Abstract
The aim of this study is to clarify the characteristics of gram-negative bacteremia (GNB), including extended-spectrum β-lactamase (ESBL)-producing pathogens, among allogeneic hematopoietic stem cell transplant (allo-HSCT) recipients on levofloxacin (LVFX) prophylaxis. A retrospective analysis on GNB at the first episode of febrile neutropenia (FN) was conducted among allo-HSCT recipients (age ≥ 20 years) on 500 mg/day of oral LVFX prophylaxis. Epidemiological and microbiological features of GNB were investigated and compared between the inappropriate and appropriate empiric therapy groups. In total, FN occurred in 414 allo-HSCT cases, and bacteremia at the first episode of FN occurred in 169 cases. Overall, 29 GNB cases were documented, and the causative organisms identified were Escherichia coli in 21 cases (including 10 ESBLs), Klebsiella pneumoniae in 2, Pseudomonas aeruginosa in 2, and other in 4. The crude 30-day mortality rate was not significantly different among cases of GNB (6.9%), gram-positive bacteremia (GPB) (7.1%), or non-bacteremia (5.4%; P = 0.78). Cefepime (CFPM) was administered in all cases in the inappropriate empiric therapy group, and all causative organisms were ESBL-producing E. coli (ESBL-EC). All patients in the inappropriate empiric therapy group had a low Pitt bacteremia score (≤ 2). Thirty-day mortality did not differ significantly between the inappropriate and appropriate empiric therapy groups (1/10 vs. 1/15, P = 0.61). In conclusion, GNB was not a significant cause of death. In LVFX breakthrough ESBL-EC bacteremia among allo-HSCT recipients, the administration of CFPM as empiric therapy did not lead to significantly poor prognosis. Empiric CFPM administration might be an acceptable strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Febrile neutropenia (FN) is the severe adverse events that can occur during chemotherapy for cancer [1]. In allogeneic hematopoietic stem cell transplantation (allo-HSCT) recipients especially, bloodstream infection (BSI) during FN remains a severe and life-threatening problem [2,3,4]. According to current guidelines, fluoroquinolone (FQ) prophylaxis, including levofloxacin (LVFX) prophylaxis, should be considered for high-risk patients, including allo-HSCT recipients, as the preventive strategy against bacterial infection [5, 6].
However, in this era where multidrug-resistant gram-negative bacteria are increasing worldwide, there are also concerns about the association between inducing or selecting multidrug-resistant strains, including extended-spectrum β-lactamase (ESBL)-producing pathogens and exposure to FQs [7, 8]. Some studies involving patients with hematological malignancy reported higher mortality rates associated with ESBL-producing Enterobacteriaceae (ESBL-E) bacteremia compared with bacteremia caused by non-ESBL-E [9]. A recent meta-analysis suggested that cefepime (CFPM) as empiric therapy for patients with FN was associated with a higher risk of all-cause death and this might be caused by inappropriate therapy for ESBL-E [10]. Reporting on the epidemiology and treatment strategy for gram-negative bacteremia (GNB), including ESBL-producing pathogens, in the setting of allo-HSCT might help to prevent poor outcome. To this end, in this study, we investigated the epidemiology of GNB and the treatment strategy used at the first episode of FN in allo-HSCT recipients on LVFX prophylaxis.
Methods
Study design
This retrospective analysis of BSI focused on GNB among allo-HSCT recipients (aged ≥ 20 years) who received standard prophylaxis with 500 mg/day oral LVFX at the 890-bed Toranomon Hospital in Tokyo between January 2011 and December 2016. Medical and microbiological records were reviewed. This study was approved by the Human Ethics Review Committee of Toranomon Hospital.
Study population
We included all recipients of allo-HSCT who fulfilled two inclusion criteria: LVFX prophylaxis 500 mg/day oral started before day − 7 (7 days before allo-HSCT); and LVFX prophylaxis stopped at the first episode of FN and changed to another antimicrobial regimen immediately after obtaining ≥ 2 sets of blood cultures.
A central venous catheter was inserted before starting the conditioning regimen. Trimethoprim-sulfamethoxazole was given from day − 7 through day − 2 for Pneumocystis pneumonia prophylaxis.
Monotherapy with an anti-pseudomonal β-lactam (APBL) agent, as recommended by the Infectious Disease Society of America guidelines, is the routine empiric antimicrobial regimen initially administered at the onset of FN [6]. Our clinicians could choose any APBL, such as CFPM, piperacillin/tazobactam (PIPC/TAZ), and meropenem (MEPM). Amikacin (AMK) could be combined with an APBL regimen for suspected severe sepsis caused by β-lactam-resistant gram-negative rod. Anti-gram-positive agents were combined with the APBL regimen for specific clinical indications, including suspected catheter-related infection, skin and soft tissue infection, or severe sepsis caused by β-lactam-resistant gram-positive organisms. Most of the clinicians chose vancomycin as the first-line anti-gram-positive agent.
Definitions
The definition of FN was body temperature ≥ 37.5 °C measured at the axillary fossa, as routinely taken in Japan [11], and absolute neutrophil count (ANC) < 500 cells/μL or ANC that decreased to < 500 cells/μL with 48 h following fever onset [6].
All hematological disorders were defined as either standard risk or high risk [12]. Conditioning regimens were classified based on a report by the Center for International Blood and Marrow Transplant Research [13]. Recipients with prior history of allo-HSCT are those who have received allo-HSCT two or more times.
BSI was defined as isolation of a bacterial or fungal pathogen from at least 1 blood culture. However, coagulase-negative staphylococci, Corynebacterium species, and unidentified gram-positive cocci were considered contaminants unless cultured from ≥ 2 separate blood culture bottles. Co-infection was defined as identification of ≥ 2 bacterial species in multiple blood culture bottles of samples collected within 24 h.
Appropriate empiric therapy was defined as treatment with an antimicrobial agent to which the causative organism was susceptible within 24 h at the first episode of FN. Severity at BSI onset was evaluated by the Pitt bacteremia score (PBS) [14]. Thirty-day mortality was defined as death within 30 days after developing FN.
Identification and antimicrobial susceptibility
Organisms cultured from blood samples were identified using the VITEK2 system (bioMérieux, Marcy l’Etoile, France) or the WalkAway 96 SI system (Siemens Healthcare, Deerfield, IL). The Clinical and Laboratory Standards Institute (CLSI) criteria were used to define susceptibility or resistance to the antimicrobial agents studied. ESBL production was detected using the Mast® D68C test (Kanto Kagaku, Tokyo, Japan) and/or the disk-diffusion method according to the CLSI recommendations [15, 16]. The breakpoint of APBL was determined according to CLSI M100-S29 [16].
Statistical analysis
Among GNB, GPB, and non-bacteremia groups, categorical variables were compared using Fisher’s exact test. Additionally, post hoc testing was performed to investigate significant group differences in variables with P values of < 0.05. Thirty-day mortality rates were estimated using Kaplan-Meier analysis and the groups were compared using the log-rank test. All statistical analysis was performed with EZR (Saitama Medical Center, Jichi Medical University, Japan), a graphical user interface for R (The R Foundation for Statistical Computing) [17].
Results
Patients characteristics
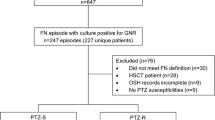
FN occurred in 414 of the 417 allo-HSCT patients who received prophylactic LVFX during the study period. Prophylactic LVFX was changed to any one of the APBLs in all 414 cases immediately after obtaining ≥ 2 sets of blood cultures at the onset of the first FN episode. Four of the 414 cases were excluded from this study due to GNB and GPB mixed infection (3/4 cases) and fungal bacteremia (1/4 case). The remaining 410 allo-HSCT cases comprised 253 (61.7%) men and 157 (38.3%) women, with a median age of 55 years (range, 20–75 years). The most common underlying disease was acute myeloid leukemia (n = 191; 46.6%). Overall, 299 (72.9%) received cord blood transplantation (CBT), 69 (16.8%) received bone marrow transplantation, and 43 (10.2%) received peripheral blood stem cell transplantation.
Incidence of BSI and isolates
Bacteremia in the first FN episode occurred in 169 (41.2%) of the 410 cases; polymicrobial bacteremia was evident in 26 of these cases. Table 1 lists the patients’ clinical characteristics. In total, 29 (17.2%) of 169 cases had GNB and 140 (82.8%) had gram-positive bacteremia (GPB). The median time to onset of BSI was 4 days (range − 7 to 12 days) after HSCT. A total of 194 causative organisms were identified from blood samples in the 169 cases; 165 (85.1%) were gram-positive bacteria and 29 (14.9%) were gram-negative bacteria.
Among the GNB, GPB, and non-bacteremia groups, there were significant differences in CBT, HLA4/6, severe neutropenia, and neutropenia of > 7 days. Post hoc testing identified CBT, HLA4/6, and neutropenia of > 7 days as risk factors for GPB only and severe neutropenia as a risk factor for both GPB and GNB.
Of the 29 GNBs documented, causative organisms were E. coli in 21 cases including 10 ESBLs (12.4% of total, 72.4% of gram-negative), Klebsiella pneumoniae in 2 cases (non-ESBLs), Pseudomonas aeruginosa in 2 cases, Helicobacter cinaedi in 2 cases, and other in 2 cases (Table 2). Among the GPBs, the common causative organisms were viridans group streptococci (38.5% of the total, and 46.4% of gram-positive) and coagulase-negative streptococci (21.3% of total, and 25.7% of gram-positive).
All isolates that had established clinical breakpoints were resistant to LVFX. No carbapenem-resistant Enterobacteriaceae (CRE) was documented.
Therapeutic regimens and outcomes
Among the 410 FN patients, the CFPM-based regimen (89%) was most frequently used for empiric therapy, followed by PIPC/TAZ-(8.8%) and MEPM-based (2%) regimens. Among the 29 GNB cases, treatment was started with CFPM monotherapy in 25 cases (86.2%), with CFPM and AMK in 2 cases (6.9%), with PIPC/TAZ monotherapy in 1 case (3.4%), and with PIPC/TAZ and AMK in 1 case (3.4%). For patients with GNB, the 30-day crude mortality rate was 6.9% (2/29), and this rate did not differ significantly among those with GNB (6.9%), those with GPB (7.1%), or those without bacteremia (5.4%; P = 0.78; Fig. 1).
Characteristics of the appropriate and inappropriate empiric therapy groups for GNB
The results of in vitro susceptibility testing indicated that empiric therapy was inappropriate in 10 of 25 GNB cases (40%; excluding 4 pathogens that do not have CLSI breakpoints [2 H. cinaedi, 1 Campylobacter spp. and 1 Fusobacterium spp.]). Table 3 shows the characteristics of patients with inappropriate empiric therapy. Inappropriate empiric therapy consisted of CFPM in all cases and all causative organisms were ESBL-producing E. coli (ESBL-EC). Appropriate therapy (AMK, PIPC/TAZ, or MEPM) was initiated in all cases as soon as the response to CFPM was confirmed or gram-negative bacteria were detected from blood culture (median 1.0 day [0–4 days] after infection onset); eventually all cases were treated with MEPM. All the 10 inappropriate empiric therapy cases showed low PBS (≤ 2), of these 1 (10%) patient died due to intracranial hemorrhage 21 days after onset of bacteremia. The crude 30-day mortality rate (1/10, 10%) was not significantly different from that of the appropriate empiric therapy group (1/15, 6.7%; P = 0.61; Fig. 2).
Discussion
Three major findings can be highlighted in this study. First, we clarified the characteristics of GNB during the first episode of FN among allo-HSCT recipients on LVFX prophylaxis. Second, GNB did not cause significant mortality. Third, the use of CFPM as empiric therapy, even if the causative organism was ESBL-EC, did not cause death in less severe cases (PBS ≤ 2).
Some previous studies have discussed the epidemiology and efficacy of FQ prophylaxis in allo-HSCT [18,19,20,21]. However, to our knowledge, this is the first study focusing on GNB in the first episode of FN under LVFX prophylaxis.
The 30-day mortality rate in cases of GNB did not significantly differ with that in cases of GPB or non-bacteremia. On the other hand, GNB is a major cause of illness and death in the previous study [22]. Girmenia et al. reported that pre-engraftment GNB after allo-HSCT represented an independent prognostic factor and was the cause of death in 39.1% of allo-HSCT recipients. The poor prognostic impact of GNB was related mainly to infection with carbapenem non-susceptible enterobacteria and P. aeruginosa [19]. There were no carbapenem non-susceptible enterobacteria and few P. aeruginosa in our study. This might be one of the reasons for the lack of significant differences among the GNB, GPB, and non-bacteremia groups. We analyzed only the first episode of FN under LVFX prophylaxis during the pre-engraftment phase and this may have affected the lack of CRE extracted as a result of exposure to various antibiotics.
Among the 25 GNB (excluding the 4 pathogens that had no CLSI breakpoints), there were 10 (40%) cases of inappropriate empiric therapy and all involved bacteremia due to ESBL-EC in which we started empiric antibiotic therapy with CFPM. Nevertheless, 30-day mortality in this group was not significant different from that in the appropriate empiric therapy group (1/10 vs. 1/15, respectively, P = 0.61). Furthermore, in only 1 recipient who died was death considered to be remotely related to ESBL-EC bacteremia (cause of death was intracranial hemorrhage). This is probably because all cases that received inappropriate empiric therapy for ESBL-EC bacteremia were of mild severity. In fact, all cases showed low PBS (≤ 2). Therefore, in empiric therapy for ESBL-EC bacteremia, inappropriate therapy for mild cases might have no effect on mortality as long as the treatment is switched to an effective regimen for ESBL-EC, such as carbapenem, immediately after documentation of gram-negative bacteria from the blood sample or soon after signs of deterioration such as hypotension.
Regarding the use of CFPM in patients with febrile neutropenia, it has been reported that patients treated with CFPM have no difference in mortality compared to patients treated with other antibiotics [23]. On the other hand, there is a report that the mortality rate was significantly higher in patients who received CFPM [24], and a most recent meta-analysis also showed that CFPM use as empiric therapy for FN might increase morbidity [10]. This was considered to be likely due to the effect of ESBL-EC bacteremia. Indeed, another study reported that patients with oncologic cancer, including those with hematological malignancy and those who undergone HSCT, had higher mortality when ESBL-EC bacteremia was confirmed during neutropenia compared with non-ESBL-EC bacteremia [25]. Patients with ESBL-EC bacteremia are more likely to receive inappropriate therapy compared with patients with non-ESBL-EC bacteremia, and in the state of neutropenia, the mortality rate is higher with ESBL-EC bacteremia than it is with non-ESBL-EC bacteremia [25]. Therefore, given the possibility of ESBL-EC bacteremia, carbapenems such as MEPM are recommended as empiric therapy for FN. Nevertheless, overuse of empiric carbapenem should be avoided because it might be associated with an increase in CRE [26].
For options other than carbapenems, β-lactam/β-lactamase inhibitors such as PIPC/TAZ can be considered. In an open-labeled randomized controlled trial, definitive therapy with PIPC/TAZ for ceftriaxone non-susceptible E. coli or K. pneumoniae was inferior to MEPM, although the study population did not include FN patients [27]. Thus, the empiric use of PIPC/TAZ might not help to avoid overusing carbapenem for treating FN.
Administering empiric carbapenem to only patients at high risk of developing ESBL-producing bacteremia among HSCT recipients could be one solution. It was reported that among HSCT recipients receiving FQ prophylaxis, stool colonization of ESBL-E prior to transplantation was associated with ESBL-E bacteremia developing, and ESBL-E colonization in stool has a 32.2% positive predictive value (PPV) and 99.6% of negative predictive value (NPV) for with ESBL-E bacteremia [28]. While the NPV is high, the PPV is relatively low, so unnecessary empiric therapy with carbapenems will be instituted for about 70% of patients who will not develop ESBL-E bacteremia. Moreover, Arnan et al. found no significant association between ESBL-EC bacteremia and colonization in stool among hematological malignancy patients, including HSCT recipients [29]. Therefore, the strategy of administering carbapenem based on stool screening may not be sufficient to ensure proper empiric carbapenem usage for FN.
Our findings indicate that empiric CFPM for less severe ESBL-EC bacteremia may not be associated with mortality. In this era of increasing ESBL-E worldwide, severity-directed empiric therapy or severity combined with ESBL-E colonization might offer a better therapeutic strategy while avoiding carbapenem overuse and preventing poor prognosis. In addition, anaerobic coverage was recently reported to be associated with risk and mortality of acute graft-versus-host disease [30]. Therefore, cefepime might be a more suitable empiric therapy of FN compared with carbapenems and PIPC/TAZ in allo-HSCT settings.
Our study has several limitations. First, it is a retrospective, single-center study. The prevalence of ESBL-E differs depending on the geographic areas, so practice in areas with high proportions of ESBL-E bacteremia remains controversial. Second, ESBL confirmatory testing was performed with the disk-diffusion method according to the Mast® D68C test and/or double-disk synergy test but did not involve PCR assay for β-lactamase genes. Third, this study has a small number of cases in the inappropriate therapy group. All the inappropriate therapy cases showed low PBS, so it was not possible to analyze the association between inappropriate therapy and worse prognosis in this group. However, in gram-negative BSI, high PBS is often associated with poor prognosis, suggesting that the inappropriate group was probably composed of milder cases [31]. Thus, the validity of the strategy where clinicians select empiric therapy on the basis of severity should be examined with a large number of patients. Fourth, although the limitations of FQ prophylaxis are recognized [4, 32, 33], it remains an accepted protocol for neutropenic patients with hematological malignancy and severe neutropenia [4, 20, 21]. Therefore, analyzing the impact of LVFX breakthrough infections is still important in real-world allo-HSCT settings currently.
In this study, we have reported on the epidemiology of GNB during the first FN episode among allo-HSCT recipients on LVFX prophylaxis. GNB was not a significant cause of death. In LVFX breakthrough ESBL-EC bacteremia among allo-HSCT recipients, the administration of CFPM as empiric therapy did not lead to significantly poor prognosis. This finding might contribute to establishing a strategy to avoid carbapenem overuse without worsening prognosis.
Data availability
The dataset generated during and/or analyzed during the current study is available from the corresponding author on reasonable request.
References
Klastersky J, Ameye L, Maertens J, Georgala F, Muanza F, Aoun M et al (2007) Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents 30(Suppl 1):S51–S59
Almyroudis NG, Fuller A, Jakubowski A, Sepkowitz K, Jaffe D, Small TN et al (2005) Pre- and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis 7:11–17
Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR (2007) Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant 40:63–70
Mikulska M, Del Bono V, Raiola AM, Bruno B, Gualandi F, Occhini D et al (2009) Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant 15:47–53
Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J (2009) Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 15:1143–1238
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious disease society of America. Clin Infect Dis 52:427–431
Kanafani ZA, Mehio-Sibai A, Araj GF, Kanaan M, Kanj SS (2005) Epidemiology and risk factors for extended-spectrum beta-lactamase-producing organisms: a case control study at a tertiary care center in Lebanon. Am J Infect Control 33:326–332
Mendelson G, Hait V, Ben-Israel J, Gronich D, Granot E, Raz R (2005) Prevalence and risk factors of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in an Israeli long-term care facility. Eur J Clin Microbiol Infect Dis 24:17–22
Kang CI, Chung DR, Ko KS, Peck KR, Song JH, The Korean Network for Study of Infectious Disease (KONSID) (2012) Risk factors for infection and treatment outcome of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae bacteremia in patients with hematologic malignancy. Ann Hematol 91:115–121
Horita N, Shibata Y, Watanabe H, Namkoong H, Kaneko T (2017) Comparison of antipseudomonal β-lactams for febrile neutropenia empiric therapy: systematic review and network meta-analysis. Clin Microbial Infect 23:723–729
Masaoka T (2004) Evidence-based recommendations for antimicrobial use in febrile neutropenia in Japan: executive summary. Clin Infect Dis 39:S49–S52
Yamamoto H, Uchida N, Matsuno N, Ota H, Kageyama K, Wada S et al (2014) Anti- HLA antibodies other than against HLA-A, B, DRB1 adversely affect engraftment and nonrelapse mortality in HLA-mismatched single cord blood transplantation: possible implications of unrecognized donor-specific antibodies. Biol Blood Marrow Transplant 20:1634–1640
Giral S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, Sandmaier B (2009) Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 15:367–369
Chow JW, Yu VL (1999) Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents 11:7–12
Nourrisson C, Tan RN, Hennequin C, Gibold L, Bonnet R, Robin F (2015) The MAST® D68C test: an interesting tool for detecting extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae. Eur J Clin Microbiol Infect Dis 34:975–983
Clinical and Laboratory Standard Institute (2019) Performance standards for antimicrobial susceptibility testing, 29th Edition: CLSI M100-ED29
Kanda Y (2013) Investigation of the freely available east-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Blennow O, Ljungman P, Sparrelid E, Mattsson J, Remberger M (2014) Incidence, risk factors, and outcome of bloodstream infections during the pre-engraftment phase in 521 allogeneic hematopoietic stem cell transplantations. Transpl Infect Dis 16:106–114
Girmenia C, Bertaina A, Piciocchi A, Perruccio K, Algarotti A, Busca A et al (2017) Incidence, risk factors and outcome of pre-engraftment gram-negative bacteremia after allogeneic and autologous hematopoietic stem cell transplantation: an Italian prospective multicenter survey. Clin Infect Dis 65:1884–1896
Bucaneve G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G et al (2005) Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med 353:977–987
Gafter-Gvili A, Fraser A, Paul M, Leibovici L (2005) Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med 142:979–995
Trecarichi EM, Pagano L, Candoni A, Pastore D, Cattaneo C, Fanci R et al (2015) Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: an Italian multicentre prospective survey. Clin Microbiol Infect 21:337–343
Kim PW, Wu Y, Cooper C, Rochester G, Valappil T, Wang Y et al (2010) Meta-analysis of a possible signal of increased mortality associated with cefepime use. Clin Infect Dis 51:381–389
Yahav D, Paul M, Fraser A, Sarid N, Leibovici L et al (2007) Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis 7:338–348
Gudiol C, Calatayud L, Garcia-Vidal C, Lora-Tamayo J, Cisnal M, Duarte R et al (2010) Bacteraemia due to extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) in cancer patients: clinical features, risk factors, molecular epidemiology and outcome. J Antimicrob Chemother 65:333–341
Schwaber MJ, Carmeli Y (2008) Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA 300:2911–2913
Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M et al (2018) Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 320:984–994
Satlin MJ, Chavda KD, Baker TM, Chen L, Shashkina E, Soave R et al (2018) Colonization with levofloxacin-resistant extended-spectrum β-lactamase-producing Enterobacteriaceae and risk of bacteremia in hematopoietic stem cell transplant recipients. Clin Infect Dis 67:1720–1728
Arnan M, Gudiol C, Calatayud L, Liñares J, Dominguez MÁ, Batlle M et al (2011) Risk factors for, and clinical relevance of, faecal extended-spectrum β-lactamase producing Escherichia coli (ESBL-EC) carriage in neutropenic patients with haematological malignancies. Eur J Clin Microbiol Infect Dis 30:355–360
Tanaka JS, Young RR, Heston SM, Jenkins K, Spees LP, Sung AD et al (2020) Anaerobic antibiotics and the risk of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant S1083-8791(20):30442–0. https://doi.org/10.1016/j.bbmt.2020.07.011
Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM (2013) Predictive scoring model of mortality in Gram-negative bloodstream infection. Clin Microbiol Infect 19:948–954
Kern WV, Klose K, Jellen-Ritter AS, Oethinger M, Bohnert J, Kern P et al (2005) Fluoroquinolone resistance of Escherichia coli at a cancer center: epidemiologic evolution effects of discontinuing prophylactic fluoroquinolone use in neutropenic patients with leukemia. Eur J Clin microbial Infect Dis 24:111–118
Hakki M, Humphries RM, Hemarajata P, Tallman GB, Shields RK, Mettus RT et al (2019) Fluoroquinolone prophylaxis selects for meropenem-nonsusceptible Pseudomonas aeruginosa in patients with hematologic malignancies and hematopoietic cell transplant recipients. Clin Infect Dis 68:2045–2052
Acknowledgments
We thank the staff of the microbiology laboratory of Toranomon Hospital (Ms Chikako Okada, Ms. Reiko Yabusaki, Ms. Mayumi Yamanaka, Ms. Hiromi Baba, Ms. Noriko Watahiki, Mr. Masaru Baba, Ms. Emiko Miyajima, Ms. Chiemi Yoshino, Ms. Ayumi Takamura, and Mr. Yusuke Endo) for performing tests for identification and antimicrobial susceptibility of ESBL-EC. This research is supported by the Okinaka Memorial Institute for Medical Research, Tokyo, Japan.
Author information
Authors and Affiliations
Contributions
SO and MK designed the study and collected data. SO, MK, and HA analyzed the clinical data. SO and MK wrote the manuscript. SO, MK, ST, TM, MY, KK, DK, AN, YT, KI, HY, YA-M, GY, NU, AW, SU-T, and HA interpreted the results and reviewed the final draft of the manuscript. All authors read and revised the final manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
The protocol of this retrospective study was evaluated by the Human Ethics Review Committee of Toranomon Hospital and waived from the requirement of patient informed consent.
Consent to publish
All the authors reviewed this study and agreed to publish this manuscript.
Ethics approval
This study was approved by the Human Ethics Review Committee of Toranomon Hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ogura, S., Kimura, M., Takagi, S. et al. Characteristics of gram-negative bacteremia during febrile neutropenia among allogeneic hematopoietic stem cell transplant recipients on levofloxacin prophylaxis. Eur J Clin Microbiol Infect Dis 40, 941–948 (2021). https://doi.org/10.1007/s10096-020-04096-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-020-04096-z