Abstract
Infective endocarditis is a growing problem with many shifts due to ever-increasing comorbid illnesses, invasive procedures, and increase in the elderly. We performed this multinational study to depict definite infective endocarditis. Adult patients with definite endocarditis hospitalized between January 1, 2015, and October 1, 2018, were included from 41 hospitals in 13 countries. We included microbiological features, types and severity of the disease, complications, but excluded therapeutic parameters. A total of 867 patients were included. A total of 631 (72.8%) patients had native valve endocarditis (NVE), 214 (24.7%) patients had prosthetic valve endocarditis (PVE), 21 (2.4%) patients had pacemaker lead endocarditis, and 1 patient had catheter port endocarditis. Eighteen percent of NVE patients were hospital-acquired. PVE patients were classified as early-onset in 24.9%. A total of 385 (44.4%) patients had major embolic events, most frequently to the brain (n = 227, 26.3%). Blood cultures yielded pathogens in 766 (88.4%). In 101 (11.6%) patients, blood cultures were negative. Molecular testing of vegetations disclosed pathogens in 65 cases. Overall, 795 (91.7%) endocarditis patients had any identified pathogen. Leading pathogens (Staphylococcus aureus (n = 267, 33.6%), Streptococcus viridans (n = 149, 18.7%), enterococci (n = 128, 16.1%), coagulase-negative staphylococci (n = 92, 11.6%)) displayed substantial resistance profiles. A total of 132 (15.2%) patients had cardiac abscesses; 693 (79.9%) patients had left-sided endocarditis. Aortic (n = 394, 45.4%) and mitral valves (n = 369, 42.5%) were most frequently involved. Mortality was more common in PVE than NVE (NVE (n = 101, 16%), PVE (n = 49, 22.9%), p = 0.042).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infective endocarditis (IE) is a growing problem in the world due to ever-increasing comorbid illnesses, invasive devices like intracardiac implants and prosthetic valves, frequent use of hemodialysis, and the increase of elderly population [1]. Despite advances in medical and surgical therapy, IE is associated with high mortality and severe complications [2, 3]. In order to achieve optimal outcomes in the management of IE, the clinical team must have an understanding of epidemiology, microbiology, and natural history of IE as well as guiding diagnostic principles and therapy. Until recently, guidelines on IE were mostly based on expert opinions due to absence of randomized trials and the limited number of meta-analyses. Hence, we performed this multinational study to depict the common presentations of IE, its microbiology, types, severity, and the complications to provide an insight into the current status of IE, and to compare the features of native valve endocarditis (NVE) and prosthetic valve endocarditis (PVE) patients as the two leading endocardial infections.
Methods
Definite IE patients > 18 years old and hospitalized between January 1, 2015, and October 1, 2018, were included. Forty-one hospitals from 13 countries were involved. The data were recruited through Infectious Diseases International Research Initiative (ID-IRI), which serves as a network for clinical research (https://infectdisiri.wordpress.com/). The data input was made through a web-based questionnaire. The questionnaire included demographic, clinical, laboratory, microbiologic, special tests, and echocardiographic findings and outcome. Therapeutic concerns were not included. Ethical approval was obtained from the Fatih Sultan Mehmet Hospital in Istanbul and participants’ respective Institutional Ethics Committees.
Definitions
Diagnoses of IE were made according to definitions below [4].
-
1.
Major criteria
-
(a)
Blood culture positive for IE
-
Typical microorganisms consistent with IE from 2 separate blood cultures (at least 2 positive cultures of blood samples drawn > 12 h apart or all 3 or a majority of ≥ 4 separate cultures of blood)
-
Single positive blood culture for Coxiella burnetii or anti-phase 1 IgG antibody titer ≥ 1:800
-
(b)
Evidence of endocardial involvement
Echocardiogram positive for IE was defined as follows: oscillating intracardiac mass on valve or supporting structures, in the path of regurgitant jets, or on implanted material in the absence of an alternative anatomic explanation; abscess; or new partial dehiscence of prosthetic valve or new valvular regurgitation.
-
2.
Minor criteria
-
(a)
Predisposition, predisposing heart condition, or injection drug use
-
(b)
Fever, temperature > 38 °C
-
(c)
Vascular phenomena, major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial hemorrhage, conjunctival hemorrhages, and Janeway lesions
-
(d)
Immunological phenomena: glomerulonephritis, Osler nodes, Roth spots, and rheumatoid factor
-
(e)
Microbiological evidence: positive blood culture but does not meet a major criterion as noted above (excludes single positive cultures for coagulase negative staphylococci and organisms that do not cause endocarditis) or serological evidence of active infection with organism consistent with IE
Definite IE
Patients with 2 major criteria, 1 major criterion and 3 minor criteria, or 5 minor criteria were classified as definite IE [4].
Ejection fraction value
A normal heart’s ejection fraction (EF) was accepted to be between 50 and 70% [5].
Pulmonary hypertension
If pulmonary artery pressure is greater than 25 mmHg at rest or 30 mmHg during physical activity, this was defined as pulmonary hypertension [6].
Timing of IE according to implantation of prosthetic valves
Early-onset illness was classified as PVE within 1 year and late onset was defined as PVE after 1 year of valve surgery.
Histopathology
Histopathological interpretation of removed cardiac vegetations was made as acute and subacute [7].
Onset timings of IE
This was determined according to start of symptoms.
Statistical analysis
Descriptive statistics were presented as frequency and percent or mean ± standard deviation (SD) and range as appropriate. Chi-square and Fisher’s exact tests were used to compare categorical variables and Student’s t test and Mann-Whitney U test were used for comparisons of continuous variables. A P value of < 0.05 was considered significant.
Results
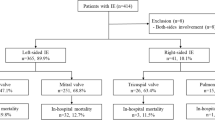
A total of 867 cases with definite IE were included from 41 referral centers in 13 countries (Albania (n = 12), Belgium (n = 26), Denmark (n = 13), France (n = 205), Israel (n = 55), Italy (n = 66), Jordan (n = 7), Pakistan (n = 27), Portugal (n = 86), Romania (n = 16), Saudi Arabia (n = 22), Slovenia (n = 10), Turkey (n = 322)). A total of 292 (33.7%) patients were females and the median age was 59.5 (16–96) years. A total of 711 cases had 2 major criteria and 136 patients had 1 major and ≥ 3 minor criteria.
Types and characteristics of endocardial infections
A total of 631 (72.8%) patients had NVE, 214 (24.7%) patients had PVE, 21 (2.4%) patients had pacemaker lead IE, and 1 patient had catheter port IE. Coexistent pacemaker lead IE was detected in 5 cases with NVE and 4 cases with PVE. In 613 NVE patients, IE was classified as community-acquired (n = 503, 82%) or hospital-acquired (n = 110, 18%). In 193 PVE patients, IE was classified as early-onset (n = 48, 24.9%) or late-onset (n = 145, 75.1%). Likely sources of IE were specified by the treating clinicians in 515 cases (NVE (n = 401), PVE (n = 107), pacemaker IE (n = 6), catheter port IE (n = 1)) (Table 1). The mean likely onset timings of IE were 34.2 ± 59 days for NVE and 52 ± 164 days for PVE (p = 0.049).
Diagnostic issues
-
1.
Embolic events
-
(a)
Arterial emboli: 385 (44.4%) patients had major embolic events. The distribution of embolisms was as follows: cerebral (n = 227, 26.3%), splenic (n = 59, 6.8%), pulmonary (n = 53, 6.1%), renal (n = 25, 2.9%), peripheral (n = 20, 2.2%), coronary (n = 4), mesenteric (n = 3). In 301 (43.4%) of 693 left-sided IE and 40 (39.2%) of 102 right-sided IE, major arterial embolism was detected (Table 3).
-
(b)
Other vascular phenomena: Janeway lesions (n = 34, 3.9%), mycotic aneurysm (n = 8), splinter hemorrhage (n = 4), and conjunctival hemorrhage (n = 4).
-
(a)
-
2.
Immunological phenomena: In 108 (12.4%) patients, one of immunological phenomena was found to be positive. Rheumatoid factor (n = 48, 5.5%), glomerulonephritis (n = 33, 3.8%), Roth spots (n = 16, 1.8%) and Osler nodes (n = 15, 1.7%).
-
3.
Microbiological data
-
(a)
Microbiological diagnosis: Blood cultures yielded 787 isolates in 766 (88.4%) patients (median, 3 sets of blood culture positivity (1–12 sets)). In 19 out 766 (2.5%) blood culture positives, multiple pathogens were recovered (double pathogens in 17, and triple pathogens in 2 patients). A total of 101 (11.6%) cases were culture-negative endocarditis. Cardiac vegetations were surgically removed in 320 (36.9%) patients, and vegetation cultures yielded the pathogen in 105/285(36.8%) patients. Although overall culture (blood+vegetation cultures) positivity was detected in 776 (89.5%) patients, blood culture positivity in accordance with the minor criteria was detected in 51 (5.9%) cases. In 9 patients, anti-phase 1 IgG antibody titer ≥ 1:800 for C. burnetii was observed. PCR detected C. burnetii in 3 of these 9 cases. Molecular testing of cardiac vegetations disclosed the pathogen in 65 of 86 (75.6%) cases tested. On the whole, 795 (91.7%) IE patients had any identified pathogen.
-
(b)
Infecting pathogens: The leading pathogens were Staphylococcus aureus (n = 267, 33.6%), Streptococcus viridans (n = 149, 18.7%), enterococci (n = 128, 16.1%), coagulase-negative staphylococci (CoNS; n = 92, 11.6%), and enteric Gram-negative bacilli (n = 50, 6.3%). The causative agents are presented in Table 2. Blood cultures yielded pathogens in 18 and vegetation cultures in 6 patients for pacemaker IE. A total of 18 of 21 cases had an identified pathogen in pacemaker IE (S. aureus (n = 12), CoNS (n = 3), Enterococcus faecalis (n = 1), Streptococcus pneumoniae (n = 1), S. viridans (n = 1)). CoNS were isolated from the blood culture of a patient with catheter port IE. The distributions and comparisons of infecting pathogens for PVE and NVE are presented in Table 2. There were 18 (2%) probable zoonotic agents (C. burnetii (9 cases), Bartonella quintana (4 cases), and Brucella spp. (5 cases)).
-
(c)
Etiology of cardiac abscesses: In 10 out of 132 patients with cardiac abscesses, the causative agents could not be recovered. A total of 128 pathogens were recovered in 122 patients. They were S. aureus (n = 36, 27.3%), enterococci (n = 24, 18.2%) (untyped Acinetobacter Iwoffii n = 5, E. faecalis n = 19), S. viridans (n = 20, 15.1%), CoNS (n = 18, 13.5%), enteric Gram-negatives (Escherichia coli (n = 3), Klebsiella oxytoca (n = 3), Enterobacter cloacae (n = 3), C. burnetii (n = 2), Enterobacter aerogenes (n = 1), Salmonella dublin (n = 1), Salmonella enteridis (n = 1)), Streptococcus dysgalactiae (n = 1), Streptococcus pseudoporcinus (n = 1)], Micrococcus luteus (n = 1), Brucella spp. (n = 1), Acinetobacter baumannii (n = 1), Pseudomonas aeruginosa (n = 1), Neisseria gonorrhoeae (n = 1), Prevotella bivia (n = 1), Bacillus cereus (n = 1), Corynebacterium jeikeium (n = 1), Lactobacillus rhamnosus (n = 1), Aggregatibacter aphrophilus (n = 1), Candida albicans (n = 2), Candida tropicalis (n = 1).
-
(d)
Major resistance issues: 73 out of 267 (27.3%) of S. aureus strains were methicillin resistant. Nine out of 128 (7%) enterococci were vancomycin resistant (VRE). Antibiotic susceptibility was recorded in 88 of 149 S. viridans strains and 24 strains were penicillin resistant (27.3%).
-
4.
Radiological data
-
(a)
Imaging modalities: Transthoracic echocardiography (n = 544), transesophageal echocardiography (n = 464), positron-emission tomography (n = 18), 3-dimensional echocardiography (n = 14), cardiac magnetic resonance imaging (n = 6), and head-to-toe multislice computed tomography (n = 5) were the radiological methods used.
-
(b)
Distributions of lesions: 693(79.9%) patients had left-sided, 102 (11.8%) had right-sided, and 18 (2.1%) patients had both-sided IE. Vegetation was not shown in 54 (6.2%) patients. A total of 893 cardiac vegetations in accordance with definite IE were observed in 810 (93.4%) patients ((NVE = 595, 73.5%), (PVE = 193, 23.8%), (pacemaker lead IE = 21, 2.6%; 5 with NVE, 4 with PVE, and 21 alone), (catheter port IE = 1, 0.1%)). There were multiple vegetations in 81 (9.4%) patients. Distributions of vegetations are as follows: aortic valves (n = 394, 45.4%), mitral valves (n = 369, 42.5%), tricuspid valves (n = 89, 10.3%), pulmonary valves (n = 7), right atrium (n = 7), left ventricle (n = 3), left atrium (n = 2), right ventricle (n = 1), and pacemaker leads (n = 21). Seven of pacemaker IE were left-sided and 14 were right-sided. There was not a difference in long diameters of vegetations between NVE (13.3 ± 7.6 mm) and PVE (13.4 ± 7.3) (p = 0.917). A total of 143 cardiac abscesses were detected in 132 (15.2%) patients. Cardiac vegetations and abscesses are presented in Table 3. Three out of 21 pacemaker IE had cardiac abscesses. Catheter port IE patient had the vegetation in the right atrium.
-
(c)
Cardiac problems: Underlying cardiac disorders are shown in Table 4. The distribution and comparison of cardiac complications in PVE and NVE patients are presented in Table 5. A total of 113 valve perforations were observed in 109 (17.2%) out of 631 patients with NVE (aortic valves (n = 56), mitral valves (n = 52), and tricuspid valves (n = 5)). Prosthetic valve dysfunction was detected in 71 (33.2%) cases with PVE.
Histopathological data
In 54 cases, histopathological analysis was available from removed cardiac vegetations. Adequate histopathology reports were provided in 39 cases. Nineteen (48.7%) cases were recorded as acute, and 20 (51.2%) cases were reported as subacute IE.
Underlying noncardiac disorders
Hypertension (n = 410, 47.2%), diabetes mellitus (n = 231, 26.6%), hyperlipidemia (n = 192, 22.1%), chronic renal failure (n = 103, 11.9%), chronic obstructive pulmonary disease (n = 85, 9.8%), hemodialysis (n = 79, 9.1%), malignancy (n = 77, 8.9%), use of extended intravenous catheter (n = 66, 7.6%), immunosuppressive drug use (n = 53, 6.1%), cerebrovascular accident (n = 53, 6.1%), IV drug addiction (n = 52, 6.1%), poor dental care (n = 35, 4%), collagenosis (n = 23, 2.7%), HIV infection (n = 14, 1.6%), alcoholism (n = 11, 1.3%), hepatitis B (n = 3, 0.3%), and hepatitis C (n = 3, 0.3%) are the underlying noncardiac disorders.
Mortality
Intrahospital mortality was 17.5% (n = 152). Treating clinicians have specified attributed 172 causes of death in 130 of 152 patients. Septic shock/multiorgan failure (n = 86, 66.2%), congestive heart failure (n = 40, 30.8%), cerebral embolism/hemorrhage (n = 23, 17.7%), myocardial infarction (n = 10, 7.7%), surgical complications (n = 6, 4.6%), atrioventricular block (n = 3, 2.3%), mesenteric ischemia (n = 3, 2.3%), and liver failure (n = 1, 0.8%) were the likely reasons of death. PVE was found to be significantly mortal than NVE (NVE (n = 101, 16%), PVE (n = 49, 22.9%), (p = 0.042)).
Discussion
Epidemiology of IE has become more complex with today’s myriad healthcare-associated factors predisposing infections. In this study, one-fourth of our cases were PVE and almost three-fourth were NVE. If left untreated, IE can rapidly destroy heart valves and may lead to life-threatening consequences [4, 8]. Our data showed that 44% of the patients had experienced major arterial embolism and 15% had cardiac abscesses. Hence, blood cultures, echocardiography, and major arterial embolism were decisive in the diagnosis of IE rather than immunological and the rest of vascular phenomena. Moreover, 17% of NVE patients had experienced valve perforations while one-third of PVE cases had prosthetic valve dysfunction as the catastrophic consequences. Finally, 17.5% of the IE cases have lost their lives and mortality was significantly higher in the PVE arm compared with that of NVE patients. Septic shock, congestive heart failure, and cerebral embolism have been the leading potential causes of death in descending order in this study.
Today, global life expectancy at the age of 60 is around 20 years [9] and this increases the population vulnerable to IE due to underlying cardiac disorders other than rheumatic diseases [10]. We have found that both coexistent valvular problems and cardiac implants made up one-third of the patients for each as the foremost cardiac problems. Historically, prosthetic heart valve implantation was first performed on aortic valves in 1960 [11]. Today, all cardiac valves can be implanted and more than half of PVE was at the aortic valves followed by mitral valve involvement in our study. Conversely, there are preliminary data of uninfected prosthetic valves in patients with coexistent NVE [12]. We have shown that 7.4% of the NVE patients had uninfected cardiac implants.
In this study, 93.4% of the patients had cardiac vegetations compatible definite IE [4]. Four-fifths, as the significant portion, of patients had left-sided IE affecting aortic and mitral valves with similar rates. Although relatively infrequent [13], we detected multivalvular involvement in one-tenth of our cases. Basically, size matters in endocarditis and bigger vegetations, over 10 mm in particular, boost embolic risks [14]. Hence, patients exceeding 10 mm of vegetations commonly become candidates for surgery [4]. In this study, the vegetation sizes were not different between NVE and PVE patients, and it was slightly more than 13 mm for both groups indicating the serious potential to produce embolism [4]. Accordingly, more than one-third of our patients experienced cardiac surgery. Besides, cardiac abscesses were detected in 15.2% of the patients. Mostly single organisms are known to cause cardiac abscesses [15] and this was typically the case in our study. S. aureus was the leading pathogen in more than one-fourth of the abscess patients followed by enterococci and CoNS. IE due to CoNS is increasingly being recognized and associated with more frequent abscess formation compared with S. aureus in PVE patients [16]. Besides, we found that patients with PVE had longer durations until the establishment of diagnosis, and abscesses and cardiac fistulas were more frequent in PVE patients [17]. Accordingly, when the removed vegetations were analyzed histopathologically, half was reported as acute, and the other half was subacute that may ease the development of suppurative complications. Hence, PVE seemingly corrodes the heart higher than NVE and this also supported by the decreased ventricular ejection fraction and higher mortality rate in this study.
Staphylococcal species have long been known as dominant causative agents in IE [4, 8]. In a recent systematic review, staphylococcal IE percentage increased in the last five decades, S. aureus from 21 to 30% in particular [1]. In this study, staphylococcal species on the whole made up more than two-fifths of all cases. In previous reports, enterococci were related to late PVE [18] or CoNS was recorded as being increasingly recognized in PVE patients [16]. We have disclosed that S. aureus was significantly more frequent in NVE while enterococci and CoNS were more common pathogens in PVE patients. Moreover, our data mark the need for extensive empirical antibiotic coverage since both a diverse group of pathogens in general and multiple microorganisms (2.5%) in particular were recovered. Since more than one-fourth of S. aureus strains were methicillin resistant and penicillin resistance was detected in more than one-fourth of S. viridans strains, the empirical use of vancomycin or daptomycin should be considered in accordance with the local susceptibility data. Although preliminary data stressed the importance of hospital-acquired pathogens [19], our data disclosed that as much as 18% of NVE cases were classified under hospital-acquired infection category. Furthermore, dental source, central catheters, and respiratory system appeared to be more significant sources in NVE while cardiac surgery was commonly associated to PVE. Consequently, these data stress the importance of infection control practices.
Culture-negative endocarditis, which has been known to increase, is a serious concern in the optimization of therapy [20]. We found that slightly more than one-tenth of the cases were culture-negative. This is mostly due to early use of antibiotics prior to blood cultures, raised incidence of zoonotic diseases, and standardization problems in microbiological tests [21]. However, zoonotic endocarditis agents, known to be common in low-income countries [20], were infrequent (2%) in our study. This datum indicates the necessity of improved diagnostic approaches in IE. Accordingly, cultures and molecular testing from the removed cardiac vegetations contributed an additional 3.3% diagnosis in this study.
IE is often accompanied by complications [2, 3], primarily the systemic embolic events due to migrating endocardial vegetations [14]. In a recent meta-analysis, embolism was significantly higher in intravenous drug users, HIV and chronic liver disease patients, during staphylococcal infections, and with multiple, mobile, or mitral valve vegetations, and when the vegetation size is over 10 mm, or in PVE patients [22]. These parameters were exceedingly common in our cohort with the resultant arterial emboli in 44.4% of the cases. Cerebral embolism was the leading cause of arterial emboli in more than one-fourth of the cases. We could not disclose a difference for embolic potential between right- and left-sided endocarditis. However, the brain was significantly the more frequent site of embolism in left-sided while lungs were primary targets in right-sided IE.
The strengths of this study are the inclusion of definite endocarditis cases and its multicenter design. Its weaknesses are the retrospective nature and potential geographical differences in microbiological procedures. In conclusion, endocardial infections are primarily reported to be managed by cardiology, cardiovascular surgery, infectious diseases, and neurology departments as a teamwork [23] and our data stresses the importance of this collaboration. Furthermore, continuous surveillance, multidisciplinary patient management, infection control, and diagnostic improvement are crucial issues in the management of disease.
References
Slipczuk L, Codolosa JN, Davila CD, Romero-Corral A, Yun J, Pressman GS et al (2013) Infective endocarditis epidemiology over five decades: a systematic review. PLoS One 8. https://doi.org/10.1371/journal.pone.0082665
Thuny F, Tribouilloy C, Giorgi R, Brahim A, Nadji G, Riberi A et al (2007) Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis : a prospective multicentre study. Eur Heart J 28:1155–1161. https://doi.org/10.1093/eurheartj/ehm005
Wadi J, Hammoudeh A, Abashour W, Naser A, Murad M, El Sha’er S (2013) Short term outcome of medical therapy in community-acquired left-sided native valve infective endocarditis. Rev Tunisienne d’Infectiologie 7:14–17
Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ et al (2015) Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. https://doi.org/10.1161/CIR.0000000000000296
Ejection Fraction Heart failure measurement. Am Hear Assoc [cited 2019 5]; https://www.heart.org/en/health-topics/heart-failure/diagnosing-heart-failure/ejection-fraction-heart-failure-measurement
Galle N, Torbicki A, Barst R, Dartevelle P, Haworth S, Higenbottam T et al (2004) Guidelines on diagnosis and treatment of pulmonary arterial hypertension. Eur Heart J 25:2243–2278. https://academic.oup.com/eurheartj/article-lookup/doi/10.1016/j.ehj.2004.09.014. https://doi.org/10.1016/j.ehj.2004.09.014
Shoen FJ, Mitchell RN (2015) The heart. In: Vinay K, Abbas A, Aster J (eds) Robins and Cotran pathologic basis of disease. Elsevier Saunders, Philadelphia, pp 523–578
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Zotti F-D et al (2015) 2015 ESC guidelines for the management of infective endocarditis The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology ( ESC ) endorsed by : European Association for Cardio-Thoracic Surgery. Eur Heart J 36:3075–3123. https://doi.org/10.1093/eurheartj/ehv319
Life expectancy and healthy life expecancy data by WHO region [Internet]. WHO [cited 2019 25]; http://apps.who.int/gho/data/view.main.SDG2016LEXREGv?lang=en
Hall R, Budaj A, Antunes M, McMurray J, Gohlke-Baerwolf C, Tamargo J et al (2007) Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 28:230–268. https://doi.org/10.1093/eurheartj/ehl428
Garver D, Kaczmarek R, Silverman B, Gross T, Hamilton P 1995 The epidemiology of prosthetic heart valves in the United States. 22:86–91
Pachirat O, Kaewkes D, Pussadhamma B, watt G (2018) Corynebacterium diphtheriae native aortic valve endocarditis in a patient with prosthetic mitral valve: a rare presentation. Cardiol Res 9:314–317. https://doi.org/10.14740/cr741w
Vahabi A, Gul F, Garakhanova S, Sipahi H, Sipahi OR Pooled analysis of 1270 infective endocarditis cases in Turkey. J Infect Dev Ctries. https://doi.org/10.3855/jidc.10056
Mohananey D, Mohadjer A, Pettersson G, Navia J, Gordon S, Shrestha N et al (2018) Association of vegetation size with embolic risk in patients with infective endocarditis a systematic review and meta-analysis. JAMA Intern Med 44195:1–9. https://doi.org/10.1001/jamainternmed.2017.8653
Ramos Tuarez FJ, Law M Cardiac abscess [internet]. StatPearls 2018 [cited 2019 5];doi: https://www.ncbi.nlm.nih.gov/books/NBK459132/
Lalani T, Kanafani ZA, Chu VH, Moore L, Corey GR, Pappas P et al (2006) Prosthetic valve endocarditis due to coagulase-negative staphylococci: findings from the International Collaboration on Endocarditis Merged Database. Eur J Clin Microbiol Infect Dis 25:365–368. https://doi.org/10.1007/s10096-006-0141-z
Nagpal A, Sohail MR, Steckelberg JM (2012) Prosthetic valve endocarditis: state of the heart. Clin Investig (Lond) 2:803–817. https://doi.org/10.4155/cli.12.70
Simsek-Yavuz S, Sensoy A, Kasikcioglu H, Ceken S, Deniz D, Atilla Y et al (2015) Infective endocarditis in Turkey : aetiology , clinical features , and analysis of risk factors for mortality in 325 cases. Int J Infect Dis 30:106–114. https://doi.org/10.1016/j.ijid.2014.11.007
McDonald J (2009) Acute infective endocarditis. Infect Dis Clin N Am 23:643–664. https://doi.org/10.1016/j.idc.2009.04.013.Acute
Gouriet F, Chaudet H, Gautret P, Pellegrin L, de Santi VP, Savini H et al (2018) Endocarditis in the Mediterranean Basin. New Microbes New Infect 26:S43–S51. doi: https://doi.org/10.1016/j.nmni.2018.05.004. https://doi.org/10.1016/j.nmni.2018.05.004
Fournier P, Thuny F, Richet H, Lepidi H, Casalta J, Arzouni J et al (2010) Comprehensive diagnostic strategy for blood culture–negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis 51:131–140. https://doi.org/10.1086/653675
Yang A, Tan C, Daneman N, Hansen MS, Habib G, Salaun E et al (2019) Clinical and echocardiographic predictors of embolism in infective endocarditis : systematic review and meta-analysis. Clin Microbiol Infect 25:178–187. doi: https://doi.org/10.1016/j.cmi.2018.08.010. https://doi.org/10.1016/j.cmi.2018.08.010
Erdem H, Tekin-Koruk S, Koruk I, Tozlu-Keten D, Ulu-Kilic A, Oncul O et al (2011) Assessment of the requisites of microbiology based infectious disease training under the pressure of consultation needs. Ann Clin Microbiol Antimicrob 10. https://doi.org/10.1186/1476-0711-10-38
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval is obtained from the Fatih Sultan Mehmet Training and Research Hospital’s Review Board.
Informed consent
Not applicable. The study has a retrospective design.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erdem, H., Puca, E., Ruch, Y. et al. Portraying infective endocarditis: results of multinational ID-IRI study. Eur J Clin Microbiol Infect Dis 38, 1753–1763 (2019). https://doi.org/10.1007/s10096-019-03607-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03607-x