Abstract
In Iran, patients showing rifampicin (RIF) resistance detected by the Xpert® MTB/RIF assay are considered as candidates for multidrug-resistant tuberculosis (MDR-TB) treatment. Despite the fact that RIF resistance has been used as a proxy for MDR-TB, little is known about the proportion of isoniazid (INH) resistance patterns in RIF-resistant TB. We systematically searched MEDLINE, Embase, and other databases up to March 2017 for studies addressing the proportion of INH resistance patterns in RIF-resistant TB in Iran. The data were pooled using a random effects model. Heterogeneity was assessed using Cochran’s Q and I2 statistics. A total of 11 articles met the eligibility criteria. Data analysis demonstrated that 33.3% of RIF-resistant isolates from new TB cases and 14.8% of RIF-resistant isolates from previously treated cases did not display resistance to INH. The relatively high proportion of INH susceptibility among isolates with RIF resistance indicated that RIF resistance may no longer predict MDR-TB in Iran. Therefore, the detection of RIF resistance by the Xpert MTB/RIF assay will require complementary detection of INH resistance by other drug susceptibility testing (DST) methods in order to establish the diagnosis of MDR-TB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) still remains a worldwide problem and ranks high alongside human immunodeficiency virus (HIV) as the leading cause of death from infectious diseases [1]. Drug-resistant strains of Mycobacterium tuberculosis have emerged as a major threat to global TB control programs [2, 3]. The World Health Organization (WHO) estimated that there were 480,000 new cases of multidrug-resistant tuberculosis (MDR-TB) in 2015, defined as strains that are resistant to at least isoniazid (INH) and rifampicin (RIF) [1]. MDR-TB arises due to the mismanagement of TB in patients with active infection [4, 5]. The management of MDR-TB should begin with the identification of cases. Although early diagnosis and proper treatment of the cases can intercept the development of MDR-TB, accurate diagnosis of drug-resistant cases remains a barrier to TB control [3, 5]. Conventional methods for detecting drug-resistant TB take weeks to months to produce results. During this time, patients may be inappropriately treated and drug-resistant strains may spread to the community [6]. Recently, the WHO endorsed the use of an automated rapid molecular assay, Xpert® MTB/RIF, for the detection of M. tuberculosis and RIF resistance [3, 7, 8]. Concomitant INH resistance is often communicated with RIF resistance [9]. In Iran, with increased prevalence of MDR-TB, some TB reference laboratories use Xpert MTB/RIF for the rapid diagnosis of TB and detection of drug-resistant TB. Based on the National Tuberculosis Control Program (NTP), patients detected as having RIF resistance on this assay are administered MDR-TB treatment. Despite the fact that RIF resistance has been used as “a surrogate marker” for MDR-TB, there is a growing concern for the increase of rifampicin monoresistance in Iran [10,11,12]. This implies that, if these patients were treated as having MDR-TB, a significant number would be treated incorrectly by excluding INH from the treatment regimen. In the present study, the extent of the INH resistance pattern in RIF-resistant TB is investigated. To our knowledge, this is the first report that investigates the INH resistance patterns in RIF-resistant cases.
Methods
Literature search
We searched MEDLINE (via PubMed), Embase, Web of Science, and Iranian databases up to 31 March 2017. We used a combination of Medical Subject Headings (MeSH) and keywords, focusing on terms to describe the relevant populations (patients with drug-resistant TB). We also screened the bibliographies of included studies for relevant articles. We included only studies published in English or Persian.
Selection criteria
We included original articles on the prevalence of drug resistance of TB in Iran. The included studies shall provide drug resistance data of either new cases or retreated cases or both and use a standard method for drug susceptibility testing (DST) of M. tuberculosis. In order to minimize the potential bias caused by too small a sample size, articles with less than 100 subjects were excluded. Each article was reviewed by two researchers independently. In case of discrepancies, the opinion of a third researcher was sought. If the study was reported in duplicate, the most informative version of the study was included. Studies that did not report the number of cases with active TB, the patterns of drug resistance, or studies on subpopulations such as studies conducted on immigrants, or HIV-positive cases, were excluded.
Data extraction
Data were extracted into a pre-designed structured Microsoft Excel® form by one reviewer and appraised for accuracy by a second reviewer. The extracted data included characteristics of the target population, settings, study designs, methods, and results.
Quality assessment
The included studies were appraised for quality using a quality assessment checklist designed by the Joanna Briggs Institute [13].
Data analysis
Data analyses were performed using Comprehensive Meta-Analysis version 2.2 (Biostat, Englewood, NJ, USA) [14]. Generally, we used fixed or random effects models, depending on statistical heterogeneity between studies, to calculate summary estimates. Statistical heterogeneity was quantified by Cochran’s Q and I2 statistics. To check for publication bias, we generated a funnel plot and used Egger’s test (p < 0.05 was considered indicative of statistically significant publication bias and funnel plot asymmetry also suggested bias in the meta-analysis).
Results
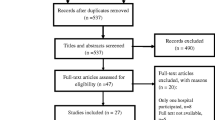
From the records identified from the MEDLINE, Embase, Web of Science, and Iranian databases (Fig. 1), 11 studies published from different regions of Iran were included in this study (Table 1) [15,16,17,18,19,20,21,22,23,24,25]. In all included studies, the standard proportional method was employed for DST, except one that used BACTEC MGIT. The WHO/International Union Against Tuberculosis and Lung Disease (IUATLD) guidelines were used for direct microscopy, culture, and drug susceptibility testing. According to the data from included studies, all clinical isolates of TB were cultured from patients with active TB. There was no information about the preventive medication, especially with INH in those with latent TB infection (LTBI).
As shown in Table 2, of the 2552 tested isolates from new cases, 156 (5.5%) were resistant to RIF. About a third (33.3%) of RIF-resistant isolates from new cases were INH susceptible. Of the 440 tested isolates from previously treated cases, 195 (36.0%) displayed resistance to RIF, with 14.8% susceptibility to INH (Table 2).
A Forest plot for the meta-analysis of any RIF-resistant TB is shown in Fig. 2. As shown in Table 2 and Fig. 3, no evidence of publication bias was observed (p = 0.2 for Egger weighted regression analysis).
Discussion
In Iran, patients detected as having RIF resistance detected by the Xpert MTB/RIF assay are given MDR-TB treatment without knowing the patterns of INH resistance. Therefore, a significant number of patients would be accidentally rejected INH. According to our findings, RIF resistance is not accompanied by INH resistance in 33.3% of new cases and 14.8% of retreatment cases in Iran. These data showed that a considerable proportion of RIF-resistant isolates were susceptible to INH. According to the results of our study, the noncompliance for frequency of resistance against RIF and INH decreased from 30.3% in new cases to 14.8% in retreatment cases. One possible reason behind this observation could be the prior long-term TB therapy in retreatment cases, which can increase the risk of drug resistance [26]. Espinal et al. [26] indicated that drug-resistant TB was significantly associated with prior treatment periods of >12 months and >6 months, respectively, compared with a treatment period of 3 months or less.
RIF resistance is considered as a proxy for MDR-TB, as a large proportion of RIF-resistant strains have INH resistance as well. Then, the detection of MDR-TB would be sufficient with a single test that detects RIF resistance [27, 28]. In areas with low RIF mono-resistant TB but high MDR prevalence, this correlation is particularly applicable. However, in countries with increasing rates of RIF monoresistance, this correlation may be questionable and not always applicable. In Iran, during 2010–2011, Velayati et al. [10] reported a high prevalence of RIF mono-resistant TB among pulmonary TB patients. The increasing rates of RIF mono-resistant TB have also been reported from different regions of the world [28,29,30,31]. Rufai et al. [32], from India, a high TB burden country, reported that 22% of TB isolates were RIF mono-resistant [32]. Likewise, another investigation from this region observed a high percentage of RIF monoresistance [33]. These high rates of RIF mono-resistant TB in studies from India may suggest that methodologies relying on RIF resistance as a marker to detect MDR-TB are not likely to be successful. Rufai et al. [32] have also indicated that the Xpert MTB/RIF assay can give false-negative and false-positive RIF resistance results. They show that relying only on the Xpert MTB/RIF results may be a disastrous step for TB control programs. False-negative results of RIF resistance can keep patients unnecessarily on anti-TB chemotherapy for a long duration, thus leaving the patients inappropriately treated. This can lead to the amplification and spread of MDR-TB [32]. Unlike studies from India, much lower RIF monoresistance levels were reported from Pakistan and Turkey [34, 35]. According to Ayaz et al. [34], the low rates of RIF monoresistance would support the use of RIF as a marker for MDR-TB in the Pakistani population. In the current study, it is important to note that we did not specifically address the reliability of RIF resistance detected by molecular tests as a proxy for MDR-TB. However, RIF resistance may no longer predict MDR-TB in a significant number of patients in Iran. Thus, we suggest that each country carries out evaluation work, to prepare guidelines for the use of the Xpert MTB/RIF at the national level. Furthermore, the detection of RIF resistance by Xpert MTB/RIF will need to be complemented by the diagnosis of INH resistance by other DST methods in order to establish the diagnosis of MDR-TB. According to the WHO guidelines for the management of drug-resistant TB, “if isoniazid susceptibility cannot be ascertained, the addition of isoniazid to the regimen may be considered” [36].
Our systematic review had some limitations. First, we could not analyze the effect of factors such as previous preventive medication on drug susceptibility status, because of the limited information obtained from the studied articles. Patients with LTBI who received preventive therapy, especially with INH, are at higher risk for acquiring anti-TB drug resistance compared to those who did not [37]. Second, although in all included studies WHO guidelines were used for drug susceptibility testing, our findings should be interpreted in the context of the variability in study quality. Finally, as with any systematic review, limitations associated with publication bias should be considered.
In conclusion, due to the high proportion of INH susceptibility among isolates with RIF resistance, RIF resistance may no longer predict MDR-TB in a significant number of cases in Iran. Therefore, Xpert MTB/RIF results must always be confirmed by DST in order to establish the diagnosis of MDR-TB. INH should also be included in the treatment regimen at least until INH resistance is proven.
References
World Health Organization (WHO) (2016) Global tuberculosis report 2016
Raviglione MC, Uplekar MW (2006) WHO’s new Stop TB Strategy. Lancet 367(9514):952–955
Daley CL, Caminero JA (2013) Management of multidrug resistant tuberculosis. Semin Respir Crit Care Med 34(1):44–59
Zignol M, Hosseini MS, Wright A, Lambregts-van Weezenbeek C, Nunn P, Watt CJ, Williams BG, Dye C (2006) Global incidence of multidrug-resistant tuberculosis. J Infect Dis 194(4):479–485
Lynch JB (2013) Multidrug-resistant tuberculosis. Med Clin North Am 97(4):553–579
Van Rie A, Page-Shipp L, Scott L, Sanne I, Stevens W (2010) Xpert® MTB/RIF for point-of-care diagnosis of TB in high-HIV burden, resource-limited countries: hype or hope? Expert Rev Mol Diagn 10(7):937–946
Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O’Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD (2010) Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363(11):1005–1015
Hillemann D, Rüsch-Gerdes S, Richter E (2007) Evaluation of the GenoType MTBDRplus assay for rifampin and isoniazid susceptibility testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol 45(8):2635–2640
Kurbatova EV, Cavanaugh JS, Shah NS, Wright A, Kim H, Metchock B, Van Deun A, Barrera L, Boulahbal F, Richter E, Martín-Casabona N, Arias F, Zemanova I, Drobniewski F, Santos Silva A, Coulter C, Lumb R, Cegielski JP (2012) Rifampicin-resistant Mycobacterium tuberculosis: susceptibility to isoniazid and other anti-tuberculosis drugs. Int J Tuberc Lung Dis 16(3):355–357
Velayati AA, Farnia P, Mozafari M, Sheikholeslami MF, Karahrudi MA, Tabarsi P, Hoffner S (2014) High prevelance of rifampin-monoresistant tuberculosis: a retrospective analysis among Iranian pulmonary tuberculosis patients. Am J Trop Med Hyg 90(1):99–105
Nasiri MJ, Dabiri H, Darban-Sarokhalil D, Rezadehbashi M, Zamani S (2014) Prevalence of drug-resistant tuberculosis in Iran: systematic review and meta-analysis. Am J Infect Control 42(11):1212–1218
Nasiri MJ, Chirani AS, Amin M, Halabian R, Fooladi AAI (2016) Isoniazid-resistant tuberculosis in Iran: a systematic review. Tuberculosis (Edinb) 98:104–109
Munn Z, Moola S, Lisy K, Riitano D (2014) The Joanna Briggs Institute Reviewers’ Manual 2014. The systematic review of prevalence and incidence data. The Joanna Briggs Institute, Adelaide
Nasiri MJ, Dabiri H, Darban-Sarokhalil D, Shahraki AH (2015) Prevalence of non-tuberculosis mycobacterial infections among tuberculosis suspects in Iran: systematic review and meta-analysis. PLoS One 10(6):e0129073
Mansoori S, Mirabolhasani Z, Farnia P, Velayati A (2003) The pattern of drug resistance among newly diagnosed and old cases of pulmonary tuberculosis in NRITLD. Arch Iranian Med 6(4):255–260
Bahrmand AR, Velayati AA, Bakayev VV (2000) Treatment monitoring and prevalence of drug resistance in tuberculosis patients in Tehran. Int J Tuberc Lung Dis 4(6):544–549
Shamaei M, Marjani M, Chitsaz E, Kazempour M, Esmaeili M, Farnia P, Tabarsi P, Amiri MV, Mirsaeidi M, Mansouri D, Masjedi MR, Velayati AA (2009) First-line anti-tuberculosis drug resistance patterns and trends at the national TB referral center in Iran—eight years of surveillance. Int J Infect Dis 13(5):e236–e240
Namaei MH, Sadeghian A, Naderinasab M, Ziaee M (2006) Prevalence of primary drug resistant Mycobacterium tuberculosis in Mashhad, Iran. Indian J Med Res 124(1):77–80
Mirsaeidi MS, Tabarsi P, Farnia P, Ebrahimi G, Morris MW, Masjedi MR, Velayati AA, Mansouri D (2007) Trends of drug resistant Mycobacterium tuberculosis in a tertiary tuberculosis center in Iran. Saudi Med J 28(4):544–550
Marjani M, Baghaei P, Tabarsi P, Shamaei M, Mansouri D, Masjedi MR, Velayati A (2012) Drug resistance pattern and outcome of treatment in recurrent episodes of tuberculosis. East Mediterr Health J 18(9):957–961
Livani S, Mirinargesi M, Nemati-Shoja E, Rafiei S, Taziki M, Tabarraei A, Ghaemi E (2011) Prevalence of multidrug resistant Mycobacterium tuberculosis by mycobacteria growth indicator tube in Golestan province, north of Iran (in Persian). Med Lab J 5(2):7–14
Nasiri MJ, Rezaei F, Zamani S, Darban-Sarokhalil D, Fooladi AAI, Shojaei H, Feizabadi MM (2014) Drug resistance pattern of Mycobacterium tuberculosis isolates from patients of five provinces of Iran. Asian Pac J Trop Med 7(3):193–196
Mohajeri P, Norozi B, Atashi S, Farahani A (2014) Anti tuberculosis drug resistance in west of Iran. J Glob Infect Dis 6(3):114–117
Farazi A, Sofian M, Zarrinfar N, Katebi F, Hoseini SD, Keshavarz R (2013) Drug resistance pattern and associated risk factors of tuberculosis patients in the central province of Iran. Caspian J Intern Med 4(4):785–789
Tavanaee Sani A, Shakiba A, Salehi M, Bahrami Taghanaki HR, Ayati Fard SF, Ghazvini K (2015) Epidemiological characterization of drug resistance among Mycobacterium tuberculosis isolated from patients in northeast of Iran during 2012–2013. Biomed Res Int 2015:747085
Espinal MA, Laserson K, Camacho M, Fusheng Z, Kim SJ, Tlali RE, Smith I, Suarez P, Antunes ML, George AG, Martin-Casabona N, Simelane P, Weyer K, Binkin N, Raviglione MC (2001) Determinants of drug-resistant tuberculosis: analysis of 11 countries. Int J Tuberc Lung Dis 5(10):887–893
Caws M, Duy PM, Tho DQ, Lan NTN, Hoa DV, Farrar J (2006) Mutations prevalent among rifampin- and isoniazid-resistant Mycobacterium tuberculosis isolates from a hospital in Vietnam. J Clin Microbiol 44(7):2333–2337
Coovadia YM, Mahomed S, Pillay M, Werner L, Mlisana K (2013) Rifampicin mono-resistance in Mycobacterium tuberculosis in KwaZulu-Natal, South Africa: a significant phenomenon in a high prevalence TB-HIV region. PLoS One 8(11):e77712
Mukinda FK, Theron D, van der Spuy GD, Jacobson KR, Roscher M, Streicher EM, Musekiwa A, Coetzee GJ, Victor TC, Marais BJ, Nachega JB, Warren RM, Schaaf HS (2012) Rise in rifampicin-monoresistant tuberculosis in Western Cape, South Africa. Int J Tuberc Lung Dis 16(2):196–202
Sanders M, Van Deun A, Ntakirutimana D, Masabo JP, Rukundo J, Rigouts L, Fissette K, Portaelst F (2006) Rifampicin mono-resistant Mycobacterium tuberculosis in Bujumbura, Burundi: results of a drug resistance survey. Int J Tuberc Lung Dis 10(2):178–183
Traore H, Fissette K, Bastian I, Devleeschouwer M, Portaels F (2000) Detection of rifampicin resistance in Mycobacterium tuberculosis isolates from diverse countries by a commercial line probe assay as an initial indicator of multidrug resistance [technical note]. Int J Tuberc Lung Dis 4(5):481–484
Rufai SB, Kumar P, Singh A, Prajapati S, Balooni V, Singh S (2014) Comparison of Xpert MTB/RIF with line probe assay for detection of rifampin-monoresistant Mycobacterium tuberculosis. J Clin Microbiol 52(6):1846–1852
Kumar P, Balooni V, Sharma BK, Kapil V, Sachdeva KS, Singh S (2014) High degree of multi-drug resistance and hetero-resistance in pulmonary TB patients from Punjab state of India. Tuberculosis (Edinb) 94(1):73–80
Ayaz A, Hasan Z, Jafri S, Inayat R, Mangi R, Channa AA, Malik FR, Ali A, Rafiq Y, Hasan R (2012) Characterizing Mycobacterium tuberculosis isolates from Karachi, Pakistan: drug resistance and genotypes. Int J Infect Dis 16(4):e303–e309
Zeka AN, Tasbakan S, Cavusoglu C (2011) Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J Clin Microbiol 49(12):4138–4141
World Health Organization (WHO) (2014) Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis
Ai J-W, Ruan Q-L, Liu Q-H, Zhang W-H (2016) Updates on the risk factors for latent tuberculosis reactivation and their managements. Emerg Microbes Infect 5(2):e10
Acknowledgments
We would like to thank the “Clinical Research Development Center of Baqiyatallah Hospital” for their kind cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval
The manuscript is a systematic review, so ethical approval was not required for the study.
Rights and permissions
About this article
Cite this article
Nasiri, M.J., Zamani, S., Pormohammad, A. et al. The reliability of rifampicin resistance as a proxy for multidrug-resistant tuberculosis: a systematic review of studies from Iran. Eur J Clin Microbiol Infect Dis 37, 9–14 (2018). https://doi.org/10.1007/s10096-017-3079-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-3079-4