Abstract
The aim of this study was to evaluate retrospectively the performance of the Xpert MRSA assay in routine practice and its current use in the intensive care unit (ICU) setting of our hospital, since a pre-emptive isolation strategy has been applied. A total of 6473 patients were routinely screened with ESwab for methicillin-resistant Staphylococcus aureus (MRSA) using three generations of rapid real-time polymerase chain reaction (PCR) (Cepheid GeneXpert) over three consecutive periods of time. Performance was evaluated using broth enrichment culture as the reference method. Our results show that the last generation of Xpert MRSA (NxG) assay is more specific (99.2% vs. 97.9%) but not more sensitive (77.8% vs. 86.9%) than the third generation. Considering the low prevalence of MRSA in our hospital, we obtained an overall low positive predictive value. In conclusion, it remains difficult to abandon the reference method in routine practice considering the possible implications of an erroneous MRSA result in the ICU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
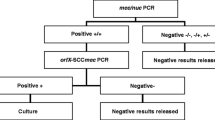
The implementation and follow-up of preventive measures against methicillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities is brain teasing. It needs to consider continually changing local epidemiology like the prevalence of MRSA, the incidence of infections, the competing effect of standard control measures and the likelihood of transmission in the concerned setting [1]. In addition, the performance of available commercial tests for screening purposes evolves constantly, which makes decision-making even more difficult. Our institution is a 1900-bed tertiary care university hospital with 106 intensive care unit (ICU) beds. Apart from pre-surgical screening on cardiac surgery, the Xpert MRSA assay is used for screening of high-risk patients on arrival in the ICU. Patients considered at high risk in our hospital are those transferred from another hospital or healthcare centre with a minimal stay of 48 h, inpatients hospitalised for at least 3 weeks who are transferred to the ICU for upgrading of care, farmers and veterinarians, patients working in the healthcare sector, patients with a history of MRSA and roommates of MRSA patients. Based on our ICU MRSA prevention strategy (Fig. 1), these patients are pre-emptively isolated until a negative molecular result is obtained. The aim of this study was to evaluate the performance of the Cepheid Xpert MRSA assay in routine practice (high-risk ICU patients and cardiac pre-surgical patients) in order to evaluate the effect of this performance on our MRSA prevention strategy in the ICU setting.
Materials and methods
This is a retrospective data analysis. In total, 6473 patients, of which 3703 were ICU patients and 2770 were pre-surgical patients, were screened for MRSA using rapid real-time polymerase chain reaction (PCR) (Cepheid GeneXpert). Three versions of Xpert MRSA were used in three consecutive periods (see Table 1). Pooled ESwabs (Amies ESwab, Copan, Brescia, Italy) (nose and perineum) were used as the screening specimen for ICU patients, while nose ESwabs were used for pre-surgical patients. For each patient, only the first test was taken into account in this data analysis. The laboratory procedure implies an MRSA screening with broth-enriched culture (18–24 h incubation; Tryptic Soy Broth) and direct inoculation on chromID MRSA (bioMérieux, Marcy-l’Étoile, France). chromID MRSA was incubated for 48 h in ambient conditions at 37 °C. Since no difference in performance was observed in our data between pooled ESwabs and nose ESwabs, the global sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for the three generations of Xpert MRSA were determined using broth-enriched culture as the reference method. The Chi-square test was used for statistical analysis (MedCalc software, version 16.8.4, p-values <0.01 were considered significant). MRSA prevalence over the three time periods was determined in the two subgroups (high-risk ICU patients/pre-surgical patients) based on the reference method.
Results and discussion
Since prevalence has no impact on the sensitivity and specificity of an assay, we calculated the global sensitivity and specificity for all patients tested (6473 patients), while the PPV, NPV and the prevalence for MRSA is only given for the ICU group (3703 patients). Our results (see Table 1) show significant evolution in the performance of the Xpert MRSA assay with marked improvement of the specificity for the last generation: 99.2% for the NxG compared with 97.9% for the third generation. This was essential considering the poor results of the third-generation assay and the low MRSA prevalence in our hospital. Considering this, the Xpert MRSA goes from 2.3% erroneous results for the third generation to 1.1% for the NxG, using broth-enriched culture as the reference method. Besides this improvement, we must note a non-significant decrease in sensitivity. Since there were no reformulations in the enrichment broth and chromogenic agar used in the reference method during the study period, the observed evolution in the analytical performance of the Xpert MRSA assay is most probably linked to an update of the molecular targets in the assay.
Based on these results, it remains difficult to get rid of the reference method in routine practice considering the possible implications of an erroneous MRSA result in the ICU [2]. Retrospective analysis of laboratory data in 2014, 2015 and 2016 showed that approximately 25% of the patients with a positive screening for MRSA in the ICU also have one or more positive clinical samples for MRSA in the days preceding or following the positive screening assay. Since our MRSA prevention strategy relies on the pre-emptive isolation of high-risk patients, giving up rapid screening and waiting upon culture results is not an option, since it would lead, on the one hand, to the overpopulation of pre-emptive isolation rooms and, on the other hand, to extra costs given that, in our setting, one day of pre-emptive isolation is considered more costly (±70 Euros/isolation day) than one molecular MRSA screening (less than 50 Euros). However, even though it is known that preventive measures are costly, further screening for MRSA in this setting with continuously decreasing MRSA prevalence will become an even more expensive activity.
Better targeting of high-risk groups for MRSA carriage is probably the best way to limit expensive molecular screening, but this is not easy to apply in routine practice. For example, during the most recent data period in our ICU (NxG), the MRSA prevalence of the high-risk ICU population was only 1.2%, while the MRSA prevalence in the non-high-risk group of the ICU was 0.72% (data not shown).
One limitation of our study is that the MRSA screening we apply in our ICU setting only considers nose and perineum MRSA carriage, while recommendations advise adding throat to nose and inguinal/axillary swabs [3]. Although this may lead to an underestimation of our MRSA prevalence, this does not change the observed analytical performance characteristics for the Xpert MRSA assay.
Besides standard control measures [4], there are no unequivocal guidelines for targeted preventive measures against MRSA. In order to prevent MRSA transmission in our ICU, we apply a strict MRSA policy (similar to that in the Netherlands and Scandinavian countries), including pre-emptive isolation and molecular screening of high-risk patients for MRSA (Fig. 1) [5]. Roisin et al. showed that the implementation of rapid molecular screening to accelerate isolation precautions did not reduce nosocomial MRSA acquisition in comparison with culture screening [6]. This suggests that, if rapid molecular MRSA screening is used, it should be done in association with pre-emptive isolation to reduce isolation time. However, because we evolve to a low MRSA prevalence setting with a more general acceptance of standard precautions and a more restrictive use of molecular tests, the question becomes whether this expensive strict MRSA policy in the ICU should be maintained or if a step backwards to classical cheaper culture screening (without pre-emptive isolation and rapid molecular screening) could be considered.
Conclusions
The analytical performance of three versions of the Xpert MRSA assay was evaluated retrospectively during a period with decreasing prevalence of methicillin-resistant Staphylococcus aureus (MRSA). The last generation (NxG) showed the lowest discrepancy rate compared to the reference method. This improvement is due to improved specificity but not improved sensitivity.
References
Kock R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, Mielke M, Peters G, Skov RL, Struelens MJ, Tacconelli E, Witte W, Friedrich AW (2014) Systematic literature analysis and review of targeted preventive measures to limit healthcare-associated infections by meticillin-resistant Staphylococcus aureus. Euro Surveill 19(29). pii: 20860
Köck R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, Mielke M, Peters G, Skov RL, Struelens MJ, Tacconelli E, Navarro Torné A, Witte W, Friedrich AW (2010) Methicillin-resistant staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill 15(41):19688
Mertz D, Frei R, Jaussi B, Tietz A, Stebler C, Flückiger U, Widmer AF (2007) Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin Infect Dis 45:475–477
Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee (2007) 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 35(10 Suppl 2):S65–S164
Wassenberg MW, Kluytmans JA, Box AT, Bosboom RW, Buiting AG, van Elzakker EP, Melchers WJ, van Rijen MM, Thijsen SF, Troelstra A, Vandenbroucke-Grauls CM, Visser CE, Voss A, Wolffs PF, Wulf MW, van Zwet AA, de Wit GA, Bonten MJ (2010) Rapid screening of methicillin-resistant Staphylococcus aureus using PCR and chromogenic agar: a prospective study to evaluate costs and effects. Clin Microbiol Infect 16(12):1754–1761
Roisin S, Laurent C, Denis O, Dramaix M, Nonhoff C, Hallin M, Byl B, Struelens MJ (2014) Impact of rapid molecular screening at hospital admission on nosocomial transmission of methicillin-resistant Staphylococcus aureus: cluster randomised trial. PLoS One 9(5):e96310
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study, formal consent is not required.
Informed consent
For this type of study, informed consent is not required.
Rights and permissions
About this article
Cite this article
Jacqmin, H., Schuermans, A., Desmet, S. et al. Performance of three generations of Xpert MRSA in routine practice: approaching the aim?. Eur J Clin Microbiol Infect Dis 36, 1363–1365 (2017). https://doi.org/10.1007/s10096-017-2939-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-2939-2