Abstract
To evaluate effect of empirical combination of a β-lactam to vancomycin and vancomycin monotherapy in Staphylococcus aureus bacteremia (MSSA-B), we conducted a retrospective cohort study. Electronic medical records of individuals who were diagnosed with MSSA-B between January 2005 and February 2015 at a tertiary care center were reviewed. Patients were classified into three groups according to empirical antibiotic regimen (BL group, β-lactam; VAN group, vancomycin; BV group, combination of β-lactam and vancomycin), and 30-day all-cause mortality of each group was compared. During the study period, 561 patients with MSSA-B were identified. After exclusion of 198 patients (36 with poly-microbial infection, 114 expired within 2 days, and 48 already received parenteral antibiotics) and a matching process, 46 patients for each group were included. Baseline characteristics were similar except for severity and comorbidity scores. The 30-day mortality for all three groups were not significantly different (BL 4.3%, VAN 6.5%, BV 8.7%; P = 0.909). In a multivariate analysis, type of empirical antibiotic regimen was not statistically associated with 30-day all-cause mortality. In comparison with the VAN group, the BV group yielded a HR of 0.579 (95% CI = 0.086–3.890, P = 0.574). Pitt bacteremia score was the only significant factor for mortality. The empirical combination of a β-lactam to vancomycin was not associated with lower mortality in treating MSSA-B, compared to vancomycin monotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to increasing prevalence of methicillin-resistant S. aureus (MRSA), empirical coverage of methicillin-resistant strains in S. aureus bacteremia (SAB) became important [1–5]. To ensure MRSA coverage before identification of methicillin susceptibility, some clinicians recommend using a combination of β-lactam antibiotics to vancomycin as an empirical regimen for SAB [6], the benefit of which is still questioned in methicillin-susceptible S. aureus bacteremia (MSSA-B). To answer this question, we evaluated the empirical combination regimen and a de-escalation strategy in which empirical vancomycin was replaced with a definitive β-lactam, in comparison with β-lactam therapy in patients with MSSA-B.
Methods
Study design and population
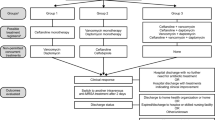
A retrospective cohort study was conducted. We reviewed the electronic medical records of adult patients (age ≥ 18) who were diagnosed with MSSA-B between January 2005 and February 2015 at Samsung Medical Center, a 1,950-bed tertiary care university hospital in Seoul, Republic of Korea. Only the first bacteremia episode for each patient was included in the analysis. Identified patients were classified into three groups according to empirical antibiotic regimen (BL group, β-lactam; VAN group, vancomycin; BV group, combination of β-lactam and vancomycin) and matched by age and source of infection (1:1:1). The VAN and BV groups underwent de-escalation of their empirical regimen to definitive β-lactam antibiotics after identification of methicillin susceptibility. Patients who had poly-microbial infection, expired within 48 h of antibiotic treatment, and/or had already been on parenteral antibiotic therapy were excluded. The primary outcome was 30-day all-cause mortality. This study was approved by the Institutional Review Board of Samsung Medical Center.
Definitions
An episode of MSSA-B was defined as a positive culture in one or more sets of blood cultures with systemic manifestations of infection. Community-acquired infection was defined as MSSA-B occurring in the community or within 48 h after hospital admission in patients without healthcare-associated risk factors. Healthcare-associated infection was defined as community-onset MSSA-B in patients with previously described healthcare-associated risk factors [7]. Hospital-acquired infection was defined as MSSA-B that occurred longer than 48 h after admission in patients who did not have symptoms or signs of infection at the time of admission [2, 8, 9]. The source of bacteremia was determined according to the presence of clinical symptoms and signs, with isolation of MSSA from the presumed source. The development of acute kidney injury (AKI) as a complication of treatment was assessed 2 weeks from the onset of bacteremia according to the RIFLE classification system [10].
Microbiologic tests
Blood cultures collected before 2006 were processed in a BACTEC-9240 system (Becton Dickinson, Sparks, MD, USA), while the BacT/ALERT 3D system (bioMérieux Inc., Marcy l’Etoile, France) was used thereafter. For identification and antimicrobial susceptibility testing, including for methicillin, the VITEK II automated system (bioMérieux Inc.) was used with a standard identification card and the modified broth microdilution method. Minimum inhibitory concentration (MIC) breakpoints and quality control protocols were used according to the standards established by the Clinical and Laboratory Standards Institute (CLSI).
Statistical analysis
To compare clinical variables, the Mann–Whitney U test and Kruskal–Wallis test were used for continuous variables, and Chi-square and Fisher’s exact tests were used for categorical variables. The Cox proportional hazard model was used to examine the association of the empirical antibiotic regimens with the 30-day mortality by adjusting potential confounding factors. All collected clinical variables with any relevance to prognosis underwent univariate analysis to find potential confounders. In addition to the type of empirical antibiotic regimen, the Pitt bacteremia score, Charlson’s WIC, and variables with statistical significance in the univariate analyses were included in the multivariate analysis. All P-values were two-tailed, and those <0.05 were considered to be statistically significant. IBM SPSS Statistics version 20.0 for Windows (IBM, Armonk, NY, USA) was used for all statistical analyses.
Results
Study population, baseline characteristics, and bacteremia acquisition
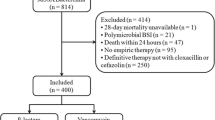
During the study period, 561 patients with MSSA-B were identified. After exclusion of 198 patients and a 1:1:1 matching process by age and infection source, 46 patients in each group were finally included in the analysis (Fig. 1). Baseline characteristics and bacteremia acquisition of patients in each group are presented in Table 1. The mean age of the patients was 60.4 years and was similar in all three groups. The BL and VAN groups showed a higher proportion of males to females than the BV group (BL 67.4%, VAN 65.2%, and BV 47.8%; P = 0.004). Patients in the VAN and BV groups had more comorbidities with higher Charlson’s weighted index of comorbidity scores than patients in the BL group (BL 0, VAN 2, and BV 2, median values; P = 0.003). Patients in the BL group had a higher proportion of community-acquired infections than patients in the VAN and BV groups (BL 56.5%, VAN 23.9%, and BV 34.8%; P = 0.005).
Selection of the study population among patients who were admitted and diagnosed with MSSA-B between January 2005 and February 2015 at a tertiary care center. Abbreviations: MSSA-B methicillin-susceptible Staphylococcus aureus bacteremia, BL β-lactam, VAN vancomycin, BV a combination of β-lactam and vancomycin
Clinical presentation, treatment, and outcomes
Clinical presentation, treatment, and outcomes of patients with MSSA-B according to empirical treatment group are presented in Table 2. The BV group had higher Pitt bacteremia score than the BL and VAN groups (BL 0.5, VAN 0, and BV 1, median values; P = 0.018) and proportion of ICU transfers within 48 h (BL 6.5%, VAN 8.7%, and BV 26.1%, median values; P = 0.012). The duration of empirical vancomycin administration in the VAN and BV groups and the total treatment duration in the three groups were similar. The vancomycin MIC in the VAN and BV groups and the oxacillin MICs in in all three groups were also statistically not different.
Although the 30-day mortality rate was highest in the BV group, no statistically significant difference was observed among the groups (BL 4.3%, VAN 6.5%, BV 8.7%; P = 0.909). The duration of bacteremia was longer in the VAN group than in the BL and BV groups (BL 2.2, VAN 3.7, BV 2.8 days, median values; P = 0.010). However, the time interval before the first follow-up blood culture was also longer in the VAN group, which makes it difficult to interpret bacteremia duration (BL 1.9, VAN 2.4, BV 2.0 days, median values; P = 0.353). Numerically, more patients in the VAN and BV groups experienced AKI, but these numbers were not statistically significantly different (BL 2.4%, VAN 13.2%, and BV 10.8%; P = 0.190).
Multivariate analysis of 30-day all-cause mortality in MSSA-B
In the multivariate analysis, only Pitt bacteremia score was significantly associated with 30-day mortality in MSSA-B (HR 1.533, 95% CI = 1.054–2.229, P = 0.025, Table 3). Although the VAN and BV groups showed slightly higher HRs than the BL group, type of empirical antibiotic regimen was not associated with 30-day all-cause mortality (in comparison with the BL group: HR 1.844, 95% CI = 0.258–13.201, P = 0.542 for the VAN group; HR 1.541, 95% CI = 0.249–9.532, P = 0.642 for the BV group). In a subgroup analysis comparing the VAN and BV groups, type of empirical antibiotic regimen was not associated with 30-day all-cause mortality (in comparison with the VAN group: HR 0.579, 95% CI = 0.086–3.890, P = 0.574 for the BV group), and only the Pitt bacteremia score showed statistical significance (HR 1.929, 95% CI = 1.097–3.391, P = 0.022, Table 4).
Discussion
In the present study, there was neither superiority nor inferiority of empirical combination regimen of β-lactam and vancomycin to the other two groups. The 30-day all-cause mortality was highest in the BV group, but BV group had also higher Pitt bacteremia score and proportion of ICU transfers within 48 h. This implies that physicians tended to choose combination therapy for severely ill patients, and such tendency was adjusted by the multivariate analysis. These findings imply that numerical difference of outcome variables in previous studies [11, 12] might be affected by disease severity and comorbidities, and emphasize that adjustment of confounding variables by multivariate analysis is essential. Our data give evidence that the combination of a β-lactam to vancomycin may not have additional benefits compared to vancomycin monotherapy as an initial empirical therapy treating MSSA-B.
The inferiority of definitive vancomycin therapy to β-lactams in treating MSSA-B is well documented [6, 11, 13–17], but it is not clear whether short-term administration of vancomycin as an empirical treatment would also be inferior [12, 18]. In our data, there was no inferiority of empirical vancomycin therapy to β-lactam treatment. No statistically significant difference among three empirical antibiotic regimens was observed in the multivariate analysis of 30-day all-cause mortality. Duration of bacteremia was longer in the VAN group than in the BL and BV groups, but the time interval before the first follow-up blood culture was also longer in the VAN group. This finding is similar to that of a previous study by Khatib et al., which showed delayed clearance of MSSA-B in the VAN group without a difference in all-cause mortality [18]. However, time-to-antibiotics was significantly longer in the VAN group, and the time interval of the first follow-up blood culture was not evaluated in that study. Another study by Lodise et al. showed significantly increased MSSA-B-related mortality in the VAN group compared to the BL group in intravenous drug users with infective endocarditis [12]. However, the severity and comorbidity of patients were neither evaluated nor adjusted in that study. As the clinical evidence to date is heterogeneous, more clinical data should be accumulated to assess the effects of a de-escalation strategy for replacing empirical vancomycin with definitive β-lactam.
Inherent to retrospective design, the three groups were different in severity and comorbidity. To compensate, we matched each group by age and source of infection, and conducted multivariate analysis to adjust for potential confounding factors. In addition, low mortality rates caused by strict exclusion limited our ability to detect statistically significant differences between groups.
In conclusion, for empirical coverage of methicillin-resistant strains, the combination of a β-lactam to vancomycin did not improve outcome of MSSA-B compared to vancomycin monotherapy.
References
El Atrouni WI, Knoll BM, Lahr BD, Eckel-Passow JE, Sia IG, Baddour LM (2009) Temporal trends in the incidence of Staphylococcus aureus bacteremia in Olmsted County, Minnesota, 1998 to 2005: a population-based study. Clin Infect Dis 49:e130–e138. doi:10.1086/648442
Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi:10.1001/jama.298.15.1763
Johnson AP, Pearson A, Duckworth G (2005) Surveillance and epidemiology of MRSA bacteraemia in the UK. J Antimicrob Chemother 56:455–462. doi:10.1093/jac/dki266
Song JH, Hsueh PR, Chung DR, Ko KS, Kang CI, Peck KR, Yeom JS, Kim SW, Chang HH, Kim YS, Jung SI, Son JS, So TM, Lalitha MK, Yang Y, Huang SG, Wang H, Lu Q, Carlos CC, Perera JA, Chiu CH, Liu JW, Chongthaleong A, Thamlikitkul V, Van PH (2011) Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother 66:1061–1069. doi:10.1093/jac/dkr024
Boucher HW, Corey GR (2008) Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 46(Suppl 5):S344–S349. doi:10.1086/533590
McConeghy KW, Bleasdale SC, Rodvold KA (2013) The empirical combination of vancomycin and a beta-lactam for Staphylococcal bacteremia. Clin Infect Dis 57:1760–1765. doi:10.1093/cid/cit560
Robinson JO, Pearson JC, Christiansen KJ, Coombs GW, Murray RJ (2009) Community-associated versus healthcare-associated methicillin-resistant Staphylococcus aureus bacteraemia: a 10-year retrospective review. Eur J Clin Microbiol Infect Dis 28:353–361. doi:10.1007/s10096-008-0632-1
Kang CI, Song JH, Chung DR, Peck KR, Ko KS, Yeom JS, Kim SW, Chang HH, Kim YS, Jung SI, Son JS, Hsueh PR, So TM, Lalitha MK, Yang Y, Huang SG, Wang H, Lu Q, Carlos CC, Perera JA, Chiu CH, Liu JW, Chongthaleong A, Thamlikitkul V, Van Pham H (2010) Clinical impact of methicillin resistance on outcome of patients with Staphylococcus aureus infection: a stratified analysis according to underlying diseases and sites of infection in a large prospective cohort. J Infect 61:299–306. doi:10.1016/j.jinf.2010.07.011
Yaw LK, Robinson JO, Ho KM (2014) A comparison of long-term outcomes after meticillin-resistant and meticillin-sensitive Staphylococcus aureus bacteraemia: an observational cohort study. Lancet Infect Dis 14:967–975. doi:10.1016/s1473-3099(14)70876-x
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004) Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. doi:10.1186/cc2872
Khatib R, Johnson LB, Fakih MG, Riederer K, Khosrovaneh A, Shamse Tabriz M, Sharma M, Saeed S (2006) Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand J Infect Dis 38:7–14. doi:10.1080/00365540500372846
Lodise TP Jr, McKinnon PS, Levine DP, Rybak MJ (2007) Impact of empirical-therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 51:3731–3733. doi:10.1128/aac.00101-07
Chang FY, Peacock JE Jr, Musher DM, Triplett P, MacDonald BB, Mylotte JM, O’Donnell A, Wagener MM, Yu VL (2003) Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 82:333–339. doi:10.1097/01.md.0000091184.93122.09
Stryjewski ME, Szczech LA, Benjamin DK Jr, Inrig JK, Kanafani ZA, Engemann JJ, Chu VH, Joyce MJ, Reller LB, Corey GR, Fowler VG Jr (2007) Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis 44:190–196. doi:10.1086/510386
Kim SH, Kim KH, Kim HB, Kim NJ, Kim EC, Oh MD, Choe KW (2008) Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 52:192–197. doi:10.1128/aac.00700-07
Schweizer ML, Furuno JP, Harris AD, Johnson JK, Shardell MD, McGregor JC, Thom KA, Cosgrove SE, Sakoulas G, Perencevich EN (2011) Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis 11:279. doi:10.1186/1471-2334-11-279
Chan KE, Warren HS, Thadhani RI, Steele DJ, Hymes JL, Maddux FW, Hakim RM (2012) Prevalence and outcomes of antimicrobial treatment for Staphylococcus aureus bacteremia in outpatients with ESRD. J Am Soc Nephrol 23:1551–1559. doi:10.1681/asn.2012010050
Khatib R, Saeed S, Sharma M, Riederer K, Fakih MG, Johnson LB (2006) Impact of initial antibiotic choice and delayed appropriate treatment on the outcome of Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 25:181–185. doi:10.1007/s10096-006-0096-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. HI12C0756).
Conflicts of interest
None of the authors have any conflicts of interest to report.
Ethical approval
This study was approved by the Institutional Review Board of Samsung Medical Center.
Informed consent
As a retrospective study, the Institutional Review Board waived informed consent in the present study.
Additional information
G.E. Park and J.-H. Ko are equally contributed to this article.
Rights and permissions
About this article
Cite this article
Park, G.E., Ko, JH., Cho, S.Y. et al. Empirical combination of a β-lactam to vancomycin may not improve outcomes of methicillin-susceptible Staphylococcus aureus bacteremia, compared to vancomycin monotherapy. Eur J Clin Microbiol Infect Dis 36, 1091–1096 (2017). https://doi.org/10.1007/s10096-016-2893-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2893-4