed description of sensitivity and specificity.
Search strategy and information sources
We searched PubMed/MEDLINE, Embase, Scopus, and Web of Science for published studies using the terms: "chronic pulmonary aspergillosis", "CPA", "simple aspergilloma", "pulmonary aspergilloma", "lung aspergilloma", "pulmonary mycetoma", "lung mycetoma", "simple aspergilloma", "complex aspergilloma", "mannans", "antigens, fungal", "galactomannan", "GM", "sensitivity", and "specificity". We did not use any language restriction. The last search was on May 12th, 2022. We searched the references of the studies for additional records.
Study selection
After duplicate removal, two qualified investigators independently evaluated the eligibility of the publications; discrepancies were resolved by consensus after discussion. Broad inclusion criteria were used for the studies. We first performed an initial screen of titles and abstracts to assess potential relevance. Afterwards, we obtained relevant full-text articles, reevaluated their eligibility, and determined their final inclusion or exclusion.
Data extraction
We extracted the following data from the studies, in a pilot-tested form: author; year of publication; definition of CPA; classification of subtypes of CPA; lung disease comparator; cutoff of serum or/and BAL galactomannan with associated true positives, false positives, true negatives, and false negatives. The extraction was done by two investigators, independently.
Quality assessment
We evaluated the risk of bias in all studies using the QUADAS-2 tool, which assessed
patient selection, the index test, the reference standard, and the flow and timing.
Statistical analysis
We performed a multi-cutoff model and a non-parametric random effect model, using
diagmeta and nsROC packages in R [20, 21]. The first has the advantage of presenting an optimal cutoff and the latter gives us a closer fit to the observed data. We estimated the optimal cutoff and the area under the curve (AUC) for the galactomannan in serum and BAL samples.
Results
Included Studies
From a total of 58 unique studies identified using the search strategy and assessed in full-text, we included 9 studies in our review [10, 11, 22,23,24,25,26,27,28]. The characteristics of the studies are summarized in Table 1. The PRISMA diagram describing the selection process is detailed in Figure 1.
Risk of bias
Figure 2 presents the risk of bias of individual studies. Regarding patient selection, five studies had consecutive sampling, while the others were case-control. A problem with the index test in the studies was that the galactomannan cutoff was not predetermined. Furthermore, the standard reference considered was the definition of CPA, which was based on the main guidelines. Finally, most studies were concerned with collecting galactomannan before starting beta-lactams, which can cause false-positives, and antifungal treatment, which can cause false-negatives.
Findings
Overall, 1347 participants were included. Publication years ranged from 1999 to 2021. Patients were from two continents: Europe (Germany) and Asia (Japan, South Korea, India, and China). The definitions of proven CPA in almost all studies were based on the criteria proposed by Denning [6, 29]. Regarding the studies that reported the subtypes of CPA, chronic cavitary pulmonary aspergillosis was the most common. Serum and BAL testing was performed using the Platelia Aspergillus enzyme immunoassay (Bio-Rad) in all participants.
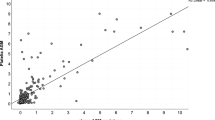
For GM in the serum, the optimal diagnostic cutoff was 0.96, with a sensitivity of 0.29 (95%CI: 0.14–0.51), specificity of 0.88 (95%CI: 0.73–0.95), and an AUC of 0.529 (with a CI: [0.415–0.682] - confidence region for specificity given sensitivity, and [0.307–0.713] - confidence region for sensitivity given specificity). AUC for the non-parametric ROC model was 0.631 (Figure 3), which contains a multi-cutoff model confidence interval.
Diagnosis of chronic pulmonary aspergillosis (CPA) using serum galactomannan (GM). Individual studies are displayed as colored lines. Each point represents a reported threshold value; points of the same color represent thresholds reported within the same study. The cross in the summary receiver operating characteristic (SROC) curve indicates the Younden-based threshold value: sensitivity=0.29 (95%CI: 0.14–0.51); specificity=0.88 (95%CI: 0.73–0.95); and AUC=0.529 (CI: [0.415–0.682] [0.307–0.713]). (A) Descriptive ROC curve. (B) SROC Curve with estimation. (C) The estimation of the Youden index. (D) ROC-random effects model
The optimal cutoff for BAL GM was 0.67, with a sensitivity of 0.68 (95%CI: 0.51–0.82), specificity of 0.84 (95%CI: 0.70–0.92), and an AUC of 0.814 (with a CI: [0.696–0.895] - confidence region for sensitivity given specificity, and [0.733–0.881] - confidence region for specificity given sensitivity). The AUC for the non-parametric ROC model was 0.789 (Figure 4), which contains a multi-cutoff model confidence interval.
Diagnosis of chronic pulmonary aspergillosis (CPA) using bronchoalveolar lavage galactomannan (GM). Individual studies are displayed as colored lines. Each point represents a reported threshold value; points of the same color represent thresholds reported within the same study. The cross in the summary receiver operating characteristic (SROC) curve indicates the Younden-based threshold value: sensitivity=0.68 (95%CI: 0.51–0.82); specificity=0.84 (95%CI: 0.70–0.92); and AUC=0.814 (CI: [0.696–0.895] [0.733–0.881] (A) Descriptive ROC curve. (B) SROC Curve with estimation. (C) The estimation of the Youden index. (D) ROC-random effects model
We performed a sensitivity analysis using only the data from studies performed in Asina countries, and we observed that the optimal cutoff for BAL GM was 0.79, with a sensitivity of 0.70 (95%CI: 0.54–0.82), specificity of 0.84 (95%CI: 0.72–0.92), and an AUC of 0.824 (with a CI: [0.696–0.895] - confidence region for sensitivity given specificity, and [0.752–0.881] - confidence region for specificity given sensitivity). The AUC for the non-parametric ROC model was 0.825. All studies of serum GM were performed in Asia.
Discussion
We evaluated the performance of the GM antigen test in the serum and BAL for the diagnosis of CPA. The performance of the serum GM antigen test was not acceptable due to low sensitivity and accuracy. However, the BAL GM antigen test had better sensitivity and accuracy, with less specificity. Overall, galactomannan alone is not an adequate assay for diagnosing CPA.
The serum GM antigen test was originally studied and approved for use in the diagnosis of invasive aspergillosis, with high sensitivity and specificity at a cut-off of 0.5 [10]. However, we observed that using this cutoff for CPA, sensitivity varied widely 22.6%-77.8%, as did specificity (43.1%-90%) [11, 23, 25,26,27]. This difference in performance was to be expected, since CPA and IA are different diseases and would probably require different cutoffs.
Already, Shin et al. had recommended that the serum GM antigen test not be used for the diagnosis of CPA [23]. In fact, the low sensitivity and accuracy we found at the optimal cutoff of 0.96 denote that it is not an adequate test. However, we found high specificity, which may be useful when used as an initial test to confirm CPA, potentially avoiding a more invasive investigation with BAL bronchoscopy [25].
BAL-GM performed better than serum GM due to better sensitivity and an excellent accuracy, using a cutoff of 0.67. Compared with serum GM, BAL GM test has higher sensitivity but a lower specificity. However, the BAL GM test is not as sensitive and specific, for CPA as it is for IA [12].
The serum and BAL GM seem to be very limited if used alone. Possibly it will be necessary to use a combination of methods such as serology and other microbiological assays for the diagnosis of CPA. For example, the combination of the GM and βDG tests on BAL was more useful than any single test for diagnosing CPA [11]. While the serum GM when combined with A fumigatus-specific IgG, it adds to the diagnostic accuracy of serological tests [25]. Sehgal et al. proposed a structured protocol using a serum GM followed by BAL GM, when the initial assays with serology and sputum culture for Aspergillus were negative [30]. Our study reinforces the sequential use of GM described in this protocol. Serum GM has high specificity and, if positive in an adequate clinical setting, confirms CPA. However, a negative result of GM does not exclude CPA, and justifies a more invasive test, the BAL.
Furthermore, sensitivity depends on the study population and the specimen [13]. We observed a difference in cutoff and AUC when modifying the analysis to include only Asian studies. This disparity may not necessarily reflect ethnic differences, but could be caused by different proportion of CPA subtypes in the included patients [31].
Our study has limitations. There are few studies of GM in CPA. Furthermore, the cutoff values varied widely. In addition, the CPA subtypes also varied in the studies which could have affected performance, especially in the subtypes with extensive involvement of the lung parenchyma, such as chronic fibrosing pulmonary aspergillosis and subacute invasive aspergillosis. Moreover, there was an imbalance in the study population only for BAL GM, as one study from Germany and eight studies from Asia were included. However, to our knowledge this is the first systematic review and meta-analysis on the subject.
Conclusion
Galactomannan antigen testing in CPA does not have the same diagnostic accuracy as in IA, and should not use the same cutoffs. It is apparent that no single serum and BAL GM antigen test is adequate. The optimal cutoff of serum GM was 0.96, with high specificity. BAL GM may be of potential use for the diagnosis of CPA using the cutoff of 0.67 with better sensitivity and an excellent accuracy when compared with serum GM.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available, because the authors elected to not share, but are available from the corresponding author on reasonable request.
References
Soubani AO, Chandrasekar PH (2002) The clinical spectrum of pulmonary aspergillosis. Chest 121:1988–1999
Rozaliyani A, Rosianawati H, Handayani D, Agustin H, Zaini J, Syam R, Adawiyah R, Tugiran M, Setianingrum F, Burhan E et al (2020) Chronic pulmonary aspergillosis in post tuberculosis patients in indonesia and the role of ldbio aspergillus ICT as part of the diagnosis scheme. J Fungi 6(4):318
Camara B, Reymond E, Saint-Raymond C, Roth H, Brenier-Pinchart M-P, Pinel C, Cadranel J, Ferretti G, Pelloux H, Pison C et al (2015) Characteristics and outcomes of chronic pulmonary aspergillosis: a retrospective analysis of a tertiary hospital registry. Clin Respir J 9:65–73
Barac A, Kosmidis C, Alastruey-Izquierdo A, Salzer HJF, CPA Net (2019) Chronic pulmonary aspergillosis update: A year in review. Med Mycol 57:S104–S109
Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC (2012) Hidden killers: human fungal infections. Sci Transl Med 4:165rv13
Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C, European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society (2016) Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 47:45–68
Denning DW, Pleuvry A, Cole DC (2011) Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ 89:864–872
The Lancet Respiratory Medicine (2016) Chronic pulmonary aspergillosis: help is on the way. Lancet Respir Med 4:83
Lowes D, Al-Shair K, Newton PJ, Morris J, Harris C, Rautemaa-Richardson R, Denning DW (2017) Predictors of mortality in chronic pulmonary aspergillosis. Eur Respir J 49(2)
Izumikawa K, Yamamoto Y, Mihara T, Takazono T, Morinaga Y, Kurihara S, Nakamura S, Imamura Y, Miyazaki T, Nishino T et al (2012) Bronchoalveolar lavage galactomannan for the diagnosis of chronic pulmonary aspergillosis. Med Mycol 50:811–817
Urabe N, Sakamoto S, Sano G, Suzuki J, Hebisawa A, Nakamura Y, Koyama K, Ishii Y, Tateda K, Homma S (2017) Usefulness of Two Aspergillus PCR Assays and Aspergillus Galactomannan and β-d-Glucan Testing of Bronchoalveolar Lavage Fluid for Diagnosis of Chronic Pulmonary Aspergillosis. J Clin Microbiol 55:1738–1746
Zou M, Tang L, Zhao S, Zhao Z, Chen L, Chen P, Huang Z, Li J, Chen L, Fan X (2012) Systematic review and meta-analysis of detecting galactomannan in bronchoalveolar lavage fluid for diagnosing invasive aspergillosis. PLoS ONE 7:e43347
Mercier T, Castagnola E, Marr KA, Wheat LJ, Verweij PE, Maertens JA (2021) Defining galactomannan positivity in the updated EORTC/MSGERC consensus definitions of invasive fungal diseases. Clin Infect Dis 72:S89–S94
Leeflang MMG, Debets-Ossenkopp YJ, Wang J, Visser CE, Scholten RJPM, Hooft L, Bijlmer HA, Reitsma JB, Zhang M, Bossuyt PMM et al (2015) Galactomannan detection for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev 2017(9)
Arvanitis M, Anagnostou T, Mylonakis E (2015) Galactomannan and Polymerase Chain Reaction-Based Screening for Invasive Aspergillosis Among High-Risk Hematology Patients: A Diagnostic Meta-analysis. Clin Infect Dis 61:1263–1272
Pfeiffer CD, Fine JP, Safdar N (2006) Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis 42:1417–1427
Ohba H, Miwa S, Shirai M, Kanai M, Eifuku T, Suda T, Hayakawa H, Chida K (2012) Clinical characteristics and prognosis of chronic pulmonary aspergillosis. Respir Med 106:724–729
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, the PRISMA-DTA Group, Clifford T, Cohen JF, Deeks JJ, Gatsonis C et al (2018) Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 319:388–396
Martínez-Camblor P (2017) Fully non-parametric receiver operating characteristic curve estimation for random-effects meta-analysis. Stat Methods Med Res 26:5–20
Steinhauser S, Schumacher M, Rücker G (2016) Modelling multiple thresholds in meta-analysis of diagnostic test accuracy studies. BMC Med Res Methodol 16:97
Kawamura S, Maesaki S, Noda T, Hirakata Y, Tomono K, Tashiro T, Kohno S (1999) Comparison between PCR and detection of antigen in sera for diagnosis of pulmonary aspergillosis. J Clin Microbiol 37:218–220
Shin B, Koh W-J, Jeong B-H, Yoo H, Park HY, Suh GY, Kwon OJ, Jeon K (2014) Serum galactomannan antigen test for the diagnosis of chronic pulmonary aspergillosis. J Infect 68:494–499
Salzer HJF, Prattes J, Flick H, Reimann M, Heyckendorf J, Kalsdorf B, Obersteiner S, Gaede KI, Herzmann C, Johnson GL et al (2018) Evaluation of Galactomannan Testing, the Aspergillus-Specific Lateral-Flow Device Test and Levels of Cytokines in Bronchoalveolar Lavage Fluid for Diagnosis of Chronic Pulmonary Aspergillosis. Front Microbiol 9:2223
Sehgal IS, Dhooria S, Choudhary H, Aggarwal AN, Garg M, Chakrabarti A, Agarwal R (2019) Efficiency of A fumigatus-specific IgG and galactomannan testing in the diagnosis of simple aspergilloma. Mycoses 62:1108–1115
Sehgal IS, Dhooria S, Choudhary H, Aggarwal AN, Garg M, Chakrabarti A, Agarwal R (2019) Utility of serum and bronchoalveolar lavage fluid galactomannan in diagnosis of chronic pulmonary aspergillosis. J Clin Microbiol 57:10
Takazono T, Ito Y, Tashiro M, Nishimura K, Saijo T, Yamamoto K, Imamura Y, Miyazaki T, Yanagihara K, Mukae H et al (2019) Evaluation of Aspergillus-Specific Lateral-Flow Device Test Using Serum and Bronchoalveolar Lavage Fluid for Diagnosis of Chronic Pulmonary Aspergillosis. J Clin Microbiol 57:10
Ye F, Zeng P, Li Z, Tang C, Liu W, Su D, Zhan Y, Li S (2021) Detection of aspergillus DNA in BALF by Real-time PCR and galactomannan antigen for the early diagnosis of chronic pulmonary aspergillosis. Ann Clin Lab Sci 51(5):698–704
Denning DW, Riniotis K, Dobrashian R, Sambatakou H (2003) Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis 37 (Suppl 3):S265–S280
Sehgal IS, Dhooria S, Muthu V, Prasad KT, Agarwal R (2020) An overview of the available treatments for chronic cavitary pulmonary aspergillosis. Expert Rev Respir Med 14:715–727
de Oliveira VF, Viana JA, Sawamura MVY, Magri ASGK, Costa N, Abdala E et al (2022) Sensitivity of antigen, serology, and microbiology assays for diagnosis of the subtypes of chronic pulmonary aspergillosis at a teaching hospital in São Paulo, Brazil. Am J Trop Med Hygiene 108(1):22–26
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Vítor Falcão de Oliveira and Guilherme Diogo Silva. The first draft of the manuscript was written by Vítor Falcão de Oliveira and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Our study did not require ethics approval.
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira, V.F., Silva, G.D., Taborda, M. et al. Systematic review and meta-analysis of galactomannan antigen testing in serum and bronchoalveolar lavage for the diagnosis of chronic pulmonary aspergillosis: defining a cutoff. Eur J Clin Microbiol Infect Dis 42, 1047–1054 (2023). https://doi.org/10.1007/s10096-023-04639-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04639-0