Abstract
The aim of the study was to investigate the epidemiology and clinical features of bloodstream infections due to Escherichia coli producing AmpC β-lactamases (AmpC-Ec-BSI). In a multi-centre case–control study, all third-generation-cephalosporin-resistant Escherichia coli BSI (3GC-Ec-BSI) isolates were analysed. Acquired bla AmpC (bla ac-AmpC) detection was done by polymerase chain reaction (PCR) and sequencing. Chromosomal bla AmpC (bla c-AmpC) expression was quantified by real-time PCR. Cases were patients with AmpC-Ec-BSI. Controls were patients with cephalosporin-susceptible E. coli BSI, matched 1:1 by sex and age. Demographics, comorbidities, intrinsic and extrinsic risk factors for antimicrobial resistance, clinical presentation and outcomes were investigated. Among 841 E. coli BSI, 17 were caused by AmpC-Ec (2 %). Eleven isolates (58.8 %) had bla ac-AmpC and six were bla c-AmpC overproducers. The mean age of cases was 66.2 years and 71 % were men. Cases were more frequently healthcare-related (82 vs. 52 % controls, p < 0.05) and presented more intrinsic and extrinsic risk factors. At least one risk factor was present in 94.1 % of cases vs. 41.7 % of controls (p = 0.002). Severity and length of stay (LOS) were higher among cases (mean Pitt Score 2.6 vs. 0.38 in controls, p = 0.03; LOS 17.5 days vs. 6 in controls, p = 0.02). Inappropriate empirical therapy (IET) was administered to 70.6 % of cases and 23.5 % of controls (p < 0.003). No differences were found in terms of cure rate at the 14th day and mortality. Bloodstream infections due to AmpC-Ec (mostly plasmid-mediated) are infrequent in our area. AmpC-Ec-BSI affects mainly patients with intrinsic risk factors and those with previous antibiotic exposure. A high proportion received IET.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteraemia represents a major cause of death in industrialised countries, with large increases in incidence and mortality being seen over the past 20 years [1, 2]. European data indicate that the burden of bacterial bloodstream infection (BSI) has been increasing for five major bacterial species (Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Enterococcus faecium and Enterococcus faecalis) during EARSS surveillance in 27 European countries from 2002 to 2008 [3]. Notably, E. coli was the most frequent causative pathogen in all age groups, representing 47 % of the global reports. BSIs due E. coli experienced an 8.7 % annual increase from 2002 to 2008 [3]. In addition, rates of BSIs caused by antimicrobial-resistant bacteria are also increasing worldwide. Worrisome, these resistant infections increase the total burden of disease rather than replace infections caused by more susceptible bacteria [4, 5]. Antibiotic therapy for E. coli bacteraemia usually relies on third-generation cephalosporins, but E. coli resistance to these drugs increased up to 82 % in a recent report from some European areas in 2014 (http://www.who.int/drugresistance/documents/surveillancereport/en).
Escherichia coli has a chromosomal AmpC (bla c-AmpC) expressed at low basal levels that does not confer resistance to β-lactams. Its overexpression, however, may lead to resistance to most β-lactams except fourth-generation cephalosporins and carbapenems [6]. This resistance may also be due to the acquisition of other AmpC genes (bla ac-AmpC), from different genera of various microorganisms by horizontal transfer [7]. The prevalence of acquired AmpC β-lactamase producing E. coli isolates has been increasing [8, 9]. Remarkably, as in extended-spectrum β-lactamases (ESBLs), plasmids carrying genes for AmpC β-lactamases often carry multiple other antimicrobial resistance determinants [6, 8, 10]. Carbapenems are frequently the only therapeutic option for E. coli overproducing AmpC. The effect of an AmpC β-lactamase in addition to porin mutations of the outer membrane can reduce susceptibility to carbapenems [11–14].
Knowledge of clinical features and outcomes of AmpC overproducing E. coli bloodstream infections (AmpC-Ec-BSI) is limited, and infections caused by AmpC overproducing E. coli are an increasing therapeutic challenge. On the other hand, it is crucial to identify risk factors for AmpC-Ec-BSI in order to appropriately design empirical therapies. The aim of the present study was to describe the epidemiology and clinical features of AmpC-Ec-BSI as compared with cephalosporin-susceptible E. coli bloodstream infections (Cph-S-Ec-BSI).
Methods
A case–control study was conducted in three acute care hospitals [Hospital Universitari Mútua de Terrassa (HUMT), Hospital de la Santa Creu i Sant Pau (HSCiSP) and Consorci Sanitari de Terrassa (CST)] and their corresponding primary care centres. Cases were recorded prospectively from June 2010 to November 2011, covering an area with 1,300,000 inhabitants and included all E. coli clinical isolates. Controls were matched 1:1 by sex and age. Data on the whole cohort have been published elsewhere [10]. In the present study, we included all adult patients with bacteraemia due to E. coli with reduced susceptibility to third-generation cephalosporins.
Variables and definitions
Cases were patients with bla AmpC producing E. coli (including both bla ac-AmpC and bla c-AmpC: AmpC-Ec-BSI) isolated from one or more sets of blood cultures, in the clinical microbiology laboratories. In patients with E. coli isolated in more than one blood culture less than 1 month apart, they were considered to be the same episode, and only the first isolate was included for analysis.
Controls were patients with bacteraemia due to a third-generation-cephalosporin-susceptible E. coli (3GC-S-Ec-BSI). The study was approved by the Ethics Committee of the participating hospitals.
Clinical data were prospectively collected [patient demographics, comorbidities (Charlson score) [15], severity (defined as the presence of septic shock and Pitt score), antimicrobial use within the previous 3 months, urinary tract infections in the previous 12 months, urinary and biliary tract abnormalities, indwelling external devices (mechanical ventilation, tracheotomy, urinary catheter, nasogastric tube, nephrostomy, external biliary drainage, endobiliary prosthesis), manipulation of the urinary, biliary, respiratory and/or gastrointestinal tracts within the previous 4 weeks, source of infection and antimicrobial susceptibility pattern]. Healthcare-related BSI was defined as a hospital-acquired or healthcare-associated infection. Hospital-acquired infection was defined as an infection acquired during hospital care that was not present or incubating at admission (infections occurring 48 h after admission were considered nosocomial) or in a patient discharged from hospital in the previous 14 days. Healthcare-associated infection was diagnosed if the patient fulfilled at least one of the following criteria: (i) resided in a nursing home or long-term care facility in the 30 days before the episode; (ii) hospitalised in an acute care hospital for ≥48 h, 90 days before the episode; (iii) attended a hospital or haemodialysis clinic or received intravenous therapy, 30 days before the episode; and/or (iv) received intravenous therapy, wound care, enteral nutrition or healthcare at home, 30 days before the episode. Otherwise, the infection was considered as community-acquired, according to the definitions by Friedman et al. [16].
The antimicrobial treatment regimen administered was recorded, including the agent or agents administered and the duration of treatment. Empirical therapy was considered to be appropriate if an active antimicrobial agent determined by in vitro susceptibility testing was administered at the usual recommended dose for AmpC-Ec-BSI or 3GC-S-Ec-BSI. Isolates were considered multidrug-resistant when they were resistant to three or more antimicrobial classes. The clinical outcome was evaluated as a cure rate at the 14th day of antibiotic and mortality until the 30th day.
Microbiological studies
Each centre selected E. coli isolates that met the following criteria: strains with resistance or exhibited reduced susceptibility (according to CLSI breakpoints and standard methodology [17]) to amoxicillin/clavulanate (<18 mm, >8/4 mg/L) and cefotaxime (<26 mm, >1 mg/L), or ceftazidime (<21 mm, >4 mg/L) or aztreonam (<21 mm, >4 mg/L). Strains with ESBLs were discarded, except those that were resistant to cefoxitin (<18 mm, >8 mg/L) or amoxicillin/clavulanate.
In each hospital, microbiological identification and susceptibility studies were determined by using Vitek [HUMT (bioMérieux Vitek, Hazelwood, MO, USA)], Microscan [CST (Dade Behring MicroScan, West Sacramento, CA, USA)] or the disc diffusion method (HSCiSP) [17, 18].
Phenotypic detection of acquired bla acAmpC (ac-AmpC) was performed by using the cefotetan/cefotetan–cloxacillin Etest (bioMérieux), if deemed appropriate. Visual examination of the antibiogram was carried out to detect colonies in the vicinity of the edge of the haloes of inhibition of cefotaxime, ceftazidime and aztreonam [19, 20].
Molecular detection of bla ac-AmpC was done by using the multiplex PCR described by Pérez-Pérez and Hanson [21]. Depending on the outcome of the multiplex PCR, additional primers were used to characterise the entire gene, as previously described [10]. Overproduction of bla c-AmpC was studied by quantitative reverse transcription PCR (qRT-PCR). Alterations of the bla c-AmpC promoter was analysed by PCR and, subsequently, sequenced, as previously described [21].
The clonal relationship of isolates was determined by pulsed-field gel electrophoresis (PFGE) [10]; phylogenetic data were obtained by multilocus sequence typing (MLST) using the Institut Pasteur tools (http://bigsdb.web.pasteur.fr/ecoli/ecoli.html).
Statistical analysis
To describe the epidemiology and clinical features of AmpC-EC-BSI as compared with cephalosporin-susceptible E. coli, the Mann–Whitney U test was used to compare continuous variables and the χ2 test or Fisher’s exact test was used to compare categorical variables. The SPSS (version 15.0) software package was used for these analyses.
Results
Among the 841 E. coli BSI episodes diagnosed during the study period, 17 (2 %) strains fulfilled the inclusion criteria and were tested by PCR and qRT-PCR. All 17 carried bla ac-AmpC or bla c-AmpC. Eleven AmpC-Ec-BSI (64.7 %) harboured ac-AmpC enzymes [81.1 % bla CMYt (six CMY2 and one CMY7) and 18.1 % bla DHAt (all DHA1)]. The remaining six isolates were AmpC overproducers. Two isolates presented a promoter with an identical mutation profile (−88; −82; −42; −18; −1; +58; +81), resulting in a promoter displacement. Two isolates presented a modified spacer region, showing a mutation at the −28 position and an insertion between positions −15 and −14. The remaining two isolates presented different mutations in the attenuator region [22].
No clonal relationship was found between isolates. Four cases were analysed by MLST, two of them carrying bla c-AmpC, being ST477 and ST494, and two carrying bla ac-AmpC, which were ST39 and ST48. Among all 17 isolates, eight belonged to less virulent phylogroups (seven to group A and one to group B1) and nine to more virulent phylogroups (four to group B2 and five to group D). Six out of eleven (54.6 %) bla ac-AmpC producing E. coli corresponded to phylogenetic group A and among bla c-AmpC, three belonged to group B, two to group D and only one to group A. No differences were found among bla CMYt and bla DHAt producing isolates in terms of phylogeny.
The demographic characteristics, epidemiological features and comorbidities are shown in Table 1. Among the whole cohort, the mean age was 65.6 years and 71 % were men. Cases were more frequently hospital-acquired than controls (p = 0.007).
Table 2 summarises AmpC-Ec-BSI risk factors and clinical features. Indwelling external devices, presence of abnormalities in the urinary or biliary tracts, and recent manipulation within the last 4 weeks prior to the episode were identified more frequently among cases as compared with controls. Almost all cases presented at least one recognised risk factor. Cases had longer length of stay (LOS) and higher severity than controls but showed similar cure rates and mortality. The sources of bacteraemia are also shown in Table 2. The biliary tract was the most frequent source of bacteraemia among cases, followed by the urinary tract.
Previous antimicrobial exposure and empirical therapy is summarised in Table 3. Previous antimicrobial exposure in the last 3 months was more frequent among cases and showed a trend towards statistical significance (p = 0.06). No specific antimicrobial class was found to be associated with the risk of AmpC-Ec-BSI.
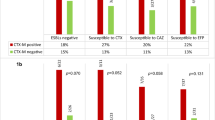
Antimicrobial resistance patterns are shown in Table 4. All cases were susceptible to carbapenems, fosfomycin, amikacin and tobramycin. Higher rates of multi-resistance were detected among E. coli producing plasmid-mediated AmpC. Rates of inappropriate empirical therapy (IET) were higher among AmpC-EC-BSI.
Discussion
The present cohort shows a low but not negligible prevalence of AmpC-Ec-BSI in a Spanish geographical area. The previously reported prevalence ranged from 1.5 to 4 % in different studies conducted in the USA [23], Europe [10, 24, 25] and Asia [26–28].
Remarkably, one-fifth of the analysed BSI due to AmpC producing E. coli were community-onset cases. This represents a therapeutic challenge, as empirical therapy for infections where E. coli should be the pathogenic agent must consider the presence of this antimicrobial resistance mechanism, depending on the local prevalence.
AmpC was mainly plasmid-mediated. A decade ago, this fact was considered a potentially epidemic threat. However, we have shown that, in our area, the prevalence of this resistance mechanism is low and stable. CMY2 was the most common ac-AmpC enzyme, as has been previously reported [6, 22, 29].
The epidemiological features of patients with BSI due to E. coli producing AmpC were somehow similar to those found among patients harbouring ESBL-producing E. coli [26, 30–32]. Cases presented significantly higher proportions of indwelling external devices, of abnormalities in the urinary or biliary tracts, recent manipulation of the urinary, biliary, respiratory and/or gastric tracts, and a trend towards a higher antimicrobial exposure. These findings suggest that bacteraemia due to AmpC-Ec occurs more frequently in patients with some comorbidities. Nevertheless, mortality was similar among cases and controls. Some authors have hypothesised that previous antibiotic use would select for these probably less virulent multidrug-resistant strains [33], which, hence, may cause invasive infections in predisposed patients [34, 35].
AmpC E. coli isolates included in the present cohort were mostly from phylogenetic groups D and A. Phylogenetic group D has been consistently recognised by many authors [36–39] as the most prevalent type together with group B1 among E. coli producing AmpC. On the other hand, in studies on BSIs caused by E. coli, phylogenetic group A has been found with increased frequency in nosocomial BSI, compromised hosts and in BSI caused by antibiotic-resistant isolates, a profile of patients that resembles that of the included cases in the present cohort [24, 40, 41]. Remarkably, the majority of E. coli isolates harbouring plasmid-mediated AmpC were identified as belonging to phylogenetic group A. Similar findings have been reported with ESBL-producing E. coli [42]. This low virulent phylogenetic group has been able to produce BSI in patients with intrinsic risk factors predisposing BSI and also in those with previous antibiotic exposure, which would have selected for AmpC-Ec because of their multidrug-resistant nature, regardless of their virulence profile.
The urinary and biliary tracts were the most common sites of infection [26, 30]. Remarkably, more than two-thirds of AmpC-Ec-BSI were of abdominal origin. On the contrary, urinary tract infections were more frequent among controls. We have no explanation for this finding. No epidemiological link was found among the included cases to suspect an outbreak in a surgical unit.
More than two-thirds of cases received an inadequate empirical therapy. This rate is similar to the data published by previous reports [34, 43]. This fact may be explained firstly because β-lactams are the first-line empirical therapy for urinary and abdominal infection and, secondly, because AmpC producing isolates were associated with higher rates of co-resistance to other antimicrobials. In Europe, some authors have reported the dissemination of plasmid-mediated quinolone resistance genes in Enterobacteriaceae, which can be associated with ESBLs or acquired AmpC β-lactamases. One mechanism for this association is incorporation on the same plasmid of qnr and genes for ESBLs or AmpC-type β-lactamases [44, 45]. In the era of carbapenem resistance, this fact represents an important therapeutic challenge, as carbapenems are frequently the only therapeutic option for E. coli producing AmpC.
The strengths of our study are the prospective and the geographically comprehensive design, including both community and hospital patients, the availability of detailed clinical information and the presence of a control group of cephalosporin-susceptible E. coli.
Our study also has several limitations. It is limited to a geographical area. Similar studies in other areas are needed to confirm our results. On the other hand, due the specific syndrome evaluated (BSIs due to AmpC producing E coli), a small sample size was recruited.
To summarise, the AmpC-Ec-BSI rates in our area are low and stable. AmpC-Ec-BSI affects mainly patients with intrinsic risk factors predisposing BSI and those with previous antibiotic exposure. Acquired enzymes are the main mechanisms implicated. Worrisome, a high proportion of patients receive IET. Despite the prevalence of AmpC-Ec still being low, in those patients with high suspicion or in those institutions with high prevalence, empiric therapy with agents active against AmpC strains may be warranted.
References
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Wilson J, Elgohari S, Livermore DM, Cookson B, Johnson A, Lamagni T et al (2011) Trends among pathogens reported as causing bacteraemia in England, 2004–2008. Clin Microbiol Infect 17:451–458
de Kraker ME, Jarlier V, Monen JC, Heuer OE, van de Sande N, Grundmann H (2013) The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect 19:860–868
Ammerlaan HS, Harbarth S, Buiting AG, Crook DW, Fitzpatrick F, Hanberger H et al (2013) Secular trends in nosocomial bloodstream infections: antibiotic-resistant bacteria increase the total burden of infection. Clin Infect Dis 56:798–805
European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2009. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC; 2010. Available online at: http://ecdc.europa.eu/en/publications/Publications/1011_SUR_annual_EARS_Net_2009.pdf
Jacoby GA (2009) AmpC beta-lactamases. Clin Microbiol Rev 22:161–182
Hawkey PM, Jones AM (2009) The changing epidemiology of resistance. J Antimicrob Chemother 64(Suppl 1):i3–i10
Miró E, Agüero J, Larrosa MN, Fernández A, Conejo MC, Bou G et al (2013) Prevalence and molecular epidemiology of acquired AmpC β-lactamases and carbapenemases in Enterobacteriaceae isolates from 35 hospitals in Spain. Eur J Clin Microbiol Infect Dis 32:253–259
Denisuik AJ, Lagacé-Wiens PR, Pitout JD, Mulvey MR, Simner PJ, Tailor F et al (2013) Molecular epidemiology of extended-spectrum β-lactamase-, AmpC β-lactamase- and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae isolated from Canadian hospitals over a 5 year period: CANWARD 2007-11. J Antimicrob Chemother 68(Suppl 1):i57–i65
Pascual V, Ortiz G, Simó M, Alonso N, Garcia MC, Xercavins M et al (2015) Epidemiology and risk factors for infections due to AmpC β-lactamase-producing Escherichia coli. J Antimicrob Chemother 70:899–904
Pallett A, Hand K (2010) Complicated urinary tract infections: practical solutions for the treatment of multiresistant Gram-negative bacteria. J Antimicrob Chemother 65(Suppl 3):iii25–iii33
Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G et al (2007) CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother 59:165–174
Mammeri H, Nordmann P, Berkani A, Eb F (2008) Contribution of extended-spectrum AmpC (ESAC) beta-lactamases to carbapenem resistance in Escherichia coli. FEMS Microbiol Lett 282:238–240
Martínez-Martínez L, Pascual A, Hernández-Allés S, Alvarez-Díaz D, Suárez AI, Tran J et al (1999) Roles of beta-lactamases and porins in activities of carbapenems and cephalosporins against Klebsiella pneumoniae. Antimicrob Agents Chemother 43:1669–1673
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP et al (2002) Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797
Clinical and Laboratory Standards Institute (CLSI) (2010) Performance standards for antimicrobial susceptibility testing: Twentieth informational supplement. CLSI document M100-S20. CLSI, Wayne, PA, USA
Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Navarro F, Calvo J, Cantón R, Fernández-Cuenca F, Mirelis B (2011) Deteccion fenotipica de mecanismos de resistencia en microorganismos gramnegativos. Enferm Infecc Microbiol Clin 29:524–534
Mirelis B, Rivera A, Miró E, Mesa RJ, Navarro F, Coll P (2006) A simple phenotypic method for differentiation between acquired and chromosomal AmpC β-lactamases in Escherichia coli. Enferm Infecc Microbiol Clin 24:370–372
Pérez-Pérez FJ, Hanson ND (2002) Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40:2153–2162
Alonso N, Miró E, Pascual V, Rivera A, Simó M, Garcia MC et al (2016) Molecular characterisation of acquired and overproduced chromosomal bla AmpC in Escherichia coli clinical isolates. Int J Antimicrob Agents 47:62–68
Alvarez M, Tran JH, Chow N, Jacoby GA (2004) Epidemiology of conjugative plasmid-mediated AmpC beta-lactamases in the United States. Antimicrob Agents Chemother 48:533–537
Courpon-Claudinon A, Lefort A, Panhard X, Clermont O, Dornic Q, Fantin B et al (2011) Bacteraemia caused by third-generation cephalosporin-resistant Escherichia coli in France: prevalence, molecular epidemiology and clinical features. Clin Microbiol Infect 17:557–565
Gude MJ, Seral C, Sáenz Y, Cebollada R, González-Domínguez M, Torres C et al (2013) Molecular epidemiology, resistance profiles and clinical features in clinical plasmid-mediated AmpC-producing Enterobacteriaceae. Int J Med Microbiol 303:553–557
Matsumura Y, Nagao M, Iguchi M, Yagi T, Komori T, Fujita N et al (2013) Molecular and clinical characterization of plasmid-mediated AmpC β-lactamase-producing Escherichia coli bacteraemia: a comparison with extended-spectrum β-lactamase-producing and non-resistant E. coli bacteraemia. Clin Microbiol Infect 19:161–168
Matsumura Y, Yamamoto M, Higuchi T, Komori T, Tsuboi F, Hayashi A et al (2012) Prevalence of plasmid-mediated AmpC β-lactamase-producing Escherichia coli and spread of the ST131 clone among extended-spectrum β-lactamase-producing E. coli in Japan. Int J Antimicrob Agents 40:158–162
Datta S, Wattal C, Goel N, Oberoi JK, Raveendran R, Prasad KJ (2012) A ten year analysis of multi-drug resistant blood stream infections caused by Escherichia coli & Klebsiella pneumoniae in a tertiary care hospital. Indian J Med Res 135:907–912
Yamasaki K, Komatsu M, Abe N, Fukuda S, Miyamoto Y, Higuchi T et al (2010) Laboratory surveillance for prospective plasmid-mediated AmpC beta-lactamases in the Kinki region of Japan. J Clin Microbiol 48:3267–3273
Pai H, Kang CI, Byeon JH, Lee KD, Park WB, Kim HB et al (2004) Epidemiology and clinical features of bloodstream infections caused by AmpC-type-β-lactamase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 48:3720–3728
Calbo E, Romaní V, Xercavins M, Gómez L, Vidal CG, Quintana S et al (2006) Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum beta-lactamases. J Antimicrob Chemother 57:780–783
Ben-Ami R, Rodríguez-Baño J, Arslan H, Pitout JD, Quentin C, Calbo ES et al (2009) A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis 49:682–690
Picard B, Garcia JS, Gouriou S, Duriez P, Brahimi N, Bingen E et al (1999) The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect Immun 67:546–553
Rodríguez-Baño J, Oteo J, Ortega A, Villar M, Conejo MC, Bou G et al (2013) Epidemiological and clinical complexity of amoxicillin-clavulanate-resistant Escherichia coli. J Clin Microbiol 51:2414–2417
Lee CH, Su LH, Li CC, Chien CC, Tang YF, Liu JW (2010) Microbiologic and clinical implications of bacteremia due to extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae with or without plasmid-mediated AmpC beta-lactamase DHA-1. Antimicrob Agents Chemother 54:5395–5398
Corvec S, Crémet L, Leprince C, Dauvergne S, Reynaud A, Lepelletier D et al (2010) Epidemiology of Escherichia coli clinical isolates producing AmpC plasmidic beta-lactamase during a 5-year period in a French teaching hospital. Diagn Microbiol Infect Dis 67:277–281
Jørgensen RL, Nielsen JB, Friis-Møller A, Fjeldsøe-Nielsen H, Schønning K (2010) Prevalence and molecular characterization of clinical isolates of Escherichia coli expressing an AmpC phenotype. J Antimicrob Chemother 65:460–464
Naseer U, Haldorsen B, Simonsen GS, Sundsfjord A (2010) Sporadic occurrence of CMY-2-producing multidrug-resistant Escherichia coli of ST-complexes 38 and 448, and ST131 in Norway. Clin Microbiol Infect 16:171–178
Oteo J, Cercenado E, Cuevas O, Bautista V, Delgado-Iribarren A, Orden B et al (2010) AmpC beta-lactamases in Escherichia coli: emergence of CMY-2-producing virulent phylogroup D isolates belonging mainly to STs 57, 115, 354, 393, and 420, and phylogroup B2 isolates belonging to the international clone O25b-ST131. Diagn Microbiol Infect Dis 67:270–276
Bukh AS, Schønheyder HC, Emmersen JM, Søgaard M, Bastholm S, Roslev P (2009) Escherichia coli phylogenetic groups are associated with site of infection and level of antibiotic resistance in community-acquired bacteraemia: a 10 year population-based study in Denmark. J Antimicrob Chemother 64:163–168
Cooke NM, Smith SG, Kelleher M, Rogers TR (2010) Major differences exist in frequencies of virulence factors and multidrug resistance between community and nosocomial Escherichia coli bloodstream isolates. J Clin Microbiol 48:1099–1104
Rodríguez-Baño J, Mingorance J, Fernández-Romero N, Serrano L, López-Cerero L, Pascual A et al (2012) Virulence profiles of bacteremic extended-spectrum β-lactamase-producing Escherichia coli: association with epidemiological and clinical features. PLoS One 7, e44238
Tam VH, Chang KT, Schilling AN, LaRocco MT, Genty LO, Garey KW (2009) Impact of AmpC overexpression on outcomes of patients with Pseudomonas aeruginosa bacteremia. Diagn Microbiol Infect Dis 63:279–285
Paltansing S, Kraakman ME, Ras JM, Wessels E, Bernards AT et al (2013) Characterization of fluoroquinolone and cephalosporin resistance mechanisms in Enterobacteriaceae isolated in a Dutch teaching hospital reveals the presence of an Escherichia coli ST131 clone with a specific mutation in parE. J Antimicrob Chemother 68:40–45
Robicsek A, Jacoby GA, Hooper DC (2006) The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect Dis 6:629–640
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. G. has accepted grants from Vifor Pharma, Bayer and Pfizer, and speaking engagements and conference invitations from Astellas, AstraZeneca, Novartis, Pfizer, GSK, Bayer, Vifor Pharma, Cubist, Durata and Theravance. E. C. has accepted grants, speaking engagements and conference invitations from Astellas, AstraZeneca, Novartis, Pfizer and MSD. All other authors: none to declare.
Informed consent and ethical approval
The study was approved by the Ethics Committee of the participating hospitals. Informed consent was not deemed necessary due to the retrospective observational design of the study.
Funding
This work was supported by the Fundació Mútua de Terrassa, Grant SQ1Number BFMT 2010/B and by REIPI, RD06 by Plan Nacional I+D+I 2008–2011 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015)—co-financed by European Development Regional Fund ‘A way to achieve Europe’ ERDF.
Rights and permissions
About this article
Cite this article
Pascual, V., Alonso, N., Simó, M. et al. Bloodstream infections caused by Escherichia coli producing AmpC β-lactamases: epidemiology and clinical features. Eur J Clin Microbiol Infect Dis 35, 1997–2003 (2016). https://doi.org/10.1007/s10096-016-2752-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2752-3