Abstract
To review the epidemiology and measures to control meticillin-resistant Staphylococcus aureus, MRSA, in Stockholm between 2000 and 2016 from the perspective of the Department of Communicable Disease Control and Prevention, Stockholm County Council, Sweden. Age, sex, and place of acquisition of their MRSA on all patients reported to the department were reviewed. Measures for control included surveillance through mandatory reporting of cases, screening patients with risk factors for MRSA, strict adherence to basic nursing hygienic principles, isolation of MRSA positive patients in single rooms in dedicated MRSA wards, and cohorting of staff. An MRSA team was created at the Department of Infectious Diseases, Karolinska University Hospital, for follow-up of all cases. Several administrative meetings and cooperative groups were formed that are still in function. From 2000 to 2016, there were 7373 MRSA cases reported. Healthcare-associated MRSA, HA-MRSA, was successfully controlled, and from 2006 onwards, very limited HA-MRSA transmission or outbreaks occurred. However, incidence increased overall, from 9.5 per 100,000 in 2000 to 37.3 per 100,000 in 2016, due to increase of MRSA acquired abroad and of MRSA acquired in the Swedish community. Surveillance and control measures have been successful in containing HA-MRSA in Stockholm, Sweden, but incidence has increased substantially due to imported cases and spread in the Swedish community. The strategy may be termed “search-and-contain” since screening, infection control, follow-up, and advice on personal hygiene were cornerstones of control, whereas eradication of carriage was not.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MRSA is an increasing health threat causing healthcare-associated as well as community-associated infections [1]. Sweden has had a low incidence of MRSA and few large-scale outbreaks [2]. This has been partly attributed to the so-called search-and-destroy strategy used by the Scandinavian countries and The Netherlands [3,4,5]. In Stockholm County, with a population of over 2 million, there has been a significant change in incidence and epidemiology of MRSA during the last 15–20 years. A substantial increase of HA-MRSA infections in the beginning of the 2000s, then prevalent in Europe, prompted public health authorities to institute several regulations to contain the spread. These actions were successful and during the last 10 years, there has been only a handful of HA-MRSA cases yearly. Instead, the now much higher incidence is due to acquisition of MRSA carriage and infections after travel and medical care abroad, and to the spread of community-associated (CA-MRSA) strains.
Here we describe the changing epidemiology and attempts to control MRSA in Stockholm between 2000 and 2016 from the perspective of the Department of Communicable Disease Control and Prevention, CDCP, Stockholm County Council, Sweden.
Materials and methods
Setting
Stockholm County Council (from 2019 named “Region Stockholm”) is the political and administrative organization responsible for providing healthcare in the Stockholm region with 2.3 million people (June 2017). There are twenty-five primary communities in Stockholm County, varying in size with Stockholm Town being the largest with almost one million inhabitants. There are six acute-care hospitals in the region. Karolinska University Hospital is the largest with two sites, a northern site at Solna, and a southern site at Huddinge. In addition, there are three middle-sized and two smaller acute-care hospitals. The CDCP department is the legal authority within the Region Stockholm for surveillance, control, and prevention of communicable diseases. There is one such authority in each of the 21 Swedish counties in accordance with the Swedish Communicable Diseases Act (latest version 2004:168).
Infection surveillance and control
Regulations and routines for the containment of MRSA were introduced at all levels of medical care and administration in Stockholm County and are summarized in chronological order in Table 1.
Reporting and surveillance
All cases of MRSA diagnosed in Sweden are, since the year 2000, to be reported to the local county department for control of infectious diseases as well as to the Swedish Public Health Authority via Sweden’s national electronic surveillance system for the reporting of communicable diseases, SmiNet [6]. For each MRSA case, there are two reports, both based on the personal identification number (PIN), which is unique for all Swedish inhabitants. One report is from the microbiology laboratory with information on patient data, the clinical unit submitting the sample, sample site or body fluid, and laboratory method used to detect MRSA. The other report comes from the clinician with information about epidemiological factors of the specific patient and advice given on personal measures to contain further spread.
In 2001, the CDCP employed a nurse to monitor the MRSA epidemiology. All cases were reviewed on the day of reporting (except on weekends), and missing data were collected, as well as additional data whether the MRSA was likely acquired abroad or in Sweden, and whether it was HA-MRSA or CA-MRSA. More detailed information was usually elicited, such as whether the HA-MRSA was acquired in an acute-care hospital or a long-term care facility, or if the patient was a healthcare worker. A database system introduced in 2003 with information on current and previous hospital admissions for each patient helped in the classification (the “MRSA system,” an Excel-based system). During the study period, these data were almost exclusively collected by one of the authors (U-B T).
Detection of MRSA patients
Screening for the detection of MRSA was introduced in 2001 for patients admitted to any hospital in Stockholm County with risk factors for MRSA carriage, or if they had been exposed to medical care abroad, as shown in Table 1. Screening samples were taken from the nose, throat, perineum, any skin lesion, and catheter urine, as well as the introduction site of indwelling catheters and skin around any stoma site. From 2003, outpatients with risk factors were screened in a similar way, as well as all infections being clinically suspected of being staphylococcal in origin. Standard laboratory methods using culture-plates with discs containing oxacillin were used. Staphylococcus aureus colonies judged to be oxacillin-resistant were confirmed to be MRSA by polymerase-chain reaction for the detection of the nuc and mecA genes. Typing of most MRSA strains was performed using pulsed-field gel electrophoresis. Laboratory costs for MRSA were since 2002 financed by the Stockholm County central administration for medical care, not by the clinical unit submitting the sample. Compliance with the screening guidelines was assessed, initially four-yearly and later twice-yearly, in all acute-care hospitals and geriatric units in Stockholm County.
Infection control
Beginning in 2001, patients were to be nursed in single rooms with strict adherence to basic hygienic principles. In 2003, two clinical units were created at the Department of Infectious Diseases (ID) at Karolinska University Hospital, one at the northern and one at the southern site. It was intended that all patients in Stockholm County infected or colonized by MRSA, and in need of hospital care, should be nursed in these wards, if medically safe. This was changed in 2012 when patients should be admitted to the medical department best suited for the clinical condition. Cohorting of nursing staff was made mandatory from 2003 and was not abandoned until 2015.
Two infection-control nurses were employed in 2003 to assist medical staff and clinical units throughout Stockholm County with advice for care of MRSA patients. Detailed practical guidelines have been issued and regularly updated from 2001 by the CDCP in cooperation with Stockholm County Infection Control Unit (IC), as summarized in Table 1.
Contact tracing
Contact tracing and information on personal hygienic measures to contain further spread is mandatory for all new cases of MRSA, according to the Swedish Communicable Diseases Act. Possible sources and secondary cases to the index case are contacted and tested for MRSA. Patients at the same ward of a newly discovered MRSA case were screened immediately and at discharge. The extent to which medical staff was screened, i.e., none, selected, or all staff, was decided after discussion with the IC department. When a known MRSA carrier was treated as an inpatient, all patients at the same ward were screened at discharge.
Eradication
An attempt to eradicate the MRSA colonization was not part of the strategy used in Stockholm. It was tried in selected patients such as medical staff prevented to return to work unless free of MRSA. Also, it was used in colonized patients before surgery to minimize the risk of post-operative infections.
Information and advisory meetings
Monthly meetings between the CDCP department, IC, and ID were instituted in 2001. In 2003, a steering group was formed, headed by the County Medical Officer at CDCP and the County’s Chief Medical Officer (CMO), with representatives for IC, ID, the microbiological departments and the 25 communities of Stockholm County. These meetings are still in operation though less frequent.
In 2003, a decree was issued by the County Director that MRSA guidelines and Basic Hygienic Principles (BHP) must be followed by all staff at all times.
Follow-up
An outpatient unit (the MRSA team) for the follow-up of all MRSA patients was organized in 2003 at the ID department at Karolinska University Hospital. Patients were referred from the attending clinician. Additional contact tracing was performed as required with screening of family members with risk factors. Detailed advice on personal hygiene related to MRSA was given to reduce the risk of further transmission, and patients were instructed to inform caregivers about their MRSA diagnosis when seeking medical or dental care. They were given a card informing about their MRSA status, to be shown when in contact with a caregiver. This is according to the Swedish Communicable Diseases Act https://www.vardgivarguide.se/globalassets/behandlingsstod/smittskydd/smittskyddsblad/patientinformation/mrsa/engelska.pdf. A warning text was introduced to the medical record. From 2010, after national regulations from the Swedish Board of Health and Welfare, patients having no medical risk factors for MRSA carriage could be relieved from these obligations, and the warning sign in the medical record removed, if followed-up at least three times during a minimum of 1 year with negative MRSA cultures. They were thereafter treated as non-MRSA patients.
Results
Epidemiology
From 2000 to 2016, there were 7373 cases of MRSA reported to the CDCP department in Stockholm County, see Fig. 1. An almost linear increase was seen in the number, and incidence, of reported MRSA cases, from 100 cases, 9.5 per 100,000, in 2000, to 846 cases, 37.3 per 100,000, in 2016. In 2015, a higher number, 865, than in 2016 was noted. This coincided with a very high influx of refugees to Sweden that year, mainly from the Middle East, many of who had acute skin infections.
Age and gender distributions show somewhat more women (51%) than men with MRSA, especially in childbearing age groups. Average age overall was 43 years. However, age and sex distribution varied depending on the background of acquisition. In HA-MRSA, average age was 69 years and there were 51% women, similar to patients with unknown background of transmission (59 years, 52% women). Average age in patients having acquired their MRSA abroad was 35 years and 51% were women. Some of this difference may be attributed to screening of pregnant women after medical care abroad. In patients with MRSA acquired in the Swedish community, average age was 34 years and 50% were women.
The epidemiological background for acquiring the MRSA infections is shown in Fig. 2. The number of patients with HA-MRSA infection or colonization had decreased substantially already in 2004. Initially, these HA-MRSA infections were usually caused by well-known European strains such as EMRSA-15 [7]. During recent years, the number of cases of HA-MRSA infections has been very low, usually between 5 and 15 per year, and caused by other types than EMRSA-15. A larger number of patients, often between 50 and 70 per year, were noted to have had several episodes of medical care as outpatients or inpatients, but with no identifiable episode of exposure to MRSA. Moreover, their specific MRSA type did not correspond to any prevalent strain likely to having been transmitted during medical care. Therefore, the origin of these MRSA infections remains obscure.
An increasing number of patients have acquired the infection abroad (Fig. 2). Patients admitted to hospital were screened if they had been exposed to inpatient or “significant” outpatient care abroad during the last 6 months, or were diagnosed via contact tracing or when presenting with clinical symptoms after travel.
Beginning around 2010, there was a sharp increase of domestic CA-MRSA cases. This was paralleled by an increase in MRSA types not previously observed, often PVL-producing strains. Detailed clinical information is often lacking in routine reporting of cases, but there appears to have been very few cases of PVL-related severe pulmonary MRSA infections in the county. Routine spa-typing was performed by the Public Health Authority of Sweden and demonstrated a very large number of different spa-types, with the dominating spa-types during recent years generally belonging to “community” types (data not shown). The transmission route of these infections, unless from a family member with known MRSA, has usually not been possible to establish.
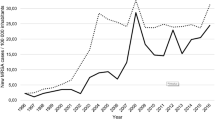
In 2002, 3% out of approximately 400 Staphylococcus aureus bloodstream infections were MRSA (Fig. 3). Although the number of patients with S.aureus bloodstream infections increased steadily during the study period, the number of MRSA bloodstream infections did not, and accounted for only 1% of more than 800 such infections in 2016.
The number of screening and clinical samples for MRSA sent to the three microbiology laboratories in Stockholm increased dramatically during the study years. In 2002, about 14,700 samples were analyzed, increasing to more than 280,000 samples in 2014. A sharp decline of submitted samples was noticed from April 2016, coinciding with the publication of renewed and joint guidelines for control of MRSA as well as vancomycin-resistant enterococci and ESBL-producing Enterobacteriaceae in hospital and outpatient care. It is likely that, before 2016, screening was used more extensively than recommended in the guidelines, perhaps due to the external financing, or misinterpretation by medical staff. However, in spite of this decreasing number of laboratory samples for MRSA, there has been no decrease of the number of reported MRSA cases.
Point-prevalence studies in hospitals and geriatric departments showed compliance with screening guidelines of between 60 and nearly 100%. Geriatric hospitals and the smaller acute-care hospitals most often had compliance above 80%, compared with around 70% for the larger acute-care hospitals.
Infection control
Infection-control practitioners have implemented the guidelines for care of suspected or verified MRSA patients (Table 1) to all medical care units in Stockholm County. These rather strict guidelines were in use until some were discontinued during 2012, 2015, and 2016, as shown in Table 1. A limited analysis of the joint effect of these guidelines was performed at Karolinska University Hospital in 2015. It was concluded that transmission of MRSA was rare if medical staff was aware that a patient was colonized by MRSA, and the patient therefore was isolated in a single room with strict adherence to basic hygienic principles and staff cohorting. However, it was noted that transmission of MRSA was rare even if cohorting was not performed during all work shifts (Ros-Mari Landqvist, personal communication).
Follow-up
The MRSA team for follow-up was soon established as one cornerstone of the MRSA control strategy. Patients were thoroughly informed about MRSA and provided with a card stating their carriage, to be shown when in contact with medical or dental care. It was secured that the medical record had been provided with a warning sign. The number of secondary infections prevented by this policy during readmissions has not been formally evaluated, but is likely to have been significant. An 11-year follow-up of MRSA carriers with no skin risk factors showed between 32 and 59% to be reliably culture negative, depending on time from initial diagnosis [8]. A total of 2081 (28%) of 7373 diagnosed MRSA carriers had, by the end of 2016, been relieved of MRSA regulations and are now treated as non-carriers when seeking medical care. Very few relapses have been identified.
Discussion
This is a retrospective study based on routine reporting of new MRSA cases to our department. Although the validity of routine reporting to the Swedish SmiNet system has been questioned [9], each case was reviewed at the time of report, and some information was routinely added, in nearly all cases by one of the authors (U-B T) throughout these years. Therefore, it is likely that our data have a high degree of consistency and validity. The guidelines on infection-control routines appear to have been effectively implemented and successful in containing outbreaks, although no systematic study has been performed in Stockholm County to assess this, except the one at Karolinska University Hospital (Landqvist, personal communication) mentioned above. However, the low number of reported cases judged to be healthcare associated and the very low number of MRSA bloodstream infections make it likely that this policy has been successful in counteracting HA-MRSA [10, 11].
The strategy used in The Netherlands and the Scandinavian countries is often referred to as “search-and-destroy” [3,4,5]. This usually involves an attempt to eradicate carriage of MRSA in the individual patient. Although thorough cleaning and disinfection of ward surfaces is used, eradication of carriage has not been part of the strategy in Stockholm. Our strategy may rather be termed “search-and-contain”; extensive screening together with strict isolation of positive cases in single rooms initially in dedicated wards, strict hygienic routines, cohorting of staff, and combined with follow-up and advice on hygienic measures for each patient. Screening was used extensively as described, with more than 280,000 submitted samples for MRSA culture in 2014, and point-prevalence studies of screening routines showed relatively good compliance of 70–80% depending on the setting.
Although systematic follow-up has not been performed, it is our impression that isolation in single rooms has been used in a consistent way through the years. However, staff cohorting may not have been possible in each case in every hospital, especially during night shifts. Changes of guidelines in 2012 allowed care in single rooms in wards with no specific MRSA profile, partly due to limitation of staff available for specialized MRSA wards at the ID department. This change has not been associated with any notable increase in HA-MRSA. Staff cohorting was abandoned in the 2015 guidelines.
Since several infection-control measures were implemented simultaneously during the period 2001–2004, and especially in 2003, evaluation of any single measure is not possible. Although this applies for most reports on MRSA infection control, infection-control measures are generally regarded as effective if implemented in a consistent way [12, 13]. The risk factor–based screening strategy used in or county is supported by a British mathematical modeling study, there termed “check-list screening,” judged to be cost-effective, at least in a relatively high-prevalence setting, and accepting relatively high costs [14].
The total cost of this “search-and-contain” strategy has not been estimated, but is likely high. Laboratory costs alone were estimated to about 106 million SEK in 2015, whereas costs for isolation routines and cohorting of staff have not been calculated. However, laboratory costs have been reduced significantly since 2016 [15], and probably costs for hospital care as well, due to the abandoned need for staff cohorting and reduced requirements for care in an ID ward, although isolation in single rooms is still mandatory. The policy of follow-up and relieving carriers of their obligations related to the Swedish Communicable Diseases Act, as well as the abandoned need for MRSA routines if admitted to hospital, is also likely to have reduced costs [16, 17]. Reports from The Netherlands on similar strategies point to high costs but favorable cost-benefit by reduced transmission and morbidity and mortality due to MRSA in hospitals [18].
Medical care, travel, and work or studies abroad are now the basis of more than 40% of MRSA cases reported in Stockholm County. Patients admitted to hospital with a history of medical care abroad during the last 6 months are nursed in single rooms until results from screening cultures are available, and few secondary cases are observed.
CA-MRSA has emerged as the other major group of MRSA positive patients, now accounting for more than 40% of cases. These MRSA belong to other types, often PVL-gene positive [19]. There have, so far, been few serious PVL-related CA-MRSA infections, and they have not been introduced and caused outbreaks in our hospitals. However, the epidemiological mechanisms of spread of CA-MRSA in the Swedish community are unknown, as reported elsewhere [20].
In conclusion, there has been a profound change of epidemiology of MRSA infections in Stockholm County during the last 15 years, from local hospital-based outbreaks to a dominance of imported and community-based cases. Hospital transmission and local outbreaks of MRSA occur but on a very limited scale. An extensive, and probably relatively costly, “search-and-contain” strategy has been used from the beginning of the 2000s and has been successful at keeping HA-MRSA and probably other aspects of MRSA epidemiology at a manageable level in Stockholm County.
References
Kock R, Becker K, Cookson B et al (2010) Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill 15(41):19688
Björholt I, Haglind E (2004) Cost-saving s achieved by eradication of epidemic methicillin-resistant Staphylococcus aureus (EMRSA)-16 from a large teaching hospital. Eur J Clin Microbiol Infect Dis 23:688–695
Spicer WJ (1984) Three strategies in the control of staphylococci including methicillin-resistant Staphylococcus aureus. J Hosp Infect 5(Supplement A:45–49
Solberg CO (2000) Spread of Staphylococcus aureus in hospitals: causes and prevention. Scand J Infect Dis 32(6):587–595
Wertheim HLM, Vos MC, Boelens HAM et al (2004) Low prevalence of methicillin-resistant Staphylococcus aureus at hospital admission in the Netherlands: the value of search and destroy and restrictive antibiotic use. J Hosp Infect 56:321–325
Rolfhamre P, Jansson A, Arneborn M, Ekdahl K (2006) SmiNet-2: description of an internet-based surveillance system for communicable diseases in Sweden. Euro Surveill : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 11(5):103–107
Holden MTG, Hsu L-Y, Kurt K et al (2013) A genomic portrait of the emergence, evolution and global spread of a methicillin-resistant Staphylococcus aureus pandemic. Genome Res 23:653–664
Lind G, Hergens M-P, Thollström U-B, Hökeberg I (2014) MRSA-carriage - a follow-up of an 11 year MRSA-cohort in Stockholm County, Sweden. Presentation K-1906, ICAAC
Stenhem M, Ortqvist A, Ringberg H et al (2009) Validity of routine surveillance data: a case study on Swedish notifications of methicillin-resistant Staphylococcus aureus. Euro Surveill 14(30):19281
Borg MA, Hulscher M, Scicluna EA et al (2014) Prevention of methicillin-resistant Staphylococcus ausreus bloodstream infections in European hospitals: moving beyond policies. J Hosp Infect 87:203–211
Widmer AF, Lakatos B, Frei R (2015) Strict infection control leads to low incidence of methicillin-resistant Staphylococcus aureus bloodstream infections over 20 years. Infect Cotrol Hosp Epidemiol 36(6):702–709
Loveday HP, Pellowe PM, Jones SRLJ, Pratt RJ (2006) A systematic review of the evidence for interventions for the prevention and control of methicillin-resistant Staphylococcus aureus (1996-2004): report to the MRSA Working Party (Subgroup A). J Hosp Infect 635:545–570
Humphreys H (2009) Do guidelines for the prevention and control of methicillin-resistant Staphylococcus aureus make a difference? Clin Microbiol Infect 15(Suppl 7):39–43
Robotham JB, Deeny SR, Fuller C, Hopkins S, Cookson B, Stone S (2016) Cost-effectivenes of mandatory screening of all admissions to English National Service hospitals for meticilin-resistant Staphylococcus aureus: a mathematical modeling study. Lancet Infect Dis 16:348–356
https://www.vardgivarguiden.se/globalassets/behandlingsstod/smittskydd/verksamhetsberattelse.pdf. Accessed 2 June 2019, page 67. (In Swedish)
Degues PA (2013) Search, destroy and confirm: how to maximize the benefit and reduce the unintended consequences of contact precautions for control of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 57(2):185–187
Shenoy ES, JiYeaon K, Rosenberg ES et al (2013) Discontinuation of contact precautions for methicillin-resistant Staphylococcus aureus: a randomized controlled trial comparing passive and active screening with culture and polymerase chain reaction. Clin Infect Dis 57(2):176–184
Souverein D, Houtman P, Euser SM, Herpers BL, Kluytmans J, Den Boer JW (2016) Costs and benefits associated with MRSA the search and destroy policy in a Dutch hospital in the region Kennemerland, The Netherlands. PLoS One 11(29):e0148175. https://doi.org/10.1371/journal.pone.0148175
Enstrom J, Froding I, Giske CG, Ininbergs K, Bai X, Sandh G et al (2018) USA 300 methicillin-resistant Staphylococcus aureus in Stockholm, Sweden, from 2008 to 2016. PLoS One 13(11):e0205761
Donker T, Bosch T, Ypma RJF et al (2016) Monitoring the spread of methicillin-resistant Staphylococcus aureus in The Netherlands from a reference laboratory perspective. J Hosp Infect 93:366–374
Acknowledgments
We wish to thank all colleagues and staff at the departments of Infection Control, Infectious Diseases, and Microbiology Laboratories as well as all clinical staff that have been involved in work with MRSA during these years.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All data were collected as required by the Swedish Communicable Diseases Act.
Informed consent
Informed consent was obtained from all patients included in the study. See also above on ethical approval.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eriksson, B.K.G., Thollström, UB., Nederby-Öhd, J. et al. Epidemiology and control of meticillin-resistant Staphylococcus aureus in Stockholm County, Sweden, 2000 to 2016: overview of a “search-and-contain” strategy. Eur J Clin Microbiol Infect Dis 38, 2221–2228 (2019). https://doi.org/10.1007/s10096-019-03664-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03664-2