Abstract
Cation-dependent inhibition of antimicrobial activity was reported for polymyxin antibiotics. Ca2+ and Mg2+ concentrations recommended by the Clinical and Laboratory Standards Institute (CLSI) for the supplementation of Müller–Hinton broth (MHB) are markedly lower than interstitial space fluid (ISF) concentrations in vivo. Hence, it was speculated that the antimicrobial activity of colistin might be overestimated if tested using conventional cation-adjusted MHB. The antimicrobial activity of colistin against n = 100 clinical isolates of Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae and Escherichia coli (n = 25 each) was evaluated by broth microdilution and, for selected isolates, by time–kill curves, in MHB without cations (MHBONLY), MHB supplemented with 25 mg/L Ca2+ and 12.5 mg/L Mg2+ according to CLSI recommendations (MHBCLSI), and in MHB adjusted to 50 mg/L Ca2+ and 20 mg/L Mg2+ simulating ISF concentrations (MHBISF). The minimum inhibitory concentration (MIC) values of colistin against the vast majority of isolates of both P. aeruginosa and A. baumannii increased significantly with higher cation concentrations. The susceptibility of K. pneumoniae isolates to colistin did not show significant changes between cation-supplemented media, while the MICs of E. coli decreased with ascending cation concentrations. These findings were confirmed in time–kill studies, where colistin killing against P. aeruginosa 1514 and A. baumannii 1485 declined with increasing cation concentrations. Contrarily, the killing of selected concentrations of colistin against K. pneumoniae 15 and E. coli 16 was enhanced in the presence of increasing cation concentrations. The present data suggest that the clinical antimicrobial activity of colistin might be misestimated in vitro if tested in conventional growth media.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colistin (polymyxin E) is a polypeptide antibiotic belonging to the antibiotic class of the polymyxins. In recent years, the clinical use of colistin has greatly increased due to its activity against a variety of multidrug-resistant Gram-negative bacteria, including Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella spp. In analogy to polymyxin B, colistin is believed to interact electrostatically with the outer membrane of Gram-negative bacteria and to competitively displace divalent cations (calcium and magnesium) from the negatively charged phosphate groups of membrane lipids [1], thereby disrupting the integrity of both the outer and the cytoplasmic membrane, and ultimately leading to cell death.
Not surprisingly, increased concentrations of both calcium (Ca++) and magnesium (Mg++) were already shown to hamper or completely abolish the antibacterial efficacy of polymyxins [2–4]. Clinical and Laboratory Standards Institute (CLSI)-recommended cation concentrations for cation-adjusted Müller–Hinton broth (CA-MHB), the standard bacterial growth medium for microbiological susceptibility testing, range from 20 to 25 mg/L for Ca++ and from 10 to 12.5 mg/L for Mg++ [5]. However, reference ranges for ionised extracellular Ca++ and Mg++ concentrations are 46–53 mg/L and 16–26 mg/L, respectively [6]. Thus, while Mg++ concentrations in CA-MHB appear to be quite close to those seen in vivo, disparity between Ca++ concentrations in human interstitial space fluid (ISF) and those in CA-MHB is more evident, with the concentrations in vivo being about twice as high.
In light of these considerations, it seems possible that microbiological tests involving polymyxin antibiotics performed with conventional CA-MHB might overestimate the antimicrobial activity of the tested compounds. However, most of the available data in this context are in regards to polymyxin B. Therefore, the present study set out to investigate the influence of different cation concentrations on the antimicrobial activity of colistin, by both broth microdilution and time–kill studies.
Materials and methods
Colistin sulphate (further referred to as ‘colistin’) was purchased from Sigma-Aldrich, Vienna, Austria. A total of 100 clinical isolates of Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae and Escherichia coli (n = 25 for each bacterial species) were kindly provided by the Clinical Division of Clinical Microbiology, Clinical Institute of Laboratory Medicine, Medical University of Vienna.
Growth media
MHB without cations was purchased from Sigma-Aldrich, Vienna, Austria, and was used as a basis for the preparation of bacterial growth media. All experiments were conducted in three sets of growth media containing different calcium (Ca2+) and magnesium (Mg2+) concentrations. One stock of pure MHB was left void of cations and will be further referred to as MHBONLY in the manuscript. For the preparation of cation-adjusted MHB according to CLSI standards (further referred to as MHBCLSI), CaCl2 and MgCl2 solutions were added to give final Ca2+ and Mg2+ concentrations of 25 and 12.5 mg/L, respectively. For the preparation of MHB meant to simulate ISF cation concentrations (further referred to as MHBISF), Ca2+ and Mg2+ levels were adjusted to 50 and 20 mg/L, respectively.
Broth microdilution
The minimal inhibitory concentrations (MICs) of colistin against 100 clinical isolates of P. aeruginosa, A. baumannii, K. pneumoniae and E. coli (n = 25 for each species) were determined in non-treated Nunc™ 96-well round bottom polystyrene microwell plates by a broth microdilution method in accordance with CLSI recommendations. Experiments were conducted in duplicate in: (I) MHBONLY, (ii) MHBCLSI and (iii) MHBISF, as specified above.
Time–kill studies
The antibacterial activity of different concentrations of colistin against selected isolates which showed the most prominent increase in MIC from MHBCLSI to MHBISF was investigated in time–kill studies. As in microdilution, the employed media were: (I) MHBONLY, (ii) MHBCLSI and (iii) MHBISF. Colistin concentrations were chosen to cover the full MIC range observed in microdilution testing for the respective organism. Experiments were performed in triplicate.
Results
Microdilution testing
The mean ± standard deviation (SD) colistin MIC values against the tested strains, including ratios between MICs in the respective media, are summarised in Table 1. The activity of colistin against P. aeruginosa isolates clearly decreased in the presence of ascending cation concentrations. The mean colistin MIC values increased significantly from 1.50 ± 0.71 mg/L in MHBCLSI to 2.72 ± 0.98 mg/L in MHBISF (p = <0.001), reflecting cation-dependent MIC increases of at least one dilution step in the vast majority of isolates. For two isolates, the MICs were identical between MHBCLSI and MHBISF, and only in two cases was the colistin activity higher in MHBISF than in MHBCLSI. The colistin activity in MHBONLY (mean MIC = 0.49 ± 0.42 mg/L) was superior to the two cation-supplemented test media in all but two cases.
A similar cation-dependent inhibition of the colistin activity was observed in the assays with A. baumannii. Against the majority of tested isolates, colistin MICs rose by at least one dilution step with increasing cation concentrations, leading to mean MIC values of 1.19 ± 0.74 mg/L in MHBCLSI and 2.52 ± 3.05 mg/L in MHBISF. In analogy to P. aeruginosa, the difference in MICs between MHBCLSI and MHBISF was statistically significant (p = <0.001). In four cases, the MIC did not change between the two cation-supplemented media, and two isolates displayed higher MICs in MHBCLSI than in MHBISF. Again, the colistin activity was constantly higher in MHBONLY (mean MIC = 0.39 ± 0.23 mg/L) than in MHBCLSI and MHBISF.
The colistin activity against K. pneumoniae was highly variable between the tested isolates and seemed virtually unaffected by differences in cation concentrations. The mean colistin MIC values did not change significantly between the two cation-supplemented growth media (p = 0.076).
A different pattern emerged for E. coli. Here, in contrast to the results observed for P. aeruginosa and A. baumannii, the colistin activity showed a linear increase with ascending cation concentrations. Accordingly, the mean MIC values were highest in MHBONLY and lowest in MHBISF, with a statistically significant drop in the MIC from MHBCLSI and MHBISF (p = 0.039).
Time–kill studies
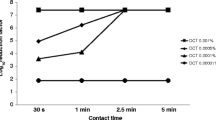
P. aeruginosa isolate 1514, A. baumannii isolate 1485, K. pneumoniae isolate 15 and E. coli isolate 16 were chosen to test the killing activity of colistin over time. The MIC values of the selected bacteria in different test media and colistin concentrations evaluated in time–kill experiments against the respective organisms are shown in Table 2. Against P. aeruginosa 1514, colistin showed a distinct pattern of reduced bacterial killing after 5 h of exposure to increasing cation concentrations (Fig. 1a). This effect was most prominent at the highest concentration tested (8 mg/L), where colistin killing was reduced by 3 log steps in MHBISF compared to MHBCLSI. This difference in activity could be observed to a lesser degree also at 4 mg/L, where colistin killing was reduced by approximately 1 log step in MHBISF. At 1 mg/L, colistin had virtually no activity in MHBISF, while it retained at least some effect in MHBCLSI, allowing only a slight growth. At the lowest concentration tested, colistin was ineffective against P. aeruginosa 1514 in both cation-supplemented media (not shown).
A similar pattern emerged for A. baumannii 1485 (Fig. 1b). Here, however, divergence in killing was highest at colistin concentrations of 3 and 1 mg/L. In both cases, the reduction in colistin killing after 5 h of incubation in MHBISF compared to MHBCLSI amounted to approximately 3 log steps. At 3 mg/L, colistin attained a colony count reduction of almost 4 log steps in MHBCLSI compared to 1 log step in MHBISF. At 1 mg/L, colistin was still bactericidal in MHBCLSI, while in MHBISF, it hardly achieved growth inhibition. At 0.2 mg/L, colistin was ineffective against the tested organism in all three media (not shown). Against both P. aeruginosa 1514 and A. baumannii 1485, colistin performed best in MHBONLY, with killing activity equal or superior to that seen in the two cation-supplemented media.
Contrarily, the killing of colistin against K. pneumoniae 15 seemed to improve with increasing cation concentrations (Fig. 2a). At the highest tested concentration (8 mg/L), colistin was rapidly bactericidal in MHBISF, achieving complete and sustained killing already at 2 h. In comparison hereto, at 5 h, killing was reduced by 3 and 6 log steps in MHBCLSI and MHBONLY, respectively. However, this discrepancy between the different media was less evident at 2 mg/L of colistin, where reduction in colony counts was only slightly superior in MHBISF compared to MHBCLSI and MHBONLY. At the two lowest concentrations, colistin was ineffective against K. pneumoniae 15 in all growth media, with colony counts identical to growth control.
Against E. coli 16, 4 mg/L of colistin were also rapidly bactericidal in MHBISF, with sterile conditions achieved at 5 h, while killing was reduced by 3 and 6 log steps in MHBCLSI and MHBONLY, respectively (Fig. 2b). Colistin concentrations of 1 mg/L still achieved a more than 3 log step colony count reduction in MHBISF and MHBCLSI, while they were ineffective in MHBONLY. Lower colistin concentrations did not show any activity against E. coli 16 in any of the employed media.
Throughout all experiments, the growth curves of all investigated organisms were not affected by differences in cation concentrations in the employed test media.
Discussion
The present study investigated the influence of different cation concentrations on the antibacterial activity of colistin as determined by broth microdilution and time–kill studies. Colistin activity was observed to decline markedly in the presence of higher cation concentrations when tested against the vast majority of P. aeruginosa and A. baumannii isolates, whereas, on the contrary, increasing cation concentrations partly enhanced colistin’s activity against K. pneumoniae and E. coli.
In general, in vitro susceptibility tests are performed in order to estimate the ability of an antimicrobial drug to treat infections caused by certain pathogens in vivo. The data presented here demonstrate that, due to cation-dependent changes in the antimicrobial activity of colistin for tissue, these estimates might not hold true, depending on the bacterial species.
The results observed for P. aeruginosa and A. baumannii indicate that colistin’s antibacterial activity against these two species in vivo might be overestimated by using conventional in vitro growth media. Importantly, it must be mentioned that colistin MIC values against P. aeruginosa did not exceed the current breakpoint of 4 mg/L, even for those isolates with the strongest cation-dependent loss in susceptibility. In contrast, for A. baumannii, in some cases, changes in the MIC between MHBCLSI and MHBISF did determine a breakpoint shift from susceptible to resistant, i.e. the MIC values of five A. baumannii isolates were above the currently established breakpoint of 2 mg/L when tested in MHBISF but would have been classified as susceptible in MHBCLSI. This might be of clinical relevance since the selection of colistin to treat a pathogen only allegedly susceptible in vitro, but resistant in vivo, could lead to treatment failure and promote resistance. Based on the present data, supplementation of employed growth media with cation concentrations adjusted to values of human ISF would seem reasonable at least for P. aeruginosa and A. baumannii.
Data derived from investigations with K. pneumoniae and E. coli point to a different direction. In time–kill studies against both species, selected concentrations of colistin showed a linear augmentation of antibacterial killing in the presence of ascending cation concentrations. While for E. coli microdilution experiments had already anticipated a pattern of cation-dependent increase in activity, colistin MIC values for K. pneumoniae were apparently unaffected by different cation concentrations. However, the mentioned variability of MIC values between K. pneumoniae isolates might have masked a de facto existing trend. These results, in overt contrast to those observed for P. aeruginosa and A. baumannii, are quite unexpected. While a cation-dependent inhibition of colistin’s activity correlates well with the current understanding of the drug’s mechanism of action, the cation-dependent augmentation of activity observed against K. pneumoniae and E. coli seems to be lacking an underlying explanation, and highlights the need for further investigations. However, the strikingly discrepant results observed for P. aeruginosa and A. baumannii on the one hand and K. pneumoniae and E. coli on the other hand suggest that the influence of divalent cations on the antimicrobial activity of colistin might be species-dependent. If confirmed, these findings might also require additional precautions in association with the antimicrobial susceptibility testing of colistin.
The need for a revision and adaptation of the current recommendations regarding antimicrobial susceptibility testing of colistin has been highlighted previously. Colistin is a polycationic molecule known to adhere to inorganic materials, including labware, which might result in considerable loss of the compound during experimental conditions [7]. In accordance with this hypothesis, the results of colistin broth microdilution experiments have been shown to differ significantly if conducted in microtitre plates with differently coated wells [8]. In analogy, colistin MICs against relevant pathogens have been observed to decline in the presence of Polysorbate 80, a surfactant often used as a dispersing agent in broth microdilution panels [8, 9]. Finally, comparison of the broth microdilution method with the Etest and the agar dilution method against selected multidrug-resistant Gram-negative organisms showed significant variability between the observed MIC results [10]. In light of the fact that the broth microdilution assay is currently the only method recommended by the CLSI and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for colistin antimicrobial susceptibility testing, incorporation of the above-mentioned factors in a revised edition of methodological recommendations for colistin antimicrobial susceptibility testing should be encouraged.
As reflected by the large SDs, some of the data presented here are affected by high variability. Also, even though a considerable number of clinical isolates was included, these experiments cannot claim to be representative for the totality of the populations of the respective bacterial strains. Moreover, it might be questioned as to what extent experiments in an artificial growth medium like MHB are representative for conditions in the human interstitial space in vivo. Nevertheless, these experiments have shown that the antibacterial activity of colistin is cation-dependent, and might be over- or underestimated in a species-dependent manner if tested in conventional, cation-poor growth media. Together with other recent reports, the findings of this study indicate the need for the adaptation of methodological recommendations regarding colistin antimicrobial susceptibility testing.
References
Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K (2005) Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents 25(1):11–25. doi:10.1016/j.ijantimicag.2004.10.001
Chen CC, Feingold DS (1972) Locus of divalent cation inhibition of the bactericidal action of polymyxin B. Antimicrob Agents Chemother 2(5):331–335
Davis SD, Iannetta A, Wedgwood RJ (1971) Activity of colistin against pseudomonas aeruginosa: inhibition by calcium. J Infect Dis 124(6):610–612
Newton BA (1953) Reversal of the antibacterial activity of polymyxin by divalent cations. Nature 172(4369):160–161
Clinical and Laboratory Standards Institute (CLSI) (2014) Performance standards for antimicrobial susceptibility testing; Twenty-fourth informational supplement. CLSI document M100-S24. CLSI, Wayne, PA
Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Vienna (2014) Parameter catalogue, reference values. Home page at: http://www.kimcl.at
Karvanen M, Malmberg C, Mohamad A, Lagerbäck P, Friberg LE, Cars O (2011) Colistin is extensively lost during normal experimental conditions. Paper presented at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Chicago, IL, September 2011
Albur M, Noel A, Bowker K, Macgowan A (2014) Colistin susceptibility testing: time for a review. J Antimicrob Chemother 69(5):1432–1434. doi:10.1093/jac/dkt503
Sader HS, Rhomberg PR, Flamm RK, Jones RN (2012) Use of a surfactant (polysorbate 80) to improve MIC susceptibility testing results for polymyxin B and colistin. Diagn Microbiol Infect Dis 74(4):412–414. doi:10.1016/j.diagmicrobio.2012.08.025
Hindler JA, Humphries RM (2013) Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant Gram-negative bacilli. J Clin Microbiol 51(6):1678–1684. doi:10.1128/JCM.03385-12
Conflict of interest
Peter Matzneller declares that he has no conflict of interest.
Sabine Strommer declares that she has no conflict of interest.
Zoe Österreicher declares that she has no conflict of interest.
Dieter Mitteregger declares that he has no conflict of interest.
Markus Zeitlinger declares that he has no conflict of interest.
Compliance with ethical requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matzneller, P., Strommer, S., Österreicher, Z. et al. Target site antimicrobial activity of colistin might be misestimated if tested in conventional growth media. Eur J Clin Microbiol Infect Dis 34, 1989–1994 (2015). https://doi.org/10.1007/s10096-015-2441-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-015-2441-7