Abstract
To determine trends in incidence and clinical relevance of rapidly growing mycobacteria (RGM) in a low-prevalence region of non-tuberculous mycobacteria. We retrospectively identified all patients with RGM-positive cultures between January 1994 and December 2015. Trends in incidence, clinical significance, and outcomes were assessed. One hundred and forty patients had RGM-positive cultures (116 respiratory and 24 extra-respiratory sources). The incidence of RGM isolates increased steadily from 2003 (0.34 per 100,000) to 2015 (1.73 per 100,000), with an average annual increase of 8.3%. Thirty-two patients (22.9%) had clinical disease, which trended to cluster in the second half of the study period. A positive acid-fast bacilli smear (odds ratio [OR] 97.7, 95 % CI 13.8–689.4), the presence of extra-respiratory isolates (OR 19.4, 95 % CI 5.2–72.7), and female gender (OR 5.9, 95 % CI 1.9–19.1) were independently associated with clinical disease. Cure rates were 73.3 and 87.5% for pulmonary and extra-pulmonary disease respectively. Although the burden of disease remains low, the presence of RGM isolates is increasing in our geographical setting. Whether this rise will be sustained over time and will coincide with an increase in clinical disease, or whether it is merely a cycle in the poorly understood epidemiological behaviour of environmental mycobacteria, will be seen in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-tuberculous (environmental) mycobacteria (NTM) are recognized as a growing infectious disease problem as the overall incidence of tuberculosis (TB) continues to decrease in low-incidence countries [1]. Infections with rapidly growing mycobacteria (RGM) are of particular concern in some countries, as they have become the second most common NTM recovered from respiratory specimens [2–4], and as for patients with Mycobacterium abscessus infection, the species that most frequently causes pulmonary disease, are associated with little chance of cure [5].
Because of their ubiquity, human RGM infections have been reported from many geographical areas in the world. However, their true incidence and prevalence are largely unknown because systematic epidemiological reporting is generally not mandatory. Most studies from different areas documenting increasing isolation frequencies are limited by the fact that not all isolates represent true clinical disease [6–8]. Recovery of RGM from patient specimens can be the result of true clinical disease, colonisation of the respiratory tract, or laboratory contamination. Although a consensus document by the American Thoracic Society (ATS) and the Infectious Disease Society of America (IDSA) [9, 10] established diagnostic criteria for the diagnosis of pulmonary disease caused by NTM, uncertainty with regard to the clinical significance of RGM isolates remains in many instances. Indeed, with the continued rise in the isolation of RGM and the profusion of new species, clinicians are increasingly likely to encounter patients with these isolates. Therefore, in settings in which RGM are still uncommon causes of human disease, providing clinicians with updated information about epidemiology, clinical significance, and prognosis may assist with decision-making in the management of patients with RGM isolates.
In this study, we investigated the longitudinal trends in the incidence and clinical significance of RGM diversity in a low-prevalence geographical area of NTM over a 22-year period.
Material and methods
Design, setting, and study population
A retrospective observational study of a 22-year period from January 1, 1994 to December 31, 2015 was conducted at the Bellvitge University Hospital (BUH), a health centre for adults. The microbiology service of the BUH accepts referrals for mycobacterial identification from the four hospitals in the area, and covered a total population of 862,495 in 2014 [data from the Catalan Health Service (http://catsalut.gencat.cat)]. Patients were identified from a database of RGM isolates of the microbiology service.
The medical records of all subjects with isolation of any RGM were identified from the microbiology service’s database. The clinical files were then retrospectively reviewed, and clinical data were entered in a standardized clinical record data (CRD), designed for the study. We collected the following data: demographic information, co-morbidities, and toxic habits, microbiological features [RGM species, nature and number of samples, and the results of acid-fast bacillus (AFB) smears and susceptibility testing], and clinical and therapeutic data.
Definitions
Clinical significance
The establishment of disease depended on whether the isolates were from the respiratory tract or not. For respiratory disease, clinical significance was established according to the criteria of the ATS/IDSA [9, 10]. In short, this comprised the following: symptomatology compatible with invasive pulmonary disease, nodular or cavitary or high-resolution computed tomography images of multifocal bronchiectasis and/or multiple small nodules, and microbiological evidence of ≥2 positive sputum cultures from different samples or ≥1 positive bronchial wash/lavage culture, or a lung biopsy from granulomatous inflammation. For osteomyelitis or skin and soft-tissue infections, one positive biopsy culture or with >1 exudate, or wound smear results were considered to be clinically significant. For all other body sites, clinical significance was established by the absence of other plausible aetiology, the presence of ≥2 positive cultures and/or characteristic pathology, and improvement of clinical signs or symptoms after treatment. Disease was considered disseminated if the same RGM species was recovered from ≥2 samples and at ≥2 different body sites.
Cure
Patients were classified as cured if, at the end of the treatment, symptoms were resolved, radiological findings had disappeared or improved, and any available samples were negative.
Failure
Persistence of positive cultures despite an active-drug regimen against the isolated microorganism was classified as treatment failure.
Relapse
Relapse was defined as reappearance of clinical disease with isolation of the same microorganism from the previous episode, but only after initial conversion to negative cultures and after completing a full treatment course.
Microbiological studies
Samples were processed according to standard procedures [11]. Sputum or bronchoalveolar wash/lavage samples were pre-treated according to conventional N-acetyl-L-cysteine 2% NaOH digestion–decontamination protocol. The following tests were then performed: 1) smears were stained by auramine-rhodamine fluorochrome, and positive slides were confirmed by Ziehl–Neelsen stain, 2) samples (0.5 ml) were inoculated on BACTEC 12B or MGIT liquid media and onto Löwenstein–Jensen solid media. The liquid cultures were processed using the BACTEC 460 radiometric method (Becton Dickinson, Sparks, MD, USA) from April 1994 to March 2009, and the MGIT 960 system (Becton Dickinson) from April 2009 onward, and 3) Identification to species level was executed by DNA probes (Accuprobe Test; Gen-Probe Inc., San Diego, CA, USA) from January 1994 to May 2004, INNO-LiPA Mycobacteria v2 assay (Innogenetics, Ghent, Belgium) from June 2004 to March 2007, and GenoType Mycobacterium CM/AS test (Hain Lifescience, Nheren, Germany) from April onwards. When mycobacteria species identification was not achieved, the 16S rRNA and hsp65 genes sequencing and phenotypic methods were performed [12, 13]. Antimicrobial studies were carried out using a broth microdilution method, following the recommendations of the Clinical Laboratory Standards Institute (CLSI) guidelines [14] in 19/111 clinical strains (11 from M. chelonae-abscessus group and eight from M. fortuitum group).
Data analysis
Annual incidence rates were calculated by dividing the number of new cases per year by the exposed population covered by the hospitals referring samples to the microbiology laboratory of the BUH. The chi-square test and Fisher exact test were used to compare qualitative variables, and the Mann–Whitney U test was used to compare continuous variables. Factors associated with the presence of clinical disease were identified by unadjusted and adjusted logistic regression analysis; odds ratios (ORs) and 95% confidence intervals (95 % CIs) are presented. P values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS, Version 15.0 (SPSS Inc., Chicago, IL, USA).
Ethics
The ethics committee of the BUH approved the study (Reference: PR130/14).
Results
One hundred and forty patients with 298 RGM isolates were identified over the study period. Of these, 116 (82.9%) had respiratory isolates (113 from sputum and/or bronchial wash/lavage, one from pleural effusion, and two from gastric aspirates), and 24 (17.1%) had extra-respiratory isolates (11 from skin or soft tissues, four from urine, five from prostheses/catheters, two from bone and joint tissues, and two from other sites). Patients were predominantly men (74%), with a mean age of 63 years. Patients with respiratory isolates were more often smokers (p < 0.001), more likely to have COPD (p < 0.001), and had less immunosuppression (p = 0.008) than patients with extra-respiratory isolates (Table 1).
Incidence trends
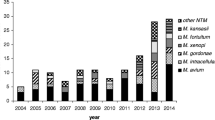
Over the 22-year period, 11 different species were isolated: M. fortuitum 63 (45%), M. chelonae 47 (33.6%), M. mucogenicum 13 (9.3%), M. abscessus eight (7.3%), M. peregrinum two (1.4%), M. mageritense two (1.4%) and M. arupense, M. cosmeticum, M. hassiacum, M. setense and M. smegmatis one (3.6%) each respectively. After stable numbers between 1994 and 2002, the incidence steadily increased from 2003 (0.34 per 100,000 population) to 2015 (1.73 per 100,000 population), with an average annual increase of 8.3% (0.11 per 100,000 population). There was a peak in 2008 (n = 18), which halved the next year. This peak in incidence was probably caused by a contamination in the lab because samples for ten patients were positive for M. fortuitum in a 16-day period from January 29 to February 13, and none had clinical disease attributable to the mycobacteria. As for clinical disease, the incidence remained fairly stable until the last 3 years when there was a trend toward sustained increase, particularly for extra-pulmonary disease (Fig. 1).
Clinical significance
Clinical disease was established in 32 of the 140 patients (22.9%). Only four species were isolated in the 32 cases with clinical disease: M. chelonae (n = 13), M. fortuitum (n = 10), M. abscessus (n = 7), and M. mucogenicum (n = 2), giving a pathogenicity of 27.7, 15.9, 87.5, and 15.4% respectively. Of patients with respiratory isolates and extra-respiratory isolates, 12.9% (15/116) and 70.8% (17/24) respectively had clinical disease. M. abscessus was the most frequent species isolated in patients with pulmonary disease (40%), whereas extra-pulmonary disease was mostly caused by M. chelonae (52.9%) and M. fortuitum (35.3%) (Fig. 2). In the adjusted analysis, AFB smear positivity (OR 97.7, 95 % CI 13.8–689.4), extra-respiratory isolates (OR 19.4, 95 % CI 5.2–72.7), and female gender (OR 5.9, 95 % CI 1.9–19.1) were associated with the presence of clinical disease (Table 2). Sixteen patients with RGM isolation had concomitant mycobacteria isolates: 11 with active pulmonary TB, four with M. avium complex, and one with M. xenopi pulmonary disease. However, the RGM were considered clinically non-significant in all cases.
Treatment and outcome
Twenty-one patients (65.6%) received antimicrobial therapy alone; four (12.5%), all with localized extra-pulmonary disease, underwent surgery alone, six (18.8%) received both antimicrobial therapy and surgery, including removal of prosthetic material, and one (3.1%) was not treated. Antimicrobial treatment, alone or in combination with surgery, was given for a mean duration of 8.7 months (11.2 months for pulmonary disease and 4.3 months for extra-pulmonary disease). Overall cure was achieved in 25 patients (78.1%), of whom two required a second course of treatment after relapsing, and six (15.6%) died during treatment. One patient who died did not receive treatment. Table 3 shows the main characteristics and outcomes of the 32 cases of clinical disease by location, and Table 4 (supplementary online material) provides detailed information for each case.
Discussion
This study documents the increasing frequency of RGM isolation in an area with a low incidence of NTM. Increasing rates of NTM infections, including RGM, have been reported from different regions over recent decades [15–18]. The trend in our area over a 22-year period shows that there has also been a steady increase in RGM isolation over the most recent period. Interestingly, while there was an overall increase of isolates due to respiratory specimens, the modest increase in clinical disease over recent years was dependent on the occurrence of extra-pulmonary disease.
New molecular sequencing methods allow better identification of different RGM species, which could explain the appearance of new species that had not been recovered before 2005 (M. mucogenicum, M. hassiacum, M. smegmatis, M. cosmeticum, and M. setense). The new culture system MGIT 960, which was implemented in the second period of the study (April 2009), does not justify the increase of NTM isolation, since the sensitivity of the two systems, in combination with solid media, is equivalent [19, 20]. Certainly, although there seems to be a true rise in the incidence of isolation of RGM in our geographical area, the burden of clinical disease remains modest when compared with countries such as US and Canada [2, 3, 15]. There is no clear or unique cause for the increase seen in rates of RGM isolates. Some authorities have postulated that the general increase in numbers of NTM may be multifactorial: population aging, improved imaging techniques (leading to sampling of more patients with pulmonary lesions that may otherwise go unnoticed), and reduced levels of TB and BCG vaccination, which may reduce herd-cross immunity [15].
In the present study, only one in five patients with RGM isolates was diagnosed with clinical disease, although this varied according to the source and the species isolated. While 71% of patients with isolates from extra-respiratory sites had clinical disease, only 13% of isolates from respiratory samples were considered clinically relevant. Of the four species causing clinical disease in our series, M. abscessus seemed to be the most pathogenic (87.5%), as well as the most common cause of respiratory disease, which is consistent with previous data [21]. The absence of paediatric patients with cystic fibrosis in our series, in whom M. abscessus is particularly common [19], may at least partly explain the low overall pathogenicity.
Distinguishing between true disease and a mere colonization or environmental contamination is challenging in cases of RGM. The ATS guidelines provide specific diagnostic criteria to aid in the diagnosis of pulmonary NTM disease [10]. However, given the large number of NTM species and the differences in virulence and host susceptibility, the ATS criteria do not allow accurate diagnoses to be established in all clinical situations, particularly when RGM are involved. We therefore looked for the factors associated with clinical disease in our series of RGM. For species other than M. abscessus isolated from the respiratory tract, clinical relevance was very low (13%), which contrasts starkly with the high pathogenicity rates of isolates from extra-respiratory specimens (71%). The other important finding was the association of AFB positivity with the presence of clinical disease in our series; this association was both expected and logical, because smear positivity excludes the presence of contamination and denotes high bacillary replication, as seen in active mycobacterial disease. Previous ATS guidelines included AFB smear positivity as a bacteriologic criterion for the diagnosis of pulmonary NTM disease [9], but it was removed in the current recommendations [10]. However, our data indicate that smear results, particularly from respiratory specimens, have a potentially important role when considering diagnosis of pulmonary disease by RGM.
Although the optimal treatment for RGM infection has not been established, it is recognized that pulmonary disease and extra-pulmonary disease represent two differentiated scenarios [1, 20]. Pulmonary disease requires combination antimicrobial therapy for at least 6 months, including an injectable agent during the first months [1, 20, 21]. In our short series of 15 cases of pulmonary disease, two patients needed retreatment because of relapse after a first course of treatment, and one patient died after 17 months with persistence of positive sputum cultures despite treatment. In contrast, all 17 cases of extra-pulmonary disease were cured with shorter treatment regimens and with the removal of prosthetic material when necessary.
This study has some weaknesses, mostly derived from its retrospective nature. First, the association of smear positivity and clinical disease may have been skewed by overdiagnosis in the presence of positive AFB specimens. Second, the small number of cases and the lack of treatment standardization preclude forming reliable conclusions concerning the response to antibiotic therapy and the true contribution of surgery in cases of extra-pulmonary disease. Finally, the entire spectrum of patients with pulmonary infection by RGM was not represented in our series because we did not include paediatric cases with cystic fibrosis.
In summary, although the burden of infections caused by RGM was still low in our area, RGM isolation is undoubtedly on the rise. Whether this will be sustained over time and be matched by an increase in clinical disease, or whether it is merely a cycle in the poorly understood epidemiology of environmental mycobacteria, will be seen in the near future. Determining the clinical relevance of RGM isolates, and making treatment decisions, can be challenging. In these circumstances, clinicians should take into account the origin of the specimen, the species isolated, and the presence of a positive AFB smear, together with established diagnostic criteria.
References
De Groote MA, Huitt G (2006) Infections due to rapidly growing mycobacteria. Clin Infect Dis 42:1756–1763
Winthrop KL, Mcnelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, Saulson A, Hedberg K (2010) Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med 182(7):977–982
Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA et al (2010) Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 182:970–976
Griffith DE (2010) It is better to light a candle…than to repeat the opinions of experts. Am J Respir Crit Care Med 182:865–866
Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL (2011) Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 52:565–571
Moore JE, Kruijshaar ME, Ormerod LP, Drobniewski F, Abubakar I (2010) Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland. BMC Public Health 10:612
Martin-Casabona N, Bahrmand AR, Bennedsen J, Thomsen VØ, Curcio M, Feldman K, Havelkova M et al (2004) Non-tuberculous mycobacteria: patterns of isolation. A multi-country retrospective survey. Int J Tuberc Lung Dis 8:1186–1193
Marras TK, Chedore P, Ying AM, Jamieson F (2007) Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997–2003. Thorax 62:661–666
This official statement of the American Thoracic Society was approved by the Board of Directors, Medical Section of the American Lung Association (1997) Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med 156:S1–S2
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F et al (2007) An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416
Pfyffer GE (2015) Mycobacterium: general characteristics, laboratory detection, and staining procedures. In: Jorgensen J, Pfaller M, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (eds) Manual of clinical microbiology, 11th edn. ASM Press, Washington, DC, pp 536–569
Simner PJ, Stenger S, Richter E, Brown-Elliott BA, Wallace RJ Jr, Wengenack NL (2015) Mycobacterium: laboratory characteristics of slowly growing mycobacteria. In: Jorgensen J, Pfaller M, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (eds) Manual of clinical microbiology, 11th edn. ASM Press, Washington, DC, pp 570–594
Brown-Elliott BA, Wallace RJ Jr (2015) Mycobacterium: clinical and laboratory characteristics of rapidly growing mycobacteria. In: Jorgensen J, Pfaller M, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (eds) Manual of clinical microbiology, 11th edn. ASM Press, Washington, DC, pp 595–612
Clinical and Laboratory Standards Institute (CLSI) (2011) Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard-second edition. CLSI document M24-A2. CLSI, Wayne, PA, USA
Marras TK, Mendelson D, Marchand-Austin A, May K, Jamieson FB (2013) Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998–2010. Emerg Infect Dis 19:1889–1891
van Ingen J, Bendien SA, de Lange WC, Hoefsloot W, Dekhuijzen PN, Boeree MJ, van Soolingen D (2009) Clinical relevance of non-tuberculous mycobacteria isolated in the Nijmegen–Arnhem region, The Netherlands. Thorax 64:502–506
Lai CC, Tan CK, Chou CH, Hsu HL, Liao CH, Huang YT, Yang PC, Luh KT, Hsueh PR (2010) Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000–2008. Emerg Infect Dis 16:294–296
Wentworth AB, Drage LA, Wengenack NL, Wilson JW, Lohse CM (2013) Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc 88:38–45
Alcaide F, Benítez MA, Escribà JM, Martín R (2000) Evaluation of the BACTEC MGIT 960 and the MB/BacT systems for recovery of mycobacteria from clinical specimens and for species identification by DNA AccuProbe. J Clin Microbiol 38(1):398–401
Cruciani M, Scarparo C, Malena M, Bosco O, Serpelloni G, Mengoli C (2004) Meta-analysis of BACTEC MGIT 960 and BACTEC 460 TB, with or without solid media, for detection of mycobacteria. J Clin Microbiol 42:2321–2325
Griffith DE, Girard WM, Wallace RJ Jr (1993) Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis 147:1271–1278
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare having no conflict of interest.
Ethical approval and informed consent
The ethical committee of the Bellvitge University Hospital approved the study (Ref.: PR130/14). Informed consent was not required due to the retrospective design of the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 89 kb)
Rights and permissions
About this article
Cite this article
Alcaide, F., Peña, M.J., Pérez-Risco, D. et al. Increasing isolation of rapidly growing mycobacteria in a low-incidence setting of environmental mycobacteria, 1994–2015. Eur J Clin Microbiol Infect Dis 36, 1425–1432 (2017). https://doi.org/10.1007/s10096-017-2949-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-2949-0