Abstract
International - predominantly American - studies undertaken in the ICUs of teaching centres show that inadequate antibiotic therapy increases mortality and length of stay. We sought to ascertain whether this also pertains to smaller ICUs in the Veneto region of north-east Italy. To the best of our knowledge, this is the first such survey in the Veneto area or in Italy as a whole. A retrospective, observational study was performed across five general-hospital ICUs to examine appropriateness of microbiological sampling, empirical antibiotic adequacy, and outcomes. Among 911 patients (mean age, 65.8 years ± 16.2 SD; median ICU stay, 17.0 days [IQR, 8.0–29.0]), 757 (83.1 %) were given empirical antibiotics. Treatment adequacy could be fully assessed in only 212 patients (28.0 %), who received empirical treatment and who had a relevant clinical sample collected at the initiation of this antibiotic (T0). Many other patients only had delayed microbiological investigation of their infections between day 1 and day 10 of therapy. Mortality was significantly higher among the 34.9 % of patients receiving inadequate treatment (48.6 % vs 18.80 %; p < 0.001). Only 32.5 % of combination regimens comprised a broad-spectrum Gram-negative β-lactam plus an anti-MRSA agent, and many combinations were irrational. Inadequate treatment was frequent and was strongly associated with mortality; moreover, there was delayed microbiological investigation of many infections, precluding appropriate treatment modification and de-escalation. Improvements in these aspects and in antibiotic stewardship are being sought.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients admitted to intensive care units (ICUs) present challenging and complex clinical problems. The estimated risk for serious infection is 5–10 times greater than for patients on general medical wards owing to three major factors: severe underlying disease, including multiple illnesses, malnutrition, extremes of age and immunosuppression; invasive medical devices, such as endotracheal tubes for mechanical ventilation and intravascular and urinary catheters, which provide entry portals for pathogens; and crowding, especially in neonatal ICUs, with consequent proximity to other colonised or infected patients, increasing the risk of cross-infection [1–3].
Antimicrobial resistance is a critical variable of ICU outcomes, co-determining patient morbidity, mortality and cost, at least in the major teaching centres where this topic has been largely investigated [4–12]. Kollef, in the USA, found an infection-related mortality rate of 17.7 % among 486 patients receiving appropriate empirical antimicrobial therapy versus 42 % among 169 receiving inappropriate antimicrobial treatment (p < 0.001) [13]. The single major reason for antibiotic therapy being classed as “inappropriate” was the presence of bacteria that had inherent or acquired resistance to the regimen. Others have found similar associations, particularly in bloodstream infections and sepsis [14–20], with mortality shown to increase progressively for each hour’s delay in initiating adequate therapy after the onset of hypotension [21]. In the few countervailing studies, where an association between antibiotic resistance and mortality was not confirmed, few patients received microbiologically inappropriate therapy, owing to early recognition of resistance and/or timely adjustment of the regimen(s) [22, 23]. Beyond its impact on mortality, initial inappropriate antibiotic therapy is also associated with an extended length of stay for ICU patients [24–26].
It is less clear whether these relationships, demonstrated in teaching centres with a complex patient mix, hold true for the smaller ICUs of district general hospitals or in the context of different countries’ cultures of prescribing and microbiological testing. We therefore present here the results of a multicentre, retrospective, observational study covering five ICUs in the Veneto region of north-east Italy, four of them in small hospitals and the fifth in a regional centre. The study had three main goals: first, to test whether, as elsewhere, there was a relationship between treatment inadequacy and clinical outcomes; second, to examine the adequacy of the first-line antimicrobial therapy prescribed and the principal reasons for any inadequacy; and, third, to verify the appropriateness of microbiological testing performed in the participating ICUs.
Patients and methods
Study location and patients
The study was conducted from 2002 to 2010 at five general hospitals in the Veneto region of north-east Italy. Four hospitals were in small towns within 50 km of Vicenza and one in Vicenza itself, which is located between Venice and Verona. When this study was performed, the Vicenza hospital (hospital 5) ICU had 14 beds, admitted approximately 700 patients per year, and was in a 1,050-bed regional hospital; a further 5-bed high-dependency provision for post-surgical care was excluded. Hospital 1 (165 beds) admitted approximately 350 patients per year to its six-bed medical–surgical ICU; hospital 2 (400 beds) had a ten-bed general ICU admitting c. 450 patients per year; hospital 3 (350 beds) had a six-bed general ICU admitting c. 240 patients per year; hospital 4 (220 beds) had a seven-bed ICU admitting c. 340 patients per year. There was a total of 43 ICU beds, accounting for 10.8 % of ICU provision in the Veneto and for 1.5 % of 3,739 Italy’s total intensive care bed provision, as of 2005 [27].
Data collection
Patients admitted into the participating ICUs from 15 May 2002 to 10 June 2010 were assessed. Data input was performed manually in Microsoft Office Excel, with the following information recorded: hospital record number; gender; date of birth; date of hospital admission; date of ICU admission (if different); age at ICU admission, and main diagnosis at admission. Any other diagnoses indicated in the clinical records and constituting: a co-morbidity, a chronic disease directly related to ICU admission, or a secondary pathological event that occurred during the ICU stay was also recorded. For statistical analysis, diagnoses were classified into main categories, all as recognised in the WHO International Statistical Classification of Diseases and Related Health Problems [28]. The date of the primary outcome (death, or transfer to another unit) was recorded. Additionally, for patients transferred from the ICU to other units in the same hospital, the dates of transfer were recorded until the final outcome (death or discharge to home). The duration of ICU stay and entire hospital stay were calculated separately. For each antibiotic course, the regimen and dates of initiation and cessation were recorded. An antibiotic treatment was defined as empirical when it was initiated on the basis of a clinical suspicion of infection and when the causative microorganism and its antibiotic susceptibility were not yet known. Fungal infections were excluded. A sample was considered clinically relevant when it had been taken from a body site related to the reported infection.
Inadequate antimicrobial treatment was defined (based upon, for example, Kollef [29] and Cosgrove and Carmeli 30]) as the microbiological documentation of infection that was not being adequately treated at the time when the causative micro-organism and its antibiotic susceptibility became known, and included:
-
1.
The absence of any agent directed against the family or genus of the micro-organism present
-
2.
The administration of an antimicrobial agent to which the particular isolate was resistant
-
3.
The complete lack of antimicrobial treatment
-
4.
The lack of adherence to minimum requirements in antibiotic administration (i.e., proper dosing, monitoring of drug levels when appropriate, and avoidance of unwanted drug interactions)
A regimen was defined as adequate if it adequately covered all pathogens present in a sample taken at the time of clinical diagnosis (T0). Adequacy was considered to be non-assessable if there was no T0 sample, if no pathogen was grown from a T0 sample, or if there was no concordance between the type of specimen sent to the laboratory and the patient’s clinical presentation (e.g. clinically diagnosed septic shock in post-surgical patients, but where the first isolates were grown from surgical wound swabs taken many days after the initiation of empirical antibiotic treatment; or cases of sepsis where the only microbiological examinations performed were on bronchial aspirates). Cases where only questionable pathogens (principally coagulase-negative staphylococci) were isolated were reviewed individually and discounted unless therapy was escalated on the basis of the microbiological result, implying that the organism was thought to be clinically significant.
Statistical analyses
Normally or near-normally distributed variables were reported as means with standard deviations and were compared using Student’s t test or analysis of variance with the Bonferroni correction for multiple comparisons. Non-normally distributed continuous data were reported as medians with interquartile ranges (IQRs) and were compared using the Mann–Whitney U test or the Kruskal–Wallis test. Spearman’s rho correlation coefficient was calculated to measure the association at the ordinal level between mortality rates and their associated rates of inadequacy of first-line antimicrobial therapy. Kaplan–Meier methods were used to estimate survival rates during follow-up, whereas the log-rank test was used to test the equality of survivor functions. Exploratory univariate analysis for several variables was performed to identify possible predictors of hospital mortality. Multivariate logistic regression analysis was conducted to investigate independent predictors of hospital mortality. Results of logistic regression analysis are reported as adjusted odds ratios (AORs) with 95 % confidence intervals (CI). All statistical analyses were performed using STATA 10.1 (StataCorp LP, College Station, TX, USA) and a two-sided p < 0.05 was routinely considered to be significant.
Ethics
The study was performed in accordance with the recommendations guiding physicians in biomedical research involving human subjects adopted by the 18th World Medical Association, Helsinki, Finland, 1964, and later revisions [31]. As it was performed retrospectively on specimens that were collected as part of the routine sampling required for the microbiological assessment of patients admitted into ICUs, there was no possible risk to any of the patients reviewed, nor any possible modification of their treatment. Consequently, individual consent was not needed. The Institutional Sanitary Board of each hospital approved the protocol and confirmed that submission to their ethics committee was not required, provided that the principal investigator (PB) was personally responsible for the security of patient-identifiable data.
Results
Patients
The study reviewed 911 patients admitted into the five ICUs (Table 1): 570 (62.6 %) were men and 341 (37.4 %) women. Eighty-eight (9.6 %) had diabetes mellitus, 45 (5.0 %) chronic renal failure, and 35 (3.9 %) cirrhosis of the liver. Five hundred and fifty-two (60.6 %) were admitted to an ICU with a medical diagnosis, 206 (22.6 %) with a surgical diagnosis, and 153 (16.8 %) following major trauma. Their mean age upon ICU admission was 65.8 ± 16.2 years (range, 14–93); those admitted to the hospital 5 ICU were significantly younger (p < 0.001) than those admitted elsewhere, partly reflecting a larger proportion of trauma patients. The median duration of ICU stay was 17.0 days (IQR, 8.0–29.0), with an inter-hospitals difference approaching significance (p = 0.079), whereas the median total length of hospital stay was 25.0 days (IQR, 14.0–44), with significant inter-centre variation (p < 0.001).
Antibiotic treatment
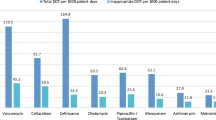
A total of 3,549 antimicrobial treatments were prescribed in the 5 ICUs over the study period. Of these, 3,470 (97.8 %) were parenteral and 79 (2.2 %) oral. Seven hundred and fifty-seven patients (83.1 %) received empiric antibiotic courses (1,223 courses in total, 34.5 % of all antimicrobial treatments; Table 2). Monotherapy was used in 30.2 % of empirical courses, with combination therapy used in 69.8 %. The commonest empirical combinations were piperacillin/tazobactam plus a glycopeptide or linezolid (52 patients, 13.4 %), a carbapenem plus a glycopeptide or linezolid (47 patients, 12.1 %), a cephalosporin plus a glycopeptide or linezolid (27 patients, 7.0 %), piperacillin/tazobactam plus a fluoroquinolone (25 patients, 6.4 %), a cephalosporin plus metronidazole (23 patients, 6.0 %), and piperacillin/tazobactam plus metronidazole (21 patients, 5.4 %).
Combination therapies included two antibiotics in 329 cases (85.0 %), three in 55 cases (14.2 %), and four in 3 cases (0.8 %). Cephalosporins (148 courses) accounted for 40.1 % of all empirical monotherapies, with cefazolin (first-generation) in 66 (44.6 %), cefotetan (second-generation) in 4 (2.7 %), cefotaxime, ceftazidime, and ceftriaxone (third-generation) in 69 (46.6 %), and cefepime (fourth-generation) in 9 (6.1 %). Other frequently prescribed monotherapies were piperacillin/tazobactam (74 courses, 20 % of all monotherapies) and other penicillin/β-lactamase inhibitor combinations (68 courses, 18.4 %).
Only 126 of the 388 combination regimens (32.5 %) comprised a broad-spectrum Gram-negative β-lactam plus an anti-MRSA agent (a glycopeptide, usually teicoplanin, or linezolid); 91 (12.2 %) of the first-line empirical regimens were irrational or redundant poly-pharmacy, commonly comprising a combination of a β-lactam with anti-anaerobe activity (i.e. a β-lactamase inhibitor combination or a carbapenem) with metronidazole.
The median duration of the first-line empirical therapy was 11.0 days (IQR, 6.7–19.0) for patients with bacteraemia, 9.0 days (5.0–14.0) for medical patients and 10.0 days (7.0–17.0) for surgical patients. Although there is a growing trend towards shortening treatment durations, particularly in Northern Europe, these longer courses are typical of Italy in the study period and are not out of line with many international guidelines [32].
Laboratory data
There was often a poor match between the site of infection indicated in the patient record and the specimens from which organisms, if any, were grown by the laboratory. Moreover, there were frequently long delays between the clinical diagnosis and any result becoming available to the treating clinician(s).
At the four smaller sites (hospitals 1–4), respiratory samples accounted for >50 % of all specimens with a pathogen grown, and for 74 % and 82 % at hospitals 2 and 3, respectively (Fig. 1). Blood and (especially) urine were rarely sampled, even when an infection was believed to involve these sites. Thus, at hospitals 2 and 3, urine accounted for only 5.6 % and 8.7 % of total microbiological investigations, respectively. These patterns seem to reflect a practice of performing surveillance cultures of respiratory secretions and basing therapy upon these, rather than of undertaking microbiological investigations of actual infections.
Clinical specimens yielding an organism were collected at the initiation of empirical antibiotics (T0) from only 251 of the 911 patients (27.6 %). Sixteen of these 251 did not receive antibiotics, as they were considered to be colonised rather than infected (n = 13) or died early (n = 3), leaving 235 patients who had a T0 specimen and an assessable empirical antibiotic treatment. This total reduced to 212 after the exclusion of 23 patients, whose T0 sample was from a body site different to the infection recorded in the patient’s notes. Samples yielding reported organisms were taken within 10 days of therapy initiation from a further 361 patients (39.6 %), whereas in the remaining 299 cases (32.8 %), the interval between the initiation of antibiotic therapy and the first sample with a reported organism was >10 days, or there was no relationship between the type of specimen from which any organism was grown and the patient’s clinical setting (Table 3). The median interval between the initiation of empirical antibiotic therapy and the availability of a first (post-infection) antibiotic sensitivity result was 7 days (IQR, 3.0–14.0), with significant variation amongst the five sites (p < 0.001). The lag between the arrival of a growth-yielding sample at the laboratory and the availability of the result varied between sites from 3 to 4.5 days, meaning that around half of this overall 7-day delay was between the clinical diagnosis of infection and the specimen being sent to the laboratory for microbiology. It follows that many of the patients were already receiving antibiotics at the time the first culture was taken, potentially compromising pathogen recovery and meaning that many were nearing the end of their antibiotic course when any microbiological results became available.
The lack of a T0 organism may be because no specimen was sent to the laboratory, or because no organism was grown by the laboratory. Discriminating between these scenarios in the hospital record systems proved difficult, but, for a random sample of 23 patients lacking culture results, we could identify 6 who had a relevant-site T0 sample from which the laboratory failed to grow a pathogen, 8 who had only a T1–T10 specimen failing to yield growth, and 9 who had no evidence of any specimen being sent to the microbiology laboratory within 10 days of clinical diagnosis.
In total, 313 isolates from clinical samples (regardless of site and apparent relevance) were collected at T0 from the 235 patients starting empirical treatment. In 147 cases (62.6 %) the organism(s) proved susceptible to the antibiotic regimen initiated, whereas 88 (37.4 %) patients had bacteria resistant to the regimen initiated. The lowest proportion of resistance was at hospital 1 (25.0 %) and the highest at hospital 2 (42.4 %). Resistance to the empirical therapy was more prevalent (192 out of 347, 53 %, p < 0.001) amongst patients who had initial isolates collected during the T1–T10 period, again with the lowest proportion (49.1 %) at hospital 4 and the highest (61.5 %) at hospital 2. The differential in resistance, between patients with a T0 vs a T1–T10 initial sample was moderately significant, even in the bacteraemia subset, where 35 out of 54 (64.8 %) isolates from patients with a T1–T10 sample were resistant to the empirical therapy given vs 14 out of 34 (41.2 %; p = 0.098) isolates from T0 samples, whereas the difference in resistance between the bacteraemic vs non-bacteraemic patients in the whole series was not significant (p = 0.1891; Table 4). Among the 212 patients who had clinically relevant T0 samples, resistance to the empirical therapy given was observed in 74 (34.9 %), with the lowest proportion (22.6 %) at hospital 1 and the highest (40.6 %) at hospital 2 (p = 0.2277). A greater proportion of resistance to empirical therapy (142 out of 266, 53.4 %), was seen in cases with an initial T1–10 sample, with the lowest rate (43.4 %) at hospital 4 and the highest (70.0 %), again, at hospital 2 (p = 0.01). Among bacteraemic cases, 6 out of 24 T0 isolates (25.0 %) were resistant compared with 13 out of 25 (52 %) among those collected from T1–10. There was little obvious demographic difference between the groups, with a first relevant-site sample at T0, T1–10 and T > 10 (or no relevant sample at all), with average ages of 66.3, 65.8, and 66.5 years and medical:surgical:trauma ratios of 73.5:15.5:11.0; 59.0:19.9:21.1; 71.2:14.8:14.0 respectively.
The frequent lateness of the microbiological data may explain the small proportion of cases (282 out of 757, 37.2 %) in whom empirical regimens were adjusted based upon susceptibility results. The vast majority of these changes (252 out of 282, 89.4 %) were escalations, meaning the addition of further agents or switches to broader-spectrum agents; first-line empirical antibiotic was stepped down in only 30 cases (10.6 %).
Outcomes
Two hundred and twenty-seven patients (24.9 %) died during their ICU stay and 316 (34.7 %) during their entire hospitalisation. One hundred and forty-three of the ICU deaths (63 % of all ICU deaths) could reasonably be related to infection. Patient primary outcome data in relation to treatment adequacy for the 212 cases with a relevant-site T0 clinical specimen is displayed in Table 5. Among the 74 (34.9 %) whose empirical antibiotic(s) failed to cover the organisms subsequently identified there were 36 ICU deaths (48.6 %) vs 26 deaths (18.8 %) among the 138 receiving therapy that covered all pathogens present (p < 0.001). This pattern was maintained among patients whose first relevant specimen was taken in the T1–T10 interval, where there was 43 % mortality among those receiving inadequate therapy vs 23 % among those receiving adequate antimicrobial therapy (OR = 1.84; 95 % CI, 1.3 to 2.5). In this case, however, it is impossible to distinguish whether inadequacy was against the initial pathogen, against its resistant progeny, or against a secondary invader. Overall mortality rates among patients with a first relevant-site sample at T0, T1–T10 and T > 10 (or no relevant sample at all) were 29.2 %, 19.9 % and 27.6 % respectively.
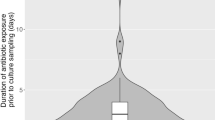
The adequacy of the initial regimen did not significantly affect the duration of ICU stay (p = 0.93; Fig. 2), partly because survivors who were hospitalised for extended periods were balanced by patients who died early.
Development of septic shock was a significant predictor of mortality as was the patient’s age (p < 0.001). Non-survivors were also more likely to have had acute renal failure upon admission (p < 0.001). By contrast, those admitted because of traumatic shock were more likely to survive (p < 0.001), perhaps owing to a higher probability of receiving adequate antibiotic treatment, given to 36.6 % of trauma patients vs 30.9 % of other patient categories.
The commonest pathogens isolated from bloodstream samples and their associated rates of inadequate antimicrobial treatment were P. aeruginosa (n = 23; 80 % inadequacy), MRSA (n = 19; 80 % inadequacy) and E. coli (n = 14; 77 % inadequacy). The large number of MRSA is unsurprising: European Antimicrobial Resistance Surveillance Network (EARS-net) data (http://www.ecdc.europa.eu) show that the MRSA rate among bloodstream S. aureus fluctuated between 33.2 and 39.4 % throughout the study period.
Despite extensive cephalosporin use (as mentioned above) only 2 cases of Clostridium difficile diarrhoea were recorded, although it should be cautioned that diarrhoeal patients were not routinely screened for this pathogen during the study period, leading to likely under-recording.
Discussion
Studies of antibiotic inadequacy and its consequences in patients who are severely ill have largely been undertaken in teaching centres [13–21, 33–35], particularly in the USA [13, 15, 17, 33]. We investigated whether their general conclusion—that inadequate empirical therapy is associated with increased mortality—also applied to smaller centres in the Veneto region.
Assessing treatment adequacy proved challenging, owing to the many patients in whom microbiology was carried out improperly or belatedly. Clinical specimens were collected at initiation of empirical antibiotics (T0) for only 31.0 % of patients (235 out of 757) starting an initial empirical antibiotic course, and only 28 % (212) had a sample taken from the reported infection site. In rather more cases (347, 45.8 %), initial samples were taken between T1 and T10, whereas for 299 cases (39.5 %), the interval to the first sample was >10 days, or there was no concordance between any laboratory specimen and the patient’s clinical setting. Resistance to the initial antibiotic therapy was significantly more prevalent amongst T1–T10 isolates than among those collected at T0, regardless of whether comparison was irrespective of body site (55.3 vs 37.4 %) or solely from isolates from the relevant site (53.4 vs 34.9 %). Similar patterns—with greater resistance in T1–T10 vs T0 samples—were seen for the subset of bacteraemia patients (64.1 % vs 41.1 % for isolates from any body site and 52.0 vs 25.0 % amongst bloodstream isolates). Greater resistance rates among “late” isolates may reflect the selection of resistance in the original pathogen, or super-infection by more resistant organisms during therapy.
When only the 212 empirically treated patients with a relevant T0 sample were analysed (Table 5), 138 treatments (65.1 %) were assessed as being adequate, with 18.8 % deaths in the ICU, vs 74 (34.9 %) being judged inadequate, with 48.6 % deaths; p = <0.001). Total mortality among these 212 patients with timely microbiological investigation was 29.2 % compared with 27.6 % among the patients who had very belated microbiological investigation (>10 days) or no investigation at all. These two groups were well matched in terms of average age and proportions of medical vs surgical vs trauma cases; the overall similarity in outcome may well reflect the fact that, even where microbiological investigation was performed, therapy was rarely changed. Mortality was lower (19.9 %) among the patients who had a first relevant sample in the T0–T10 period, but this group contained a higher proportion of trauma patients, who tended to have better outcomes anyway.
This study, covering five small ICUs in the Veneto region, thus confirms a significant association between inadequate empirical antimicrobials and ICU mortality. S. aureus and resistant Gram-negative bacteria were the pathogens most frequently associated with poor outcomes, also as seen elsewhere [14, 15, 17, 21, 33–35]. Furthermore, and in keeping with a recent meta-analysis [36], ICU infections following trauma had lower mortality, perhaps because most trauma patients are younger and have fewer co-morbidities. In contrast to several published studies [8, 14, 18, 20, 24–26], we found no relationship between treatment adequacy and duration of ICU stay, although this calculation may be confounded by how duration is counted for patients who die early.
The frequent lack of prompt microbiological investigation is the core finding of this study. In many cases, clinicians’ decision seemed to depend upon surveillance sampling for bacterial colonisation of the lower airways rather than direct microbiological investigation of the clinically diagnosed infections, often with an excess of antibiotics. Such overtreatment seems widespread in Italy [37, 38] and elsewhere [39, 40].
Even when relevant specimens were collected, they were often collected late, meaning that the organisms grown may have been secondary colonists, and that susceptibility results only became available around the time when primary empirical treatment was ending, or even afterwards. This may explain the infrequency of treatment de-escalation based on laboratory results. Even hospital 5—where laboratory results were available earlier—in all but 2 cases, changes to initial empirical antibiotic treatment were escalations, not de-escalations.
In summary, despite its limitations (e.g. being retrospective, exclusion of fungal infections and the difficulty of evaluating empirical therapy in patients whose microbiological investigation was inadequate), this study provided a clear picture of sub-optimal microbiological testing and antibiotic use in the five ICUs. There was frequent antibiotic misuse, inappropriate empirical treatment, and high variability in (generally overlong) treatment duration and a considerable need for the ICUs to improve specimen-taking and the use of the microbiology laboratory. Notably, the ICUs lacked local antibiotic practice guidelines, which represent one tool for clinicians to manage patient and stewardship needs. Clinicians should be aware that any transient clinical benefit achieved by overtreatment is counterbalanced by collateral damage and detriment to the community as a whole via increased selection pressure for resistance. These issues are becoming even more serious and urgent with the recent and extensive dissemination in Italy of Klebsiella pneumoniae with KPC carbapenemases [20, 41, 42]. Poorly directed antibiotic use may have helped to drive this dissemination, which saw the proportion of carbapenem-resistant bloodstream K. pneumoniae in Italy rise from 1–2 % in 2006–2009 to over 30 % in 2013–2014 (http://www.ecdc.europa.eu). Most of these isolates are, however, clonal [43] and it therefore seems likely that infection control failures are a greater issue, a view supported by the observation that near-identical strains of carbapenemase-producing K. pneumoniae were reduced in prevalence in Israel by improved infection control rather than stewardship changes. Carbapenemase-producing K. pneumoniae remain rare at the ICUs included here (5–8 isolates, hospital-wide, per annum from 2010–2012 in hospital 5, rising to 14 in 2013, 18 in 2014 and 34 in the first half of 2015), but are hugely more prevalent in a major teaching centre just 50 km away (data not shown).
References
Kollef M (2008) SMART approaches for reducing nosocomial infections in the ICU. Chest 134:447–456
Joram N, de Saint Blanquat L, Stamm D, Launay E, Gras-Le Guen C (2012) Healthcare-associated infection prevention in pediatric intensive care units: a review. Eur J Clin Microbiol Infect Dis 31:2481–2490
Osman MF, Askari R (2014) Infection control in the intensive care unit. Surg Clin North Am 94:1175–1194
Cohen ML (1992) Epidemiology of drug resistance: implications for a post-antimicrobial era. Science 257:1030–1055
Goldmann DA, Weinstein RA, Wenzel RP, Tablan OC, Duma RJ, Gaynes RP et al (1996) Strategies to prevent and control the emergence and spread of antibiotic-resistant organisms in hospitals - a challenge to hospital leadership. JAMA 275:234–240
Carbon C (1999) Costs of treating infections caused by methicillin-resistant staphylococci and vancomycin-resistant enterococci. J Antimicrob Chemother 44:31–36
Vincent JL (2000) Microbial resistance: lessons from the EPIC study. Intensive Care Med 26 [Suppl 1]:S3–S8
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H et al (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34:344–353
Raymond DP, Pelletier SJ, Crabtree DT, Evans HL, Pruett TL, Sawyer RG (2003) Impact of antibiotic-resistant Gram-negative bacilli infections on outcome in hospitalized patients. Crit Care Med 31:1035–1041
Cosgrove SE (2006) The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 42 [Suppl 2]:82–89
Ammerlaan H, Seifert H, Harbarth S, Brun-Buisson C, Torres A, Antonelli M et al (2009) Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European Countries. Clin Infect Dis 49:997–1005
Paruk F, Richards G, Scribante J, Bhagwanjee S, Mer M, Perrie H et al (2012) Antibiotic prescription practices and their relationship to outcome in South Africa: findings of the prevalence of infection in South African intensive care units (PISA) study. S Afr Med J 102:613–618
Kollef MH, Sherman G, Ward S, Fraser VJ (1999) Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462–474
Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD (1998) The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med 244:379–386
Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH (2000) The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146–155
Vallés J, Rello J, Ochagavía A, Garnacho J, Alcalá MA (2003) Community-acquired bloodstream infection in critically ill adult patients. Impact of shock and inappropriate antibiotic therapy on survival. Chest 123:1615–1624
Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y et al (2005) The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol 26:166–174
Garnacho-Montero J, Ortiz-Leyba C, Herrera-Melero I, Aldabó-Pallás T, Cayuela-Dominguez A, Marquez-Vacaro JA et al (2008) Mortality and morbidity attributable to inadequate empirical antimicrobial therapy in patients admitted to the ICU with sepsis: a matched cohort study. J Antimicrob Chemother 61:436–441
Retamar P, Portillo MM, López-Prieto MD, Rodríguez-López F, de Cueto M, García MV et al (2012) Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: a propensity score-based analysis. Antimicrob Agents Chemother 56:472–478
Girometti N, Lewis RE, Giannella M, Ambretti S, Bartoletti M, Tedeschi S, Tumietto F, Cristini F, Trapani F, Gaibani P, Viale P (2014) Klebsiella pneumoniae bloodstream infection: epidemiology and impact of inappropriate empirical therapy. Medicine (Baltimore) 93:298–309
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S et al (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596
Carmeli Y, Troillet N, Karchmer AW, Samore MH (1999) Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch Intern Med 159:1127–1132
Moroney JF, Fiore AE, Harrison LH, Patterson JE, Farley MM, Jorgensen JH et al (2001) Clinical outcomes of bacteremic pneumococcal pneumonia in the era of antibiotic resistance. Clin Infect Dis 33:797–805
Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO (2001) Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 32:1162–1171
Shorr AF (2009) Review of studies of the impact on Gram-negative bacterial resistance on outcomes in the intensive care unit. Crit Care Med 37:1463–1469
Shorr AF, Micek ST, Welch EC, Doherty JA, Reichley RM, Kollef MH (2011) Inappropriate antibiotic therapy in Gram-negative sepsis increases hospital length of stay. Crit Care Med 39:46–51
Italian Association of Hospital Anaesthetists. National Census of ICU beds, as of June 30th 2005. www.aaroi.it
World Health Organization. International Statistical Classification of Diseases and Related Health Problems—10th revision. http://apps.who.int/classifications/apps/icd/icd10online/
Kollef MH (2000) Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis 31 [Suppl 4]:131–138
Cosgrove SE, Carmeli Y (2003) The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis 36:1433–1437
World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by the 55th WMA General Assembly, Tokyo 2004 www.wma.net/en/30publications/10policies/b3/17c.pdf
Barrett J, Edgeworth J, Wyncoll D (2015) Shortening the course of antibiotic treatment in the intensive care unit. Expert Rev Anti-Infect Ther 13:463–471. doi:10.1586/14787210.2015.1008451
Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H et al (2004) Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum β-lactamases. Clin Infect Dis 39:31–37
Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B et al (2007) Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 51:1987–1994
Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE et al (2009) Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 136:1237–1248
Siempos II, Vardakas KZ, Kyriakopoulos CE, Ntaidou TK, Falagas ME (2010) Predictors of mortality in adult patients with ventilator-associated pneumonia: a meta-analysis. Shock 33:590–601
Malacarne P, Rossi P, Bertolini G (2004) Antibiotic usage in intensive care units: a pharmaco-epidemiological multicentre study. J Antimicrob Chemother 54:221–224
Vaccheri A, Silvani MC, Bersaglia L, Motola D, Strahinja P, Vargiu A et al (2008) A 3 year survey on the use of antibacterial agents in five Italian hospitals. J Antimicrob Chemother 61:953–958
Vlahovic-Palcevski V, Francetic I, Palcevski G, Novak S, Abram M, Bergman U (2007) Antimicrobial use at a university hospital: appropriate or misused? A qualitative study. Int J Clin Pharmacol Ther 45:169–174
Kariv G, Paul M, Shani V, Muchtar E, Leibovici L (2013) Benchmarking inappropriate empirical antibiotic treatment. Clin Microbiol Infect 19:629–633
Nordmann P (2014) Carbapenemase-producing Enterobacteriaceae: overview of a major public health challenge. Med Mal Infect 44:51–56. doi:10.1016/j.medmal.2013.11.007
Corcione S, Rocchetti A, Argentero PA, Raso R, Zotti CM, De Rosa FG, Ghisetti V (2015) A one-year survey of carbapenemase-producing Klebsiella pneumoniae in Italy: beyond the ICU. Clin Microbiol Infect 21:e11–e13. doi:10.1016/j.cmi.2014.09.012
Albiger B, Glasner C, Struelens M, Grundmann H, Monnet D, European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) working group (2015) Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries. Euro Surveill 20(45)30062. doi: 10.2807/1560-7917.ES.2015.20.45.30062
Acknowledgements
We are indebted to the Institutional Sanitary Boards of the participating hospitals for assistance with performing the study. We are also grateful to Dr P. Piccinni, Dr D. Mastropasqua, Dr M. Baiocchi, Dr G. Zanardo and Dr L. Ongaro for giving permission to examine patients’ clinical records in the participating ICUs. Special thanks to Prof A. Holmes and to Prof P. Wilson for their careful reading of the PhD thesis upon which this manuscript is based.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding of any kind has been received for this work, and data have been generated as part of the routine work. No financial support is ongoing for any of the authors. The manuscript has been written without any external professional support.
Conflicts of interest
David M. Livermore has performed on advisory boards or ad-hoc consultancy for Accelerate, Achaogen, Adenium, Allecra, AstraZeneca, Auspherix, Basilea, BioVersys, Centauri, Cubist, Cycle, Discuva, Meiji, Nordic, Pfizer, Roche, Shionogi, Tetraphase, VenatoRx, Wockhardt; paid lectures for AOP Orphan, AstraZeneca, Merck, Nordic, Pfizer; relevant shareholdings in Dechra, GSK, Merck, Perkin Elmer, Pfizer amounting to <10 % of portfolio value; contract research for Achaogen, Allecra Antiinfectives, AstraZeneca, Cubist Pharmaceuticals, GlaxoSmithKline, Merck, Meiji Melinta, and Wockhardt Ltd.
All the other authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required. Please see also: Patients and methods.
Rights and permissions
About this article
Cite this article
Benedetti, P., Sefton, A.M., Menegozzo, M. et al. Antimicrobial use and microbiological testing in district general hospital ICUs of the Veneto region of north-east Italy. Eur J Clin Microbiol Infect Dis 35, 1627–1638 (2016). https://doi.org/10.1007/s10096-016-2701-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2701-1