Abstract
Complicated intra-abdominal infections (cIAIs) are commonly associated with multimicroorganisms and treatment choices are becoming narrower due to developing resistance, especially in the gram-negative Enterobacteriaceae species. Eravacycline is a newly developed, fully synthetic tetracycline derivative that has shown potent broad-spectrum activity against a wide variety of microorganisms, including those such as extended spectrum β-lactamase producing Enterobacteriaceae and Acinetobacter. Eravacycline has shown activity against many gram-positive organisms such as methicillin-resistant S. aureus and vancomycin resistant Enterococcus faecalis and Enterococcus faecium (VRE), gram-negative organisms such as Escherichia coli, and anaerobic species of microorganisms such as Bacteroides. This fluorocycline has been compared to ertapenem and meropenem for the treatment of complicated intra-abdominal infections and levofloxacin for the treatment of complicated urinary tract infections. Eravacycline was shown to be noninferior to ertapenem but did not meet noninferiority criteria in comparison to levofloxacin. Oral and IV formulations on eravacycline were tested in clinical trials, but at this time, only the IV formulation is FDA approved. Eravacycline has been noted to have a half-life of 20 h with protein binding around 80%; AUC over minimum inhibitory concentration (MIC) has also been shown to be eravacycline’s best predictor of efficacy. Of note, eravacycline does not require any renal dose adjustments, as the majority of its clearance is by nonrenal pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rise of multidrug-resistant microorganisms not only has become a public health concern but also has complicated how we treat different hospital and community acquired infections [1]. The Centers for Disease Control and Prevention (CDC) has stated that each year in the USA alone, approximately 2 million people acquire an infection due to multidrug-resistant bacteria and at least 23,000 people each year die from these infections; the World Health Organization (WHO) has reported that in Europe the yearly number of deaths is even higher, with an estimated 25,000 deaths due to multidrug-resistant bacteria per year [2, 3]. In order to combat this threat, the WHO has advocated for a global health plan that includes five objectives: enhance awareness and perception of antimicrobial resistance, increase knowledge in areas such as surveillance and research, decrease the rate of infections, promote appropriate use of antimicrobials, and take into account the needs of all countries which includes the creation and expansion of new antimicrobial agents, diagnostic tools, and vaccines [4]. In 2012, the Generating Antibiotic Incentives Now (GAIN) act was passed as part of the Food and Drug Administration Safety and Innovation Act (FDASIA) which helped to direct attention to antimicrobial resistance by encouraging the development and approval of new antimicrobials [5]. This act also allowed for new antimicrobials to be designated as qualified infectious disease products (QIDPs) which lets the FDA grant Fast Track designation to these new agents, amongst other incentives [6].
A new, fully synthetic fluorocycline has been developed to treat infections caused by multidrug-resistant microorganisms, such as carbapenem-resistant Enterobacteriaceae, methicillin-resistant Staphylococcus aureus, extended spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, and vancomycin-resistant enterococci species; in 2014, this fluorocycline was designated a QIDP by the FDA, granting it fast track eligibility [5, 7]. Eravacycline (Xerava®), formerly TP-434, was designed to overcome two of the main resistance mechanisms common to the tetracycline class: ribosomal protection, commonly seen in gram-positive organisms, and active drug efflux, common in both gram-positive and gram-negative organisms [7, 8]. Modifications at the C-7 and C-9 positions on the tetracycline core lead to this enhanced, broad-spectrum bacterial activity (Fig. 1) [9]. Eravaycline’s mechanism of action is similar to other members of the tetracycline class as it inhibits protein synthesis by binding to the ribosome.
Data sources
A search of PubMed (1945–March 2019) and International Pharmaceutical Abstracts (1970–March 2019) was conducted using the terms eravacycline, TP-434, and intra-abdominal infections. All articles available in English were reviewed. Additional sources were the Clinicaltrials.gov website, International Pharmaceutical Abstracts, the Centers for Disease Control and Prevention (CDC) website, and the Tetraphase Pharmaceuticals website.
Medicinal chemistry
As mentioned previously, eravacycline has modifications at key points in the tetracycline core that have enhanced its antibacterial activity in comparison to others of the tetracycline class [8]. Previous studies examining structure–activity relationships of tetracycline analogs have shown that the northwest region of the tetracycline structure does not have direct involvement with ribosomes; therefore, this region can be altered without risking compromising antibacterial activity. The southeast portion of the molecule, however, is directly involved in interactions with bacterial ribosomes, and modifications here lead to loss of activity. Substitutions at C9 can lead to improvements in both antibacterial potency and activity against organisms with tetracycline specific resistance mechanisms. Eravacycline has a pyrrolidine on the C9 side chain and a fluoro group at C7, both of which grant its potent, broad-spectrum activity that will be discussed further in this manuscript.
Microbiological coverage
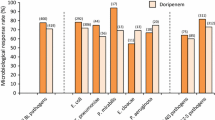
Table 1 details eravacycline’s microbiological coverage in comparison to other agents according to Clinical Laboratory Standards Institute (CLSI) testing. In vitro studies have shown that eravacycline has activity against a wide variety of organisms, including multidrug-resistant Acinetobacter species and ESBL-producing Enterobacteriaceae [9].
In vitro studies have evaluated eravacycline’s gram-positive activity and found MIC90 values to be between 0.016 and 0.05 μg/mL against organisms such as methicillin-resistant S. aureus (MRSA), methicillin susceptible S. aureus, coagulase negative staphylococci, vancomycin susceptible Enterococcus faecium and Enterococcus faecalis (VSE), vancomycin resistance Enterococcus faecium and Enterococcus faecium (VRE), Streptococcus pneumoniae, and Streptococcus pyogenes. Eravacycline’s activity also includes coverage against gram-negative organisms such as Escherichia coli (including ESBL-producing strains), Salmonella species, Shigella species, Haemophilus influenzae, Moraxella catarrhalis, and Acinetobacter lwoffii; MIC90 values for gram-negatives have been shown to be ≤ 0.5 μg/mL. Anaerobe coverage was also noted with eravacycline as it showed activity against Clostridium difficile, Peptostreptococcus species, Actinomyces species, Anaerococcus species, Bacteroides species, Bifidobacterium species, Eggerthella species, Lactobacillus species, and Propionibacterium acnes [9, 11].
Additionally, compared to another tetracycline related antibiotic, tigecycline, eravacycline has shown greater in vitro activity [9]. In an in vitro study done by Sutcliffe et al., eravacycline showed to have MIC90 values for a variety of gram-negative organisms, including Acinetobacter baumannii, Acinetobacter lwoffii, Klebsiella oxytoca, Moraxella catarrhalis, Morganella morganii, Proteus mirabilis, Proteus vulgaris, and Salmonella spp., which were about 2-fold lower than those of tigecycline, a glycylcycline. The study also showed that eravacycline had a 2-fold greater gram-positive and anaerobic activity than tigecycline. Gram-positives assessed in this study included the following: E. faecalis (VRE and VSE), E. faecium, Enterococcus spp., MRSA, coagulase negative staphylococci, and S. pneumoniae. When assessing activity against S. aureus isolates with quinolone resistant genes, eravacycline retained its activity to these isolates, while tigecycline’s activity was significantly reduced.
Atypical organisms that most often are implicated in community acquired pneumonia include Chlamydophila pneumoniae, Mycoplasma pneumoniae, and Legionella species [12, 13]. Another member of the tetracycline class, doxycycline, is commonly used to treat community acquired pneumonia, but at this time, only in vitro studies have also evaluated eravacycline against some of these pathogens. When tested against Legionella pneumophila, eravacycline was found to have a MIC90 between one and two, depending on the serogroup, in comparison to tetracycline which has an MIC90 of eight for this microorganism [14].
Pharmacokinetics/pharmacodynamics
Pharmacokinetic (PK) studies have shown an average protein binding of 71.4–82.5% with a nonlinear, concentration-dependent relationship [15, 16]. Oral bioavailability of eravacycline is low, with an average around 28% [17]. Eravacycline has a half-life of 20 h that increases with repeated doses and a median time to maximum plasma concentration (Tmax) of about 30 min [18]. A dose escalation study over 10 days showed that there is some systemic accumulation, but over the 10 days, the greatest amount of accumulation was 45% when dosed at 1 mg/kg every 12 h. Eravacycline is metabolized mainly by CYP3A4 and FMO-mediated oxidation. It not only is primarily excreted in the feces but also has some minor renal elimination as well. Data from the phase 1, open-label, safety and pharmacokinetic study on eravacycline is summarized in Table 2. Data examining pharmacodynamic (PD) parameters identified fAUC/MIC as the PK/PD index that in the best associated with eravacycline efficacy [19]. Eravacycline does not require dose adjustments in renal impairment or in patients with mild to moderate hepatic impairment (Child Pugh A and Child Pugh B), but in those with severe hepatic impairment (Child Pugh C), it is recommended to adjust the dose [20]. The fact that eravacycline does not require renal dose adjustments is promising for its applicability in critically ill patients with augmented renal clearance. Additionally, eravacycline has been shown to be active in vitro against biofilms produced by uropathogenic E. coli, which could potentially lead to a role in treatment of urinary tract infections [21].
As mentioned previously, eravacycline’s half-life increases with cumulative dosing, and in a study examining eravacycline’s antibacterial efficacy, it was shown that this cumulative dosing leads to an enhanced killing effect, specifically when tested against E. coli and MRSA strains [22]. Pharmacokinetic studies have also shown that steady state for eravacycline is not reached until at least day 5 of dosing [18]. When comparing eravacycline’s pharmacokinetic properties to other members of the tetracycline class differences in volume of distribution are evident. Eravacycline’s volume of distribution at steady state (Vss) is approximately 4 L/kg, which is suggestive of wide tissue distribution. Comparatively, tigecycline, omadacycline, tetracycline, and doxycycline have volumes of distribution at steady state of 6–9 kg/L, 2.6 L/kg, 1.3 L/kg, and 0.7 L/kg, respectively [18].
Phase 1 study
A phase 1 study by Connors, et al. examined the safety and pharmacokinetics of eravacycline in healthy men and women [16]. Twenty healthy adults participated in the study for about 8 weeks, which included a 30-day screening period, 6-day dosing/observation period, and 20-day follow-up period. These individuals received 7 IV doses of 1 mg/kg eravacycline every 12 h as a 1 h infusion. This study evaluated plasma and pulmonary pharmacokinetics as well as protein binding. Plasma samples taken immediately before and after 12 h after the seventh dose showed no statistical difference, indicating steady state was achieved. Protein binding was found to range from 79.3 to 87.1% with an average of 82.5 ± 1.7% and steady-state plasma fAUC0–12 was found to be 0.77 ± 0.14 μg·h/mL. The calculated AUC0–12 for the epithelial lining fluid (ELF) and alveolar macrophages (AM) were 4.93 and 39.53 μg·h/mL, respectively. Previously, studies have shown concentrations in the ELF of 500 mg levofloxacin to be 11.01 ± 4.52 μg/mL at 4 h and 2.5 ± 0.97 μg/mL at 12 h. Also, concentrations of 500 mg levofloxacin were reported to be 83.9 ± 53.2 μg/mL at 4 h and 18.3 ± 6.7 μg/mL at 12 h in the AM. On the other hand, concentrations of 500 mg azithromycin were found to be 1.7 ± 0.74 μg/mL at 4 h and 1.27 ± 0.47 μg/mL at 12 h in the ELF and 649.9 ± 259.1 μg/mL at 4 h and 669.4 ± 310.5 at 12 h [23]. All study participants tolerated eravacycline, and no serious adverse effects were noted. The most common adverse effects reported were nausea (90%), infusion related irritation (65%), vomiting (35%), and headache (30%), but all were reported to be mild or moderate in severity. Because this was a phase 1 study, sample size was small, but this trial demonstrated good tissue concentrations, with concentrations 6-fold and 50-fold higher in the ELF and AM, respectively, and can lead to future study in respiratory infections.
Phase 2 study
A randomized phase two, double-blind, active control study, published in 2014, was conducted at 19 sites in 6 countries [24]. Patients were included in the study if they had a BMI ≤ 30 kg/m2, were aged 18–75, and diagnosed with a complicated intra-abdominal infection (cIAI) requiring surgical intervention with an anticipated antibiotic duration of 14 days or less. Major exclusion criteria varied from symptoms of complicated appendicitis within less than 24 h of the current hospitalization, previous hospitalization in the past 6 months, an Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II score greater than 25, requirement of vasopressors, abnormal renal or liver function, and known or suspected inflammatory bowel disease (IBD). One hundred forty-three eligible patients were randomized in a 2:2:1 fashion to receive an IV infusion of eravacycline at 1.5 mg/kg every 24 h, eravacycline at 1 mg/kg every 12 h, or 1 g of ertapenem every 24 h. Fifty-three patients received eravacycline at 1.5 mg/kg, 56 received eravacycline at 1 mg/kg, 30 received ertapenem, and 4 patients do not receive a study drug. The primary efficacy end point was clinical response, defined as complete resolution or significant improvement in signs and symptoms of initial infection, at the test of cure (TOC) visit, in the microbiologically evaluable (ME) population. The ME population, a subset of the clinically evaluable population which encompassed all randomized patients who received any amount of drugs studied, were those who also had a baseline pathogen identified and were assessed for microbiological response. A majority of pathogens identified were gram-negative aerobes (67.9%), with the most common organism being E. coli (60.3%), and approximately 9% of pathogens isolated were anaerobes. A large majority of the study population’s primary site of infection was appendicitis (54%). Clinical success rates were as follows: 92.9% in the group receiving eravacycline 1.5 mg/kg, 100% in the group receiving eravacycline 1 mg/kg, and 92.3% in the ertapenem group. The difference between ertapenem and the group receiving eravacycline 1.5 mg/kg was 0.5% (95% CI − 23.1 to 25.2%). The difference between the group receiving eravacycline 1 mg/kg and ertapenem was 7.7% (95% CI − 6.7 to 40.9%). Specifically, for patients with isolated ESBL-producing pathogens, there were clinical success rates of 80%, 100%, and 100% in the 1.5 mg/kg eravacycline group, 1 mg/kg, and ertapenem groups, respectively. As the results show, there was no statistically significant difference between either dose of eravacycline and ertapenem, and interestingly, it showed that there was a higher rate of clinical success in the 1 mg/kg group over the 1.5 mg/kg group, but this was not shown to be statistically significant. There were also lower rates of total adverse effects in the 1 mg/kg eravacycline group when compared to the 1.5 mg/kg group, which will be discussed further in the safety section.
Safety
The percentage of patients who experienced a treatment–emergent adverse effect was 35.8% in the group receiving eravacycline 1.5 mg/kg, 28.6% in the group receiving eravacycline 1 mg/kg, and 26.7% in the ertapenem group. The most commonly reported adverse effects were nausea and vomiting and were of mild severity. No serious adverse effects, such as death, life threatening events, or events that lead to significant disability, were reported to be related to the study drug. Nausea occurred in 1.9% of those in the 1.5 mg/kg eravacycline group and 10.7% of the 1 mg/kg eravacycline group as compared to 6.7% in the ertapenem group and when comparing these results to previous studies of tigecycline which show much higher rates of nausea, 24.4% of patients, eravacycline has a favorable side effect profile [25].
Phase 3 study—IGNITE1
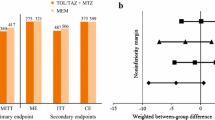
In 2016, a randomized phase 3 study was conducted to evaluate the safety and efficacy of eravacycline [7]. The Investigating Gram-Negative Infections Treated with Eravacycline (IGNITE1) trial was a randomized, double-blind, double dummy, multicenter study that compared eravacycline to ertapenem in the management of cIAI which required surgical or percutaneous intervention plus antibiotics. Five hundred and forty-one patients were randomized to receive either eravacycline 1 mg/kg IV every 12 h or ertapenem 1 g IV every 24 h. The primary efficacy end point was clinical response at the TOC visit. A noninferiority margin of 10% was implemented. Clinical cure rates 87.0% for the eravacycline group and 88.8% for the ertapenem group in the modified intent to treat (MITT) population. Noninferiority criteria was met with a 95% CI of − 7.4 to 3.8% for this population. The microbiologically evaluable population also met noninferiority; 91.4% of the eravacycline group and 95% of the ertapenem group had clinical cure (95% CI − 8.9 to 1.5%). There were 3 deaths in the eravacycline group and 6 in the ertapenem group, but none were considered related to study therapy. More treatment emergent adverse events occurred in the eravacycline group (113 of 270 versus 75 of 268 in the ertapenem group); nausea and phlebitis occurred more frequently in the eravacycline group. Study results show that eravacycline did meet the Food and Drug Administration (FDA) and European Medicines Agency (EMA) criteria for noninferiority and that the microbiology in this study was typical of the larger population of patients seen in clinical practice with these types of infections. However, the average patient in this study was under 65 years of age and had a low risk of mortality as evidence by a low APACHE score.
When comparing the study populations in the IGNITE1 trial, the most common pathogen in both the eravacycline group and the ertapenem group was Enterobacteriaceae, with 168 isolates in the eravacycline group (76.36% of the study population) and 171 in the ertapenem group (75.66% of the study population). A total of 12.5% of the Enterobacteriaceae were confirmed to be ESBL-producing in the eravacycline group, and 10.5% were ESBL-producing in the ertapenem group. A total of 90.5% of the patients with ESBL-producing Enterobacteriaceae in the eravacycline group had a favorable response, while 83.3% in the ertapenem group had a favorable response. About 8% of the patients receiving eravacycline had Pseudomonas isolates and 8.8% of the ertapenem group has Pseudomonas isolates, but the ertapenem group had a higher favorable response rate with this pathogen (90.0% to 83.3%). As mentioned previously, eravacycline is not known to have reliable coverage to Pseudomonas species, so this favorable response rate was critiqued to be due to pre-operative antipseudomonal β-lactams used for source control [26]. Both treatment groups had similar durations of therapy (average 7.6 days for each). Table 3 compares data from the phase 1, phase 2, and phase 3 studies.
Phase 3 study—IGNITE4
A recently published study discussed results from the IGNITE4 trial which compared eravacycline to meropenem for the treatment of cIAI [27]. This study was a randomized, double-blind, double-dummy, multicenter, prospective trial aimed to address the safety and efficacy of eravacycline compared to meropenem. Five hundred patients from 65 sites in 11 countries were randomized to receive either 1 mg/kg eravacycline IV every 12 h or 1 g meropenem daily. The primary end point for this study was similar to the INGITE1 study, which was clinical response at the TOC visit. As eravacycline had demonstrated noninferiority in the IGNITE1 trial with a 10% margin, the noninferiority margin for this study was set at 12.5%. The primary disease for each group was complicated appendicitis, with 48.2% in the eravacycline group and 43.9% in the meropenem group. At the TOC visit, 92.4% of the eravacycline group and 91.6% of the meropenem group met the criteria for clinical cure (95% CI, − 4.1 to 5.8), indicating noninferiority. Gram-negative aerobes were the most common pathogen isolated at baseline, with 89.2% of the eravacycline group and 92.2% of the meropenem group reporting these pathogens. Additionally, 90% of the eravacycline group and 93.7% of the meropenem group had anaerobes isolated at baseline; polymicrobial infections occurred in 71% of total patients. Treatment emergent adverse events occurred in 37.2% of the eravacycline group compared to 30.9% of the meropenem group, with the majority of side effects being gastrointestinal in nature; nausea was only reported in 4.8% of patients in the eravacycline group and 0.8% of the meropenem group.
Additional studies
IGNITE2, a trial studying IV to PO regimens of eravacycline, compared eravacycline to levofloxacin in the treatment of complicated UTIs (cUTIs). The maker of eravacycline, Tetraphase Pharmaceuticals, announced results of the trial, but results showed eravacycline to be inferior to levofloxacin. Those in the study who remained on IV therapy alone had a higher response rate, so it was hypothesized that the problem lay with the oral formulation due to low oral bioavailability as mentioned previously [28]. IGNITE3, a trial again studying eravacycline, compared IV eravacycline to IV levofloxacin for the treatment of cUTIs. Eravacycline again failed to meet the FDA’s criteria for noninferiority [29].
Conclusion
Eravacycline, a newly approved tetracycline derivative, has shown broad-spectrum activity for gram-positive, gram-negative, anaerobic, and multidrug-resistant microorganisms. Eravacycline also does not require renal dose adjustments, which could potentially make it an appealing treatment option in populations such as the critical ill or others with impaired renal function. As eravacycline has shown in vitro superiority to tigecycline, has met noninferiority criteria to ertapenem and meropenem, and has broad-spectrum activity, it can be reasonable to suggest eravacycline to be an alternative treatment option for the treatment of mild to moderate severity cIAI as a single-agent particularly in those with confirmed or highly suspected resistant infections.
References
Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. (2018) Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev 31(2)
Antibiotic/Antimicrobial Resistance. Centers for Disease Control and Prevention website. https://www.cdc.gov/drugresistance/index.html. Last reviewed August 18, 2017. Last Updated March 29, 2018. Accessed 6 Sept 2018
Antimicrobial Drug Resistance. World Health Organization website. http://www.who.int/csr/disease/OP_AMR_FINAL.pdf. Accessed 6 Sept 2018
Global action plan on antimicrobial resistance. World Health Organization website. http://www.who.int/antimicrobial-resistance/global-action-plan/en/. Accessed 6 Sept 2018
Generating antibiotic incentives now. Food and Drug Administration website. https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/UCM595188.pdf. Accessed 6 Sept 2018
FDA awards fast track status to tetraphase pharmaceuticals for IV and oral formulations of eravacycline [news release]. Tetraphase Pharmaceuticals, Watertown, MA. April 2, 2014. https://ir.tphase.com/news-releases/news-release-details/fda-awards-fast-track-status-tetraphase-pharmaceuticals-iv-and#. Accessed 6 Sept 2018
Solomkin J, Evans D, Slepavicius A et al (2017) Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the investigating gram-negative infections treated with eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg 152(3):224–232
Xiao XY, Hunt DK, Zhou J et al (2012) Fluorocyclines. 1. 7-fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline: a potent, broad spectrum antibacterial agent. J Med Chem 55(2):597–605
Sutcliffe JA, O’Brien W, Fyfe C, Grossman TH (2013) Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother 57(11):5548–5558
CLSI (2016) Performance Standards for Antimicrobial Susceptibility Testing, 26th edn. CLSI Supplement M100S. Clinical and Laboratory Standards Institute, Wayne
Goldstein EC, Citron DM, Tyrrell KL (2018) In vitro activity of eravacycline and comparator antimicrobials against 143 recent strains of Bacteroides and Parabacteroides species. Anaerobe 52:122–124
Mandell LA, Wunderink RG, Anzueto A et al (2007) Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(suppl 2):S27–S72
Zhanel GG, Cheung D, Adam H et al (2016) Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs 76:567–588
Dubois J, Dubois M, Martel JF, Grossman T, Sutcliffe J (2010) In vitro activity of fluorocyclines against Legionella pneumophila, poster 178. 50th ICAAC, Boston
Thabit AK, Monogue ML, Nicolau DP (2016) Eravacycline pharmacokinetics and challenges in defining humanized exposure in vivo. Antimicrob Agents Chemother 60(8):5072–5075
Connors KP, Housman ST, Pope JS et al (2014) Phase I, open-label, safety and pharmacokinetic study to assess bronchopulmonary disposition of intravenous eravacycline in healthy men and women. Antimicrob Agents Chemother 58(4):2113–2118
Leighton A, Zupanets I, Bezugla N, Plamondon L, Macdonald G, Sutcliffe J (2011) Broad spectrum fluorocycline TP-434 has oral bioavailability in humans, poster 1509. 21st ECCMID/27th ICC, Milan, Italy
Newman JV, Zhou J, Izmailyan S, Tsai L (2018) Randomized, double-blind, placebo-controlled studies of the safety and pharmacokinetics of single and multiple ascending doses of eravacycline. Antimicrob Agents Chemother 62(11):e01174–e01118
Thabit AK, Monogue ML, Newman JV, Nicolau DP (2018) Assessment of in vivo efficacy of eravacycline against Enterobacteriaceae exhibiting various resistance mechanisms: a dose-ranging study and pharmacokinetic/pharmacodynamic analysis. Int J Antimicrob Agents 51(5):727–732
Eravacycline (2018) Package insert. Tetraphase Pharmaceuticals, Watertown
Grossman TH, O’Brien W, Kerstein KO, Sutcliffe JA (2015) Eravacycline (TP-434) is active in vitro against biofilms formed by uropathogenic Escherichia coli. Antimicrob Agents Chemother 59(4):2446–2449
Monogue ML, Thabit AK, Hamada Y, Nicolau DP (2016) Antibacterial efficacy of eravacycline in vivo against gram-positive and gram-negative organisms. Antimicrob Agents Chemother 60(8):5001–5005
Rodvold KA, Danziger LH, Gotfried MH (2003) Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob Agents Chemother 47(8):2450–2457
Solomkin JS, Ramesh MK, Cesnauskas G et al (2014) Phase 2, randomized, double-blind study of the efficacy and safety of two dose regimens of eravacycline versus ertapenem for adult community-acquired complicated intra-abdominal infections. Antimicrob Agents Chemother 58(4):1847–1854
Babinchak T, Ellis-Grosse E, Dartois N, Rose GM, Loh E (2005) The efficacy and safety of tigecycline for the treatment of complicated intraabdominal infections: analysis of pooled clinical trial data. Clin Infect Dis 41(Suppl 5):S354–S367
Honore PM, Spapen HD (2017) Eravacycline for treatment of complicated intra-abdominal infections: the fire is not ignited! Ann Transl Med 5(21):425
Solomkin JS, Gardovskis J, Lawrence K et al (2018) IGNITE4: results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs. meropenem in the treatment of complicated intra-abdominal infections. Clin Infect Dis
Tetraphase announces top-line results from IGNITE2 phase 3 clinical trial of eravacycline in cUTI [news release]. Tetraphase Pharmaceuticals, Watertown, MA. September 8, 2015. http://ir.tphase.com/news-releases/news-release-details/tetraphase-announces-top-line-results-ignite2-phase-3-clinical. Accessed 7 March 2019
Tetraphase announces top-line results from IGNITE3 phase 3 clinical trial of eravacycline in complicated urinary tract infections (cUTI). Tetraphase Pharmaceuticals, Watertown, MA. February 13, 2018. http://ir.tphase.com/news-releases/news-release-details/tetraphase-announces-top-line-results-ignite3-phase-3-clinical. Accessed 6 Aug 2018
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Eravacycline is a newly approved tetracycline derivate, a fluorocycline, which is active against gram-positive organisms, gram-negative organisms, anaerobes, and certain microorganisms that have developed resistance mechanisms.
• Eravacycline is currently only available in an IV formulation which does not require renal dose adjustments.
• Eravacycline met noninferiority criteria when compared to both ertapenem and meropenem for the treatment of complicated intra-abdominal infections.
Rights and permissions
About this article
Cite this article
Lee, Y.R., Burton, C.E. Eravacycline, a newly approved fluorocycline. Eur J Clin Microbiol Infect Dis 38, 1787–1794 (2019). https://doi.org/10.1007/s10096-019-03590-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03590-3