Abstract
The aim of this study is to see the frequency, clinical presentation, and therapeutic response of extensively drug-resistant Salmonella enterica serovar Typhi and current susceptibility pattern of typhoidal Salmonella strains in our setup. This study was carried out at the Department of Medical Microbiology and Immunology and Department of Medicine, Pakistan Navy Ship (PNS) Shifa Hospital, Karachi, from January 1 to December 31, 2018. All the blood culture samples of patients (indoor and outdoor) with suspicion of enteric fever were processed. Isolates were cultured and identified using standard microbiological procedures. The antimicrobial sensitivity against the typhoidal Salmonellae was determined using Kirby-Bauer disc diffusion method as per the guidelines of Clinical and Laboratory Standards Institute (2018) and all the extensively drug-resistant (XDR) isolates were confirmed by Vitek 2 system. Clinical presentation and response to treatment of patients were followed. A total of 292 typhoidal Salmonella isolates were cultured. Resistance to ciprofloxacin against both Salmonella Typhi and Salmonella Paratyphi A was found to be very high (91%). Percentage of multidrug-resistant (MDR) isolates in Salmonella Typhi was 76% (182 isolates) and in Salmonella Paratyphi it was 34% (18 isolates). XDR isolates in Salmonella Typhi were significant that is 48% (115 isolates). Only 10 cases were given azithromycin who responded to treatment in mean 4.3 days. Out of 115 cases of XDR Salmonella Typhi, 103 patients were given parenteral meropenem and clinical response was seen in mean 5 days. The emergence and rapid spread of extensively drug-resistant Salmonella Typhi is alarming and highlights the significance of strict antimicrobial susceptibility surveillance programs with antimicrobial stewardship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enteric fever is caused by typhoidal strains of Gram-negative bacterium Salmonella enterica including Salmonella enterica subsp. enterica serovars Typhi (S. Typhi) and Paratyphi (S. Paratyphi) A, B, and C. Transmission of S. Typhi is by the fecal-oral route commonly through contaminated water and food [1,2,3]. Typhoid usually presents with non-specific fever initially and is often misdiagnosed as other common causes of acute fever in our region such as malaria, dengue, chikungunya, and brucellosis resulting in inappropriate therapy and a prolonged febrile illness that can last for many weeks [2, 4, 5]. The frequency of S. Typhi and S. Paratyphi as causative agents of typhoid fever is highly variable depending on the geography. It remains to be a substantial public health problem worldwide with over 21.6 million cases and at least 250,000 deaths occurring every year. The disease poses a more significant public health threat in developing countries, including Pakistan. According to an estimate, incidence of enteric fever is 110 cases/100000 population in South Asia [6, 7].
Antimicrobial agents are the foremost treatment modality in enteric fever so as to prevent the complications and mortality but prevalence of antibiotic-resistant S. Typhi strains has increased significantly in recent times [1, 8]. Previously first-line antimicrobial drugs for the treatment of typhoid included chloramphenicol, ampicillin, and trimethoprim-sulfamethoxazole. However, MDR S. Typhi defined as strains resistant to all three first-line antibiotics that is ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole emerged in the late 1980s. This resulted in the use of fluoroquinolones as major treatment option for MDR S. Typhi cases. With widespread and injudicious use of these agents, reports of treatment failure and isolates showing resistance to fluoroquinolones started coming in, and the first case was finally reported in 1992 [7, 9,10,11].
Ceftriaxone, a third-generation cephalosporin, and azithromycin, a macrolide, were now being considered magic drugs until November 2016, as a large outbreak of ceftriaxone-resistant cases has been reported in the province of Sindh, Pakistan, mostly from the cities of Hyderabad and Karachi. These XDR S. Typhi strains were resistant to first-line drugs, fluoroquinolones, and third-generation cephalosporins. Three hundred thirty-nine XDR S. Typhi were isolated from the Sindh region of Pakistan between November 2016 and September 2017 [1, 12,13,14]. For most XDR strains of S. Typhi, only treatment options include azithromycin and carbapenems. Cases resistant to azithromycin have already been reported in South Asia [1, 5, 15].
In this backdrop, when the therapeutic options for enteric fever are dwindling, widespread workup and assessment of alternative choices for operative management of enteric fever cases is becoming important. We aimed this study to see the burden of extensively drug-resistant typhoidal isolates, clinical presentation, susceptibility profile, and response to therapy in XDR typhoid patients.
Methodology
This prospective study was carried out in the Department of Microbiology in collaboration with Department of Medicine, PNS Shifa Hospital, Karachi, from January 1 to December 31, 2018. This hospital is a 700-bedded tertiary care setup, which receives patients from districts Balochistan, Sindh, and Southern Punjab. Permission was obtained from the Hospital Ethical Committee and informed consent was taken from all the patients. All the blood culture samples submitted from medical wards and outpatient department of patients with suspicion of enteric fever were processed for culture. Clinical presentations and relevant investigations were recorded by senior registrar medicine. All the samples were incubated in automated BacT/ALERT blood culture system (Biomerieux). Duplicate samples of same patient were excluded from the study. Positive signaled culture bottles were plated directly on blood and MacConkey agar. In case of contamination or samples yielding growth of skin colonizers, additional blood cultures were requested while the original bottles were subcultured as per the protocol. The isolates were identified based on colony morphology, serotyping, and biochemical tests using API 20E (Biomerieux, France).
Antimicrobial susceptibility was performed by the Kirby-Bauer disc diffusion method on Muller-Hinton agar. Antimicrobial discs used were as follows: ampicillin (10 μg), ceftriaxone (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), trimethoprim-sulfamethoxazole (1.25/23.75 μg disk), azithromycin (15 μg), and meropenem (10 μg). Salmonella ATCC 700931 and Salmonella paratyphi ATCC 9150 were used for QC of anti-sera, and Escherichia coli ATCC 25922 was used for QC of discs. The interpretation of zone diameters was done according to Clinical and Laboratory Standards Institute (CLSI) guidelines 2018 [16]. All the ceftriaxone-resistant results were confirmed by VITEK 2 system (Biomerieux, France). As there are no specific guidelines for meropenem breakpoints for S. Typhi and S. Paratyphi, so we followed CLSI general guidelines for Enterobacteriaceae for meropenem breakpoints and further confirmed by Vitek-2 breakpoints/MICs.
Clinical presentation and therapeutic response of patients with XDR S. Typhi were followed. Antibiotic response was measured by settling of fever and improvement of associated signs and symptoms. Response day was counted from day of last fever spike. The data was analyzed using SPSS 24.0. Descriptive statistics were used to calculate frequencies and percentages of the qualitative variables.
Results
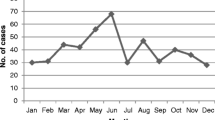
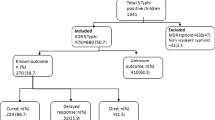
A total of 430 blood culture specimens of provisionally diagnosed enteric fever patients were submitted in microbiology department in a 1-year time period. Out of these, 292 (67.9%) specimens yielded growth of typhoidal Salmonella isolates. Characteristics of these 292 patients and isolates species are shown in Table 1. Mean time of culture positivity from insertion of bottle in system to positive flag indicator was 24 h (4–96 h). Susceptibility pattern of both S. Typhi and S. Paratyphi is shown in Table 2.
Clinical presentation of these 115 cases of XDR S. Typhi and their clinical response with antibiotics along with number of days are shown in Table 3. Only 10 cases were given azithromycin [1 g orally 1st dose and then 500 mg orally once daily dose (adults), 10–20 mg /kg orally once per day (children)], who responded to treatment in mean 4.3 days. Out of 115 cases of XDR S. Typhi, 103 patients were given injection meropenem [1 g intravenous 8 hourly (adults), 20–40 mg/kg 8 hourly (children)] and response was seen in mean 5 days. All patients treated with injection meropenem were managed in indoor setting.
Discussion
Typhoid fever continues to be one of the most common infectious diseases in resource constrained countries like Pakistan. Its spread is multifactorial which includes unsafe sources of potable water supplies, inadequate sanitary practices, and scarce antimicrobial susceptibility data. Typhoid fever is a notifiable disease in the Sindh province of Pakistan [1, 11]. Most cases of enteric fever–related morbidity and mortality have been reported from Asia. Injudicious antibiotic prescribing practices have significantly contributed in developing resistance to multiple antibiotics [17]. Emergence of multidrug-resistant and extensively drug-resistant Salmonella enterica serovars demands ongoing surveillance to type antibiogram trends for empirical therapy and prevention of spread [18].
In this study out of total 292 cases, 81% were male patients. Males were found to be more predisposed to contracting enteric fever likely as a result of outdoor working and eating and drinking food from outside. In our study, majority of patients (52%) had age less than 15 years. Studies from some Asian countries have demonstrated that incidence of enteric fever is highest among children aged < 15 years [17]. In our study, S. Typhi was the dominant isolate (82%) followed by S. Paratyphi A (18%). This pattern was seen in some other local studies as well [11, 19].
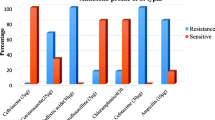
In our study, percentage of MDR isolates in S. Typhi was quite high, 76% (182 isolates) followed by S. Paratyphi A 34% (18 isolates). Resistance to ampicillin, trimethoprim-sulfamethoxazole, and chloramphenicol in S. Typhi was 82%, 83%, and 79% respectively. Resistance to ampicillin, trimethoprim-sulfamethoxazole, and chloramphenicol in S. Paratyphi was 34%, 47%, and 34% respectively. Resistance of S. Typhi and S. Paratyphi against 1st-line antimicrobials was lesser in other local studies done at various cities of Pakistan [6, 11, 17, 19, 20]. Prevalence of MDR isolates in other regional and international studies was significantly low as compared with our study [2, 3].
Resistance to ciprofloxacin against both S. Typhi and S. Paratyphi was alarming (91%). There were only 7 isolates of S. Typhi and 2 isolates of S. Paratyphi which showed intermediate susceptibility to ciprofloxacin. Resistance was comparable with other local studies but was more than regional and international studies [2, 8, 9, 11, 17, 19, 20]. The main reason for this very high resistance is easy availability of this drug over the counter and injudicious use especially in diarrheal diseases. Consequently, this drug is no longer recommended empirically in typhoid fever in our setup.
The frequency of XDR isolates in S. Typhi was alarming in our study that is 48%. These XDR isolates are resistant to first-line agents, quinolones, and in addition to third-generation cephalosporins. Meropenem and azithromycin were the only options for these cases. Ceftriaxone resistance was not reported in local studies until 2016 when a large outbreak of XDR S. Typhi was reported from Hyderabad, Pakistan [1, 7]. This outbreak is still ongoing and so far 5372 XDR S. Typhi isolates have been reported. Cases of XDR S. Typhi have also been reported in foreigner who traveled to Pakistan [15]. To best of our knowledge, this is the first reported study on susceptibility pattern of XDR cases of Salmonella isolates. There were 7 isolates of S. Typhi with azithromycin resistance, two of them were XDR isolates, two were MDR isolates, and three had only resistance to ciprofloxacin. Resistance to azithromycin in S. Typhi has already been reported [1, 18] but its resistance in XDR isolates is a new and alarming finding.
Mean time to fever at presentation in our study was more than 1 week that is more than fever days at presentation in an international study. The possible reason could be because all cases we followed were culture positive for XDR S. Typhi which was not the case in that study. Associated clinical features in our cases were not different from usual typhoid fever presentation [6, 21].
There was only one case that presented with multi-organ failure and one case in third trimester of pregnancy presented with breast pain and swelling along with fever [22]. There were two cases who responded to ceftriaxone which was started empirically despite being XDR on culture results. Ten XDR cases responded to empiric azithromycin and 103 cases responded to parenteral meropenem. The emergence of XDR cases in recent years has presented a serious therapeutic challenge. In Pakistan, ceftriaxone was the drug of choice empirically in suspected cases of typhoid fever requiring hospital admission. Ceftriaxone resistance against S. Typhi in our study was very high (48%).
The questions arise: Should we give a drug with a such high resistance as empirical therapy? Should we give meropenem and azithromycin empirically to those suspected cases who require admission or at risk for complication of typhoid fever in XDR outbreak areas? or Should we wait for culture results? There is limited clinical experience and evidence regarding other potential antimicrobials options like piperacillin-tazobactam, carbapenems (imipenem or ertapenem), ceftazidime-avibactam, tigecycline, fosfomycin, and colistin [23, 24]. Many a times, culture is not positive and not all the hospitals in this area have culture and sensitivity facilities. Some hospitals have developed their own guidelines for empirical therapy of typhoid fever based on patient clinical condition and risk of complications. An advisory for prevention and treatment of XDR typhoid cases has been published by the National Institute of Health (NIH) Pakistan recently [25].
Diagnostic problem and local production of many different antimicrobial drugs with doubtful quality along with poor compliance of patients to antimicrobials add to the threat of resistance [7]. Appropriate control measures along with vaccines can also prevent typhoid fever. Currently, two vaccines are available, i.e., Ty21a vaccine, a live attenuated oral vaccine, and the Vi parenteral vaccine. Both vaccines have comparable efficacy and are given to children aged 2 years [6, 17, 23].
There were certain limitations of our study. We checked azithromycin susceptibility by disk diffusion method following CLSI breakpoints for which are still investigational (based on MIC distribution data and limited clinical response). Moreover, azithromycin was given to very small number of patients, which were treated in outdoor; this finding may not be helpful in finding its clinical efficacy in XDR S. Typhi cases.
More multi center studies are required to know the exact prevalence of MDR and XDR cases, their therapeutic response to different antimicrobials, and to formulate a national guideline for empirical therapy of suspected cases.
Conclusion
Our study concludes that cases of XDR Salmonella Typhi are increasing. The treatment options are very limited leaving azithromycin and meropenem the only options. The emergence of azithromycin resistance among XDR isolates is alarming and will further limit the treatment options. This highlights the importance of antimicrobial susceptibilities surveillance for typhoid which would be helpful in the development of effective prevention and control measures as empirical treatment with ceftriaxone is no longer reliable in our setup.
References
Klemm EJ, Shakoor S, Page AJ (2018) Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. MBio 9:105–118
Britto CD, Wong VK, Dougan G (2018) A systematic review of antimicrobial resistance in Salmonella enterica serovar Typhi, the etiological agent of typhoid. PLoS Negl Trop Dis 12:6779
Khatun H, Islam SB, Naila NN (2018) Clinical profile, antibiotic susceptibility pattern of bacterial isolates and factors associated with complications in culture-proven typhoid patients admitted to an urban hospital in Bangladesh. Tropical Med Int Health 23:359–366
Gibani MM, Britto C, Pollard AJ (2018) Typhoid and paratyphoid fever: a call to action. Curr Opin Infect Dis 31:440
Levine MM, Simon R (2018) The gathering storm: is untreatable typhoid fever on the way? MBio 9:482–418
Das JK, Hasan R, Zafar A (2018) Trends, associations, and antimicrobial resistance of Salmonella typhi and paratyphi in Pakistan. Am J Trop Med Hyg 99:48–54
Aziz S, Malik L (2018) Emergence of multi-resistant enteric infection in a Paediatric unit of Karachi, Pakistan. J Pak Med Assoc 5:2–84
Bhetwal A, Maharjan A, Khanal PR (2017) Enteric fever caused by Salmonella enterica Serovars with reduced susceptibility of fluoroquinolones at a community based teaching Hospital of Nepal. Int J Microbiol 2017:2869458
Mutai WC, Muigai AW, Waiyaki P (2018) Multi-drug resistant Salmonella enterica serovar Typhi isolates with reduced susceptibility to ciprofloxacin in Kenya. BMC Microbiol 18:187
Tanmoy AM, Westeel E, De Bruyne K (2018) Salmonella enterica Serovar Typhi in Bangladesh: exploration of genomic diversity and antimicrobial resistance. MBio 9:2112–2118
Zehra NM, Irfan F, Mirza IA (2017) Current trends of antimicrobial susceptibility of Typhoidal Salmonellae isolated at tertiary care hospital. J Coll Physicians Surg Pak 27:690–692
Djeghout B, Saha S, Sajib MS (2018) Ceftriaxone-resistant Salmonella Typhi carries an IncI1-ST31 plasmid encoding CTX-M-15. J Med Microbiol 67:620–627
Qamar FN, Yousafzai MT, Khalid M (2018) Outbreak investigation of ceftriaxone-resistant Salmonella enterica serotype Typhi and its risk factors among the general population in Hyderabad, Pakistan: a matched case-control study. Lancet Infect Dis 18:1368–1376
Narasanna R, Chavadi M, Chandrakanth K (2018) Prevalence of multidrug-resistant Salmonella typhi in typhoid patients and detection of blaCTX-M2 and blaCTX-M9 genes in cefetoxime-mediated extended spectrum β-lactamase-producing Salmonella typhi isolates. Biomed Res 29
Chatham-Stephens K (2019) Emergence of extensively drug-resistant Salmonella Typhi infections among travelers to or from Pakistan—United States, 2016–2018. Morb Mortal Wkly Rep 68:11–13
Clinical and Laboratory Standards Institute (2018) Performance standards for antimicrobial susceptibility testing 28th ed, document M100. CLSI, Wayne
Qamar FN, Yousafzai MT, Sultana S (2018) A retrospective study of laboratory-based enteric fever surveillance, Pakistan, 2012–2014. J Infect Dis 218:201–205
Makkar A, Gupta S, Khan ID (2018) Epidemiological profile and antimicrobial resistance pattern of enteric fever in a tertiary care hospital of North India-a seven year ambispective study. Acta Med (Hradec Kralove) 61:125–130
Shujat U, Ikram A, Hashmi IQ (2016) Current antimicrobial sensitivity pattern of typhoidal salmonellae in a referral diagnostic centre. Microbiologia Medica 31
Malik N, Ahmed M (2016) In vitro effect of new antibiotics against clinical isolates of Salmonella Typhi. J Coll Physicians Surg Pak 26:288–292
Habte L, Tadesse E, Ferede G (2018) Typhoid fever: clinical presentation and associated factors in febrile patients visiting Shashemene Referral Hospital, southern Ethiopia. BMC Res Notes 11:605
Zehra NM, Satti L, Hanif F (2019) Unilateral breast abscess by an extremely drug resistant Salmonella enterica serovar Typhi: first case report from Pakistan. J Clin Diagn Res 13
Ryan ET, Andrews J Treatment and prevention of enteric (typhoid and paratyphoid fever). In: Post TW (ed) UpToDate. Waltham, UpToDate Inc. http://www.uptodate.com. Accessed on 22 April 2019
Parry CM, Ribeiro I, Walia K (2019) Multidrug resistant enteric fever in South Asia: unmet medical needs and opportunities. BMJ 364:5322
National Institute of Health Pakistan. Advisory for the treatment and prevention of XDR typhoid. Pakistan 2018. No.F.1-31/Misc/FEDSD/2018. Retrieved from https://www.nih.org.pk/wp-content/uploads/2019/02/Advisory-for-Typhoid-5-oct.pdf
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval to conduct this study from was obtained from Institutional Ethical Committee, PNS SHIFA Hospital.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hussain, A., Satti, L., Hanif, F. et al. Typhoidal Salmonella strains in Pakistan: an impending threat of extensively drug-resistant Salmonella Typhi. Eur J Clin Microbiol Infect Dis 38, 2145–2149 (2019). https://doi.org/10.1007/s10096-019-03658-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03658-0