Abstract
The aim of the present work was to study the epidemiology of Salmonella enterica serovar Enteritidis (S. Enteritidis) in Greece, comparing all the food and food animal isolates during a 3-year period with clinical isolates. Submission of the generated data to the PulseNet Europe database was carried out in order to study the population structure of this particular serovar and indicate possible connections with European strains. One hundred and sixty-eight (168) S. Enteritidis strains of human, animal, and food origin, isolated during the period 2008–2010 in Greece, were studied. Strains were characterized by phenotypic (antibiotic resistance) and molecular [pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST)] methods. PFGE revealed 39 XbaI, 48 BlnI, and 80 XbaI–BlnI distinct pulsotypes, suggesting several clones circulating through the food chain and multiple sources of transmission. Submission to the PulseNet Europe database indicated that PFGE profile SENTXB.0001, the most common PFGE profile in Europe, was also predominant in Greece (33.3 %). MLST showed that all the strains studied shared the same sequence type (ST11), representing the most common ST in Europe. High rates of resistance to nalidixic acid were observed among human and poultry isolates (~25 %), indicating the potential fluoroquinolone treatment failure. Our data suggest that strains originating from multiple reservoirs circulated in Greece through the food chain during the study period. Predominant profiles in Greece were common to PulseNet Europe profiles, indicating similarities between the S. Enteritidis populations in Greece and Europe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-typhoidal salmonellae (NTS) are commonly acquired from contaminated food products and are responsible for numerous foodborne infections worldwide. Although 2659 serovars have been identified worldwide [1], Salmonella enterica serovar Enteritidis (S. Enteritidis) is responsible for the majority of reported cases of salmonellosis [2]. Poultry and poultry products are the main reservoir and most of the strains are often isolated from broiler meat, laying hens, and breeders [2]. In Greece, S. Enteritidis ranked first during the period 2007–2012 among human isolates, while the notification rate in 2012 was among the lowest in 27 European Union member States (≤5 per 100,000) [2–4]. Most NTS infections result in gastroenteritis, but, sometimes, severe invasive infections require antibiotic treatment, such as fluoroquinolones (ciprofloxacin) and third-generation cephalosporins (the drug of choice for children) [5]. Resistance to these drugs have been widely reported for the past two decades [6, 7].
Several molecular methods, such as pulsed-field gel electrophoresis (PFGE) coupled with phage typing or multiple locus variable number of tandem repeats analysis (MLVA), are often used where further strain discrimination is required [8–10], mainly during outbreaks investigations. PulseNet Europe has set up a standardized protocol to ensure that PFGE fingerprints from various laboratories are reproducible and comparable [11]. Additionally, multilocus sequence typing (MLST) is used for epidemiological purposes in order to study the population structure of Salmonella spp. [12].
The aim of the present work was to study the epidemiology of S. Enteritidis in Greece during a 3-year period. Strains isolated from patients, animals, and food products were characterized by (i) antimicrobial susceptibility testing and (ii) genotyping using PFGE. A subset of strains was studied using MLST.
Methods
A total of 88 isolates isolated in Greece between 2008 and 2010 during the official control programs for Salmonella were serotyped as S. Enteritidis. Sixteen isolates were from food of Greek origin (poultry products), originating from six regions, and 72 isolates were from poultry, representing 57 farms and 17 regions. During the same period, 1039 human isolates were serotyped by the National Reference Centre for Salmonella (NRCS) as S. Enteritidis. Eighty of them were randomly selected for this study [3]. Identification of the Salmonella isolates was carried out at the NRCS by serotyping in accordance to the White–Kauffmann–Le Minor scheme [13, 14]. Susceptibility to a panel of 20 antimicrobials was determined using the agar dilution method in Iso-Sensitest agar (Oxoid, Basingstoke, UK). The final plate concentrations (mg/L) were: ampicillin (A) 8 and 128; chloramphenicol (C) 8; colomycin (Co) 8; gentamicin (G) 4; kanamycin (K) 16; neomycin (Ne) 8; streptomycin (S) 16 and 128; spectinomycin (Sp) 64; sulfonamide (Su) 64; tetracycline (T) 8 and 128; trimethoprim (Tm) 2; ciprofloxacin (Cp) 0.125 and 1; nalidixic acid (Nx) 16; furazolidone (Fu) 8; amikacin (Ak) 4; cephalexin (Cx) 16; cephradine (Cr) 16; cefuroxime (Cf) 16; ceftriaxone (Cn) 1; cefotaxime (Ct) 1. Multidrug resistance was defined as previously proposed [15]. PFGE was performed after the digestion of genomic DNA with XbaI and BlnI (Takara, Tokyo, Japan), according to the PulseNet protocol, as previously described [16], with S. enterica serovar Braenderup (H9812) as a DNA size marker. All profiles following XbaI digestion were submitted to the PulseNet Europe database for official PulseNet profile designation; BlnI and composite XbaI–BlnI profile designations were not official. MLST was performed by sequencing seven core genes, as previously described [12]. Data from MLST were submitted to the Salmonella enterica MLST Database (http://mlst.warwick.ac.uk/mlst/dbs/Senterica) in order to define the sequence types (STs). Statistical comparison between resistance rates among isolates was analyzed by the Chi-square test (Microsoft Excel 2007). Fingerprints were analyzed using BioNumerics v6.1 (Applied Maths, Sint-Martens-Latem, Belgium). Dendrograms were constructed using the Dice similarity coefficient and the unweighted pair group method with arithmetic mean (UPGMA), optimization, and position tolerance set at 1.5%. The composite analysis was based on equal weighting of the XbaI and BlnI data and UPGMA clustering.
Results
Based on the antimicrobials tested, high resistance levels to nalidixic acid were observed among human and poultry isolates (25 %), and lower resistance levels (p < 0.05) among food isolates (6.25 %). Resistance to ampicillin was observed in two isolates, and resistance to streptomycin, trimethoprim, kanamycin, and spectinomycin was observed in one isolate each. Fifty-nine out of 80 (73,7 %) human isolates, 54 out of 72 (75 %) poultry isolates, and 15 out of 16 (93.7 %) food isolates were pan-susceptible to the 20 antimicrobials tested. Resistance to ciprofloxacin was not observed.
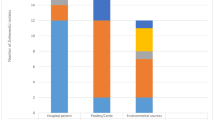
PFGE analysis identified 39 unique profiles using XbaI macrorestriction endonuclease. Submission to the PulseNet Europe database showed that 109 out of 168 isolates (64.9 %) were represented by the eight PulseNet-identified profiles SENTXB.0001, SENTXB.0002, SENTXB.0004, SENTXB.0005, SENTXB.0007, SENTXB.0023, SENTXB.0010, and SENTXB.0040 (Fig. 1). The remaining 32 profiles were represented by one to seven isolates, but did not correspond to PulseNet profiles. The most prevalent PFGE profile was SHADXB.0001 (55/168, 32.7 %). This profile was identified among human, poultry, and food isolates, and was present in every year from 2008 to 2010. The second highest occurring profile was SENTXB.0005 (29/168, 17.3 %), and was also identified during each year from all sample categories (Table 1). PFGE using BlnI restriction endonuclease revealed 48 distinct BlnI pulsotypes among the 168 strains. The predominant BlnI profile was BlnI-0007 and was present in 60 out of 168 (35.7 %) profiles (two from food, 26 from poultry, and 32 from humans). The second most dominant profile was BlnI-0010, with 21 (12.5 %) strains (four from foods, nine from poultry, and eight from humans). The remaining 46 BlnI profiles corresponded to one to nine isolates. The composite dataset divided strains into 80 distinct XbaI–BlnI profiles. The most common patterns were XbaI–BlnI-0028 (13.1 %), XbaI–BlnI-0026 (10.1 %), and XbaI–BlnI-0039 (7.1 %). All the remaining patterns were represented by one to ten isolates (Fig. 1).
A subset of eight strains were chosen for MLST; five of them had been isolated from humans and three from poultry. These belonged to eight different XbaI pulsotypes, with similarity values ranging from 77 to 95 %. Five of them were identical to five PulseNet Europe profiles and three did not correspond to any of them. All eight isolates belonged to ST11 (allelic profile 5-2-3-7-6-6-11). ST11 includes the majority of S. Enteritidis isolates uploaded to the Salmonella enterica MLST Database (Fig. 2).
Discussion
To our knowledge, this is the first study that describes the population structure of S. Enteritidis based on PFGE types submitted to the PulseNet Europe database and drug resistance surveillance of this serovar in Greece. Even though the isolates cover the period 2008–2010, we believe that the general condition has not changed substantially during the last several years and the main conclusions are still an ongoing issue.
In general, S. Enteritidis is a serovar with low resistance rates and, thus, multidrug resistance is rarely observed [17]. Our data showed resistance to nalidixic acid mainly among human and poultry isolates (~25 %). There were variations in resistance to nalidixic acid among human isolates observed in European countries for 2012. High rates were reported from Spain (60.3 %), Romania (37 %), and France (31.7 %), whilst low rates were reported in Germany (1 %) and Hungary (2.3 %) [18]. Poultry population data from 2012 in the European Union reported resistance to nalidixic acid ranging from 0 % in Austria to 39.3 % in Poland and 92.6 % in Portugal [18]. Resistance to nalidixic acid in salmonellae is of great concern, as it has been reported to be an indicator for reduced susceptibility to the drug of choice, ciprofloxacin, resulting in possible treatment failure [19]. Consequently, the 25 % of nalidixic acid-resistant S. Enteritidis in this study must be considered as possibly having reduced susceptibility to ciprofloxacin, and, thus, potentially treatment-resistant, even though our results indicated no resistance/partial resistance to ciprofloxacin. The lack of or partial resistance to ciprofloxacin in this study could be explained as resistance to ciprofloxacin was studied for the breakpoints of 1 and 0.125 mg/L and not 0.064 mg/L, which is the current European Union clinical breakpoint [20]. This observation differed in some other European Union countries, where most S. Enteritidis strains, especially PT4, PT1, and PT6, were susceptible to nalidixic acid and other antimicrobial agents. It must be stressed that, in 2012, Greece had the highest levels of antimicrobial consumption in primary care in Europe [21]; this could explain the 25 % of resistance to nalidixic acid among humans in our study.
SENTXB.0001 was, according to our results, the most common PFGE profile in Greece, comprising 32.7 % of the isolates. This profile is also dominant in Europe; 62.5 % (n = 2210) in England and Wales during 2001–2012 and 56.4 % (n = 6734) of human isolates from other European countries (Austria, Denmark, Spain, Finland, Scotland, England and Wales, Italy, Netherlands, and Germany) (data not shown). The second most prevalent profile, SENTXB.0005 (17.3 %), is also common in Europe; 3.75 % (n = 133) in the UK and 9.3 % (n = 1111) in Europe. An interesting observation is that the third most common profile, SENTXB.0002, in the UK with 13 % incidence (n = 459) and also in Europe with 15.7 % incidence (n = 1880), is relatively rare in Greece, where the rate is only 3.6 %. It is also to be noted that SENTXB.00014, the fourth highest occurring profile in the UK and in Europe (~2.7 %), was absent in Greece, and profile SENTXB.0007 was rare; 0.6 % (n = 1) in Greece, 2 % (n = 71) in the UK, and 1.7 % (n = 205) in Europe. In general, our data suggest mainly similarities but also some differences among Greek and European populations of S. Enteritidis. This diversity may suggest that strains originated from multiple reservoirs and, also, non-appearance of point-source outbreaks. The absence of some human S. Enteritidis PFGE patterns, in particular from food and food animals, suggests that sources other than food animals, such as fresh foods or vegetables, may play a role in clinical illness. Furthermore, some differences among occurring profiles could also be explained by the PulseNet database structure, which is biased towards isolates from outbreak investigations and, consequently, not accurately representative. However, as a strong correlation between SENTXB.0001 and PT4, PT1, and PT6 exists [10], future work involving phage typing could be undertaken in order to further define S. Enteritidis populations in Greece.
Due to the clonal nature of S. Enteritidis [22], the use of two to three additional restriction enzymes for PFGE or combinations of multiple methods like phage typing and MLVA have been proposed when enhanced strain discrimination is needed [9, 10, 23]. BlnI is mainly recommended as the second enzyme for discriminating epidemiologically unrelated strains, as well as partly discriminating XbaI-indistinguishable profiles [8, 24, 25]. Τhis last observation has also been noticed in our study, with 48 different BlnI pulsotypes being generated. The predominant BlnI pulsotype was SENTBL.0007, which was seen in 35.7 % of all isolates. The composite dataset using XbaI and BlnI revealed that the SENTXB.0001–SENTBL.0007 clone was predominant, with an occurrence rate of 12 % (n = 21). Seventeen out of these 21 strains shared the same R-type (nalidixic acid) and were isolated during each year from humans, animals, and foods. This clone was present in Greece throughout the study period and had spread through the food chain among eight different regions in Greece.
MLST has been suggested to be a good discriminatory tool for typing S. Enteritidis [26]. Our results do not support this suggestion, as all the isolates belonged to ST11, which is the most common sequence type for S. Enteritidis [12, 26]. The conserved DNA sequence of the internal fragments of the seven housekeeping genes tested in this study explains the lack of diversity among the strains studied.
The last few years has seen a revolution in the application of whole genome sequencing (WGS) for routine microbiology, as it is replacing current conventional phenotypic and molecular methods [27, 28]. WGS is a promising method that represents the opportunity for a step-change in diagnostic microbiological practice, as it can be used not only for research, but also as an alternative typing method for public health surveillance and outbreak investigation in routine practice [29]. Newer, higher resolution typing methods based on single nucleotide polymorphisms (SNPs) can be/have been developed to discriminate this closely related S. Enteritidis serovar [30]. One of the drawbacks of the current study was that, due to the lack of resources, we were not able to apply WGS methods to conduct the surveillance, but the PFGE and susceptibility data generated showed enough diversity to ensure that future S. Enteritis surveillance should involve both newer WGS-based typing methods and good epidemiological and clinical data to detect sources of infections. This, in turn, will allow the evaluation of control measures to be carried out to reduce resistance in Salmonella, specifically targeting the prudent use of antimicrobial agents by farmers and veterinarians.
References
Issenhuth-Jeanjean S, Roggentin P, Mikoleit M, Guibourdenche M, de Pinna E, Nair S, Fields PI, Weill FX (2014) Supplement 2008–2010 (no. 48) to the White–Kauffmann–Le Minor scheme. Res Microbiol 165(7):526–530. doi:10.1016/j.resmic.2014.07.004
European Food Safety Authority and European Centre for Disease Prevention and Control (2014) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2012. EFSA J 12(2):3547. doi:10.2903/j.efsa.2014.3547
National School of Public Health and Hellenic Center for Disease Control and Prevention (2015) The Greek system for the surveillance of antimicrobial resistance. Home page at: http://www.mednet.gr/whonet/. Accessed 2 Nov 2015
European Food Safety Authority and European Centre for Disease Prevention and Control (2015) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J 13(1):3991. doi:10.2903/j.efsa.2015.3991
Acheson D, Hohmann EL (2001) Nontyphoidal salmonellosis. Clin Infect Dis 32(2):263–269. doi:10.1086/318457
Threlfall EJ, Ward LR, Skinner JA, Graham A (2000) Antimicrobial drug resistance in non-typhoidal salmonellas from humans in England and Wales in 1999: decrease in multiple resistance in Salmonella enterica serotypes Typhimurium, Virchow, and Hadar. Microb Drug Resist 6(4):319–325
Parry CM (2003) Antimicrobial drug resistance in Salmonella enterica. Curr Opin Infect Dis 16(5):467–472
Hyytiä-Trees EK, Cooper K, Ribot EM, Gerner-Smidt P (2007) Recent developments and future prospects in subtyping of foodborne bacterial pathogens. Future Microbiol 2(2):175–185. doi:10.2217/17460913.2.2.175
Hopkins KL, Peters TM, de Pinna E, Wain J (2011) Standardisation of multilocus variable-number tandem-repeat analysis (MLVA) for subtyping of Salmonella enterica serovar Enteritidis. Euro Surveill 16(32). pii: 19942
Peters TM, Berghold C, Brown D, Coia J, Dionisi AM, Echeita A, Fisher IST, Gatto AJ, Gill N, Green J, Gerner-Smidt P, Heck M, Lederer I, Lukinmaa S, Luzzi I, Maguire C, Prager R, Usera M, Siitonen A, Threlfall EJ, Torpdahl M, Tschäpe H, Wannet W, van der Zwaluw WK (2007) Relationship of pulsed-field profiles with key phage types of Salmonella enterica serotype Enteritidis in Europe: results of an international multi-centre study. Epidemiol Infect 135(8):1274–1281. doi:10.1017/S0950268807008102
Barrett TJ, Gerner-Smidt P, Swaminathan B (2006) Interpretation of pulsed-field gel electrophoresis patterns in foodborne disease investigations and surveillance. Foodborne Pathog Dis 3(1):20–31
Achtman M, Wain J, Weill FX, Nair S, Zhou Z, Sangal V, Krauland MG, Hale JL, Harbottle H, Uesbeck A, Dougan G, Harrison LH, Brisse S; S. Enterica MLST Study Group (2012) Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog 8(6):e1002776
Guibourdenche M, Roggentin P, Mikoleit M, Fields PI, Bockemühl J, Grimont PAD, Weill F-X (2010) Supplement 2003–2007 (No. 47) to the White-Kauffmann-Le Minor scheme. Res Microbiol 161(1):26–29. doi:10.1016/j.resmic.2009.10.002
Grimont PAD, Weill F (2007) Antigenic formulae of the Salmonella serovars. World Health Organization Collaborating Center for Reference and Research on Salmonella (ed). Institut Pasteur, Paris
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
Papadopoulos T, Petridou E, Zdragas A, Nair S, Peters T, de Pinna E, Mandilara G, Passiotou M, Vatopoulos A (2015) Phenotypic and molecular characterization of multidrug-resistant Salmonella enterica serovar Hadar in Greece, from 2007 to 2010. Clin Microbiol Infect 21(2):149.e1–149.e4
Medalla F, Hoekstra RM, Whichard JM, Barzilay EJ, Chiller TM, Joyce K, Rickert R, Krueger A, Stuart A, Griffin PM (2013) Increase in resistance to ceftriaxone and nonsusceptibility to ciprofloxacin and decrease in multidrug resistance among Salmonella strains, United States, 1996–2009. Foodborne Pathog Dis 10(4):302–309. doi:10.1089/fpd.2012.1336
European Food Safety Authority and European Centre for Disease Prevention and Control (2014) The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2012. EFSA J 12(3):3590. doi:10.2903/j.efsa.2014.3590
Aarestrup FM, Wiuff C, Mølbak K, Threlfall EJ (2003) Is it time to change fluoroquinolone breakpoints for Salmonella spp.? Antimicrob Agents Chemother 47(2):827–829. doi:10.1128/aac.47.2.827-829.2003
European Committee on Antimicrobial Susceptibility Testing (EUCAST) (2014) European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Home page at: http://www.eucast.org/
European Centre for Disease Prevention and Control (ECDC) (2014) Surveillance of antimicrobial consumption in Europe 2012. ECDC, Stockholm
Allard MW, Luo Y, Strain E, Pettengill J, Timme R, Wang C, Li C, Keys CE, Zheng J, Stones R, Wilson MR, Musser SM, Brown EW (2013) On the evolutionary history, population genetics and diversity among isolates of Salmonella Enteritidis PFGE pattern JEGX01.0004. PLoS One 8(1):e55254. doi:10.1371/journal.pone.0055254
Zheng J, Keys CE, Zhao S, Ahmed R, Meng J, Brown EW (2011) Simultaneous analysis of multiple enzymes increases accuracy of pulsed-field gel electrophoresis in assigning genetic relationships among homogeneous Salmonella strains. J Clin Microbiol 49(1):85–94. doi:10.1128/jcm.00120-10
Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ (2006) Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis 3(1):59–67
Murase T, Nakamura A, Matsushima A, Yamai S (1996) An epidemiological study of Salmonella enteritidis by pulsed-field gel electrophoresis (PFGE): several PFGE patterns observed in isolates from a food poisoning outbreak. Microbiol Immunol 40(11):873–875
Noda T, Murakami K, Asai T, Etoh Y, Ishihara T, Kuroki T, Horikawa K, Fujimoto S (2011) Multi-locus sequence typing of Salmonella enterica subsp. enterica serovar Enteritidis strains in Japan between 1973 and 2004. Acta Vet Scand 53(1):38
Köser CU, Ellington MJ, Cartwright EJP, Gillespie SH, Brown NM, Farrington M, Holden MTG, Dougan G, Bentley SD, Parkhill J, Peacock SJ (2012) Routine use of microbial whole genome sequencing in diagnostic and public health microbiology. PLoS Pathog 8(8), e1002824. doi:10.1371/journal.ppat.1002824
den Bakker HC, Allard MW, Bopp D, Brown EW, Fontana J, Iqbal Z, Kinney A, Limberger R, Musser KA, Shudt M, Strain E, Wiedmann M, Wolfgang WJ (2014) Rapid whole-genome sequencing for surveillance of Salmonella enterica serovar enteritidis. Emerg Infect Dis J 20(8):1306–1314. doi:10.3201/eid2008.131399
Ashton P, Nair S, Peters T, Tewolde R, Day M, Doumith M, Green J, Jenkins C, Underwood A, Arnold C, de Pinna E, Dallman T, Grant K (2015) Revolutionising public health reference microbiology using whole genome sequencing: Salmonella as an exemplar. bioRxiv. doi:10.1101/033225
Quick J, Ashton P, Calus S, Chatt C, Gossain S, Hawker J, Nair S, Neal K, Nye K, Peters T, De Pinna E, Robinson E, Struthers K, Webber M, Catto A, Dallman TJ, Hawkey P, Loman NJ (2015) Rapid draft sequencing and real-time nanopore sequencing in a hospital outbreak of Salmonella. Genome Biol 16(1):114. doi:10.1186/s13059-015-0677-2
Acknowledgments
The authors gratefully acknowledge the personnel of the National Veterinary Reference Centre for Salmonella in Chalkis, Greece, and also the personnel of the National Reference Centre for Salmonella in Vari, Greece, for providing and serotyping all the veterinary and human isolates, respectively. We also thank the administration and personnel of the Veterinary Research Institute (VRI-NAGREF, Thessaloniki) for their for laboratory assistance in the PFGE typing. Acknowledgments are also extended to the Salmonella Reference Service in Colindale, London, UK, for all the laboratory assistance during MLST and for submission to the PulseNet Europe database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No specific funding was used for this research.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This research did not involve human participants and/or animals.
Informed consent
Not applicable.
Rights and permissions
About this article
Cite this article
Papadopoulos, T., Petridou, E., Zdragas, A. et al. Comparative study of all Salmonella enterica serovar Enteritidis strains isolated from food and food animals in Greece from 2008 to 2010 with clinical isolates. Eur J Clin Microbiol Infect Dis 35, 741–746 (2016). https://doi.org/10.1007/s10096-016-2591-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2591-2