Abstract
Chlamydia pneumoniae causes diseases in humans, including community-acquired pneumonia, bronchitis, and sinusitis. It is also associated with atherosclerosis, coronary heart disease, and hyperlipidemia. In this study, we investigated novel materials with which to develop a sensitive and specific method to identify early C. pneumoniae infection, to allow more effective clinical treatment and prevention. We prepared novel monoclonal antibodies (mAbs) against a recombinant protein equivalent to the immunodominant region of chlamydial protease-like activity factor (CPAF) from C. pneumoniae. The mAbs specifically reacted with the endogenous CPAF antigen of the C. pneumoniae type strain in immunoblotting and indirect immunofluorescence (IIF) assays, but did not react with C. trachomatis type strains or genital secretions from patients with acute C. trachomatis infection. The mAb with the highest titer was used to develop a new IIF assay and enzyme-linked immunosorbent assay (ELISA) to detect the C. pneumoniae antigen in clinical specimens from child patients suspected of pneumonia. The sensitivity, specificity, and concordance rate of the mAb-based IIF and ELISA tests were compared with those of polymerase chain reaction (PCR). Our results show that these mAbs have excellent specificity and may be used to develop new screening tools for the diagnosis of early pediatric pneumonia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chlamydia pneumoniae causes diseases in humans, accounting for approximately 10 % of community-acquired pneumonia and 5 % of bronchitis and sinusitis in adults and children [1, 2] C. pneumoniae-induced respiratory infections are often asymptomatic or mild, although severe pneumonia can develop in older patients and those with coexisting cardiopulmonary disease. C. pneumoniae may also be a risk factor for lung cancer [3]. Seroepidemiological studies have shown antibody prevalence of 50–70 %, suggesting a high frequency of previous infections. Nearly all humans can expect to be infected with C. pneumoniae at least once in their lifetimes. Reinfection is common, and persistence of the agent in the host after the primary infection is a potential risk factor for chronic infection. The associations between C. pneumoniae and atherosclerosis and coronary heart disease have attracted the attention of many investigators [4, 5]. The bacterium appears to be associated with hyperlipidemia and might, therefore, represent a further risk factor for cardiovascular disease in human immunodeficiency virus (HIV)-positive patients [6].
The laboratory methods currently used for the diagnosis of acute C. pneumoniae infection include culture, serological assays [including microimmunofluorescence (MIF) and enzyme-linked immunosorbent assay (ELISA)] and polymerase chain reaction (PCR), although the latter two are the most frequently used. However, it is still difficult to reliably diagnose C. pneumoniae infection because standardized and commercially available diagnostic tests that are both sensitive and specific are rare. Although infections with C. pneumoniae can be identified by the direct isolation of the agent, it is difficult to culture this chlamydial species in vitro, because the culture procedure for C. pneumoniae is laborious and time-consuming. The MIF test is currently considered the “gold standard” for the serodiagnosis of C. pneumoniae infection. However, the need for paired serum samples and the extended persistence of immunoglobulin G (IgG) antibodies in some adult populations make this test retrospective in nature and unsuitable for an early diagnosis [7]. MIF testing is also hampered by poor specificity because of cross-reaction with other chlamydial species. ELISA is often used to diagnose C. pneumoniae infection because the results can be interpreted objectively, and it is convenient and rapid [8]. However, some cross-reactions occur among chlamydial species because the antigens mainly used are whole C. pneumoniae bacteria or antigens common to the genus Chlamydia, such as the major outer membrane protein (MOMP), outer membrane protein 2, and lipopolysaccharide [9, 10]. Molecular-analysis-based assays, such as PCR, have recently been developed for the rapid and sensitive detection of C. pneumoniae. However, the overall diagnostic utility of PCR-based assays is currently limited by the lack of type specimens, an appropriate nucleic acid extraction method, standardized amplification protocols, and the special instruments and trained professional personnel required [2].
In general, methods that detect pathogenic antigens are more direct and rapid than serological diagnostic methods in identifying the causes of infections, allowing early and effective treatment or disease prevention. Therefore, it is extremely important to identify new C. pneumoniae antigens, especially potentially immunogenic peptides, that can be used to develop highly sensitive and specific assays for the early diagnosis of C. pneumoniae infections.
Chlamydial proteasome/protease-like activity factor (CPAF), encoded in the chlamydial genome, is secreted into the host cell cytosol and has proteolytic activity that is important during chlamydial infection [11]. CPAF is immunogenic during human infection with live chlamydial bacteria. CPAF-specific antibodies are produced during CPAF synthesis, which requires the replication of live chlamydial organisms in chlamydia-infected cells. Humans infected with chlamydia produce a dominant antibody response to CPAF [12]. Therefore, CPAF can be used as an effective immunodominant antigen with which to diagnose early C. pneumoniae infection. Zhong et al. previously reported that a chlamydia-secreted protein, designated CPAF, is present in the cytosol of chlamydia-infected cells. They reported that CPAF does not preexist in chlamydial organisms and that the replication of the live organism is required for CPAF synthesis in cultured cells [11]. Mice inoculated with live chlamydial organisms produced a strong antibody response to CPAF, whereas mice inoculated with dead chlamydial organisms did not, and CPAF-specific antibody production correlated with CPAF synthesis in the animals. That study showed that the levels of CPAF antibodies were higher than antibodies directed against either chlamydial MOMP or heat shock protein 60 (HSP60) in humans infected with chlamydia. Specific anti-CPAF antibodies in human sera can neutralize endogenous CPAF and its glutathione S-transferase (GST) fusion protein [12], suggesting that CPAF is both produced and immunogenic during human chlamydial infection. All known chlamydial organisms produce CPAF, and the CPAF gene is highly conserved among chlamydial strains, although it has no significant overall homology with any other known gene. The intraspecies identity of the CPAF protein is as high as 99 % and the interspecies identity is as low as 46 % [13]. Zhong et al. demonstrated that CPAF produced by C. pneumoniae consists of 619 amino acids, and its middle region, covering residues 200–338, is the immunodominant region, which is predominantly recognized by human neutralizing antibodies [14].
It is well known that a monoclonal antibody (mAb) directed against an antigen can distinguish and specifically combine with that antigen. Therefore, mAbs have become a new focus of research into the prevention and diagnosis of pathogenic infections [15, 16]. We produced mAbs directed against a recombinant protein containing the immunodominant region epitopes of CPAF (designated “CPAFm”) from C. pneumoniae. The mAb with the highest titer was selected to develop a new indirect immunofluorescence (IIF) assay and indirect ELISA. These assays were used to detect the C. pneumoniae type strain and to analyze sputum pharyngeal swabs from pediatric patients suspected of C. pneumoniae infection, respectively. We also assessed the diagnostic value of the new IIF assay and ELISA by comparing their results with the detection of C. pneumoniae by PCR in the same samples. We thus established several novel materials to develop a method that is both sensitive and specific for the rapid and specific identification of early C. pneumoniae infections.
Materials and methods
Bacterial strains and clinical samples
The C. pneumoniae type strain CWL029 and its genome template and the C. trachomatis D, F, and I type strains were gifts from Prof. G. M. Zhong at the Department of Microbiology and Immunology, University of Texas Health Science Center, San Antonio, TX, USA. HeLa cells, Escherichia coli JM109 and BL21, mouse myeloma cells (SP2/0), and the expression vector pGEX6p-2 are stored in our laboratory. Clinical sputum pharyngeal swab samples (n = 300) were obtained from symptomatic pediatric patients who had a persistent dry cough, moderate or slight fever, even slight bronchial rale, and X-ray-confirmed pneumonia between January, 2010 and May, 2013 in the pediatric departments of four affiliated hospitals in China. No patients had serological evidence of C. trachomatis infection. Normal sputum pharyngeal swab samples (n = 120) were collected from children with no reported illness or symptoms, who had undergone a medical examination in the physical examination center. Forty genital secretion samples were collected from patients with acute C. trachomatis infection, who had positive results on urethral or endocervical C. trachomatis DNA amplification with the AMPLICOR test (Roche Diagnostic Systems, USA). None of these patients had serological evidence of C. pneumoniae infection. Parents/guardians of pediatric patients gave their informed consent.
Production, purification, and identification of recombinant protein GST–CPAFm
The recombinant and purification of recombinant protein GST–CPAFm were as according to Zheng et al. [17]. The gene sequence encoding the CPAFm immunodominant epitopes of CPAF (amino acids 181–400) (gi: 15617929; bp 1,167,582–1,168,241) was selected, and amplification primers were designed. The sense primer sequence was 5′-CCGGGATCCGATGCGGTTCCTTCAG-3′, and the antisense primer sequence was 5′-TTGCGGCCGCTTAGACTTCATCCTGAGTG-3′. The purified PCR products were subcloned into the expression vector pGEX6p-2 and transferred into the bacterium E. coli BL21. The expression products were analyzed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
The induced product from whole bacteria and the purified protein were both identified with SDS-PAGE and immunoblotting assays. C. pneumoniae IgG antiserum from patients was used as the primary antibody (confirmed with commercially available reagents for C. pneumoniae MIF from EUROIMMUN Medizinische Labordiagnostika AG, Germany). Coomassie Brilliant Blue solution and the enhanced chemiluminescence detection system were used to visualize specific straps.
Production of mAbs
To produce the mAbs, 4-week-old BALB/c mice were subcutaneously immunized with 50 mg of the prepared recombinant protein in complete Freund’s adjuvant. The production of mAbs was according to Fazekas de St Groth and Scheidegger [18]. The cutoff value (COV), defined as the mean A 450 value (mean = 0.10) plus two standard deviations (s = 0.051) for the 120 donor sera, was calculated to be 0.202. The A 450 per well was determined three times and the mean value calculated. The supernatants of the growing hybrids were considered positive if the mean A 450 value was more than the COV, which implied that the supernatants contained GST–CPAFm antibodies. If A 450 > COV, the result was negative and the sample contained no anti-GST–CPAFm antibodies. The purification effect was determined with an immunoblotting analysis with the C. pneumoniae IgG antiserum described above as the primary antibody.
Biological characteristics of mAbs
The isotype of each antibody was determined with the Mouse Monoclonal Antibody Isotyping Kit (Sigma Chemical Co.). The specificity of each antibody was identified with an IIF test and an immunoblotting analysis based on the C. pneumoniae type strain. The affinity constant was determined with the method described by Beatty et al. [19]. The Ultramicro Analytical System was slightly modified. The Ultramicro ELISA plates were sensitized with four concentrations of GST–CPAFm: 0.5, 1, 2, and 4 mg/mL. Every mAb was used at the highest and lowest dilutions, ranging from 2 to 0.007 mg/mL.
Identification of mAb specificity and construction of a new IIF test
HeLa cells were cultured with Dulbecco’s Modified Eagle Medium (DMEM) containing 10 % fetal bovine serum (Sigma Chemical Co.) and infected with the C. pneumoniae type strain CWL029, as described above. The supernatants were collected from the infected HeLa cells lysed with SDS cell lysis buffer (Beyotime Institute of Biotechnology) and diluted to use as the antigens. One of the mAbs was used as the primary antibody.
To determine the specificity of the mAbs, a new indirect IIF assay was developed as described below. Briefly, the membranes of infected HeLa cells were disrupted with 2 % bovine serum albumin (BSA; Gibco BRL, Rockville, MD) solution containing 0.1 % Triton X-100. The supernatants, as antigens, were added to clean microscope slides (15 holes). In one group, each of the four mAbs (dilutions ranging from 1:100 to 1:5,000) was used as the primary antibody and added to 12 parallel antigen samples (each mAb was tested in triplicate), and incubated for 30 min in a moist chamber at 37 °C. Another group of samples, in which a specific mouse mAb directed against C. pneumoniae MOMP (diluted 1:2,000; Sigma) was used as the primary antibody, was the positive control. PBS was used as the negative control, and no mAb was added to another three parallel samples as the blank control in the procedure described above. After the slides were washed three times with PBS, the mouse mAbs were visualized with a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody (Sigma, 1:5,000). The mAb produced with the strongest response was selected as the primary antibody to detect the pathogen in clinical samples.
Application of the new IIF test
The IIF method developed was used to analyze 120 normal sputum pharyngeal swab samples and 300 clinical sputum pharyngeal swab samples lysed with SDS cell lysis buffer (Beyotime Institute of Biotechnology). The GST–CPAFm-specific mouse mAb with the highest titer was selected as the primary antibody.
A commercially available PCR-based assay (DaAn Gene Technology Corp., Sun Yat-Sen University, China) was used to isolate and detect C. pneumoniae DNA in the clinical sputum pharyngeal swab samples, according to the manufacturer’s instructions. This real-time fluorescent method for the quantitative detection of C. pneumoniae and the kit are based on the amplification of the conserved region of the MOMP gene of C. pneumoniae, followed by the colorimetric detection of the amplification products on microplates coated with specific probes. The regions targeted by the primers and probes are in the gene encoding the MOMP protein of C. pneumoniae. The forward primer sequence was 5′-gctactggaacaaagtctgcga-3′ and the reverse primer sequence was 5′-cattgtactccaatgtatggcacta-3′. The probe sequence is 5′-ttatcatgaatggcaagtaggagcctctctatctt-3′. The diagnostic specificity of this PCR assay is highly consistent with that of the “gold standard” MIF test, according to the specifications supplied by the manufacturer.
The sensitivity and specificity of the IIF method developed here were then calculated from the experimental results. The differences between the two methods (IIF and PCR) were measured with a χ2 analysis and p < 0.05 was considered significant. The prevalence of patients infected with C. pneumoniae was analyzed.
Construction of a new indirect ELISA
The membranes of HeLa cells infected with C. pneumoniae strain CWL029 were broken, and the supernatant containing the antigen to be detected was used as the positive control. Normal sputum pharyngeal swab samples were used as the negative controls. The GST–CPAFm-specific mouse mAb with the highest titer was used as the primary antibody. The collected supernatants were diluted as the antigen to be detected and used to coat enzyme immunoassay microtiter plates (Costar, Cambridge, MA) for 2 h at 37 °C or at 4 °C overnight. The wells were then washed with phosphate-buffered saline with Tween 20 (PBST). After the binding sites of other antigens were blocked with PBST containing 5 % BSA (100 μL; Gibco), the mAb (diluted 1:100) was then added to the wells. The mixtures were incubated for 2 h at 37 °C with shaking, and the wells were then washed with PBST. Horseradish peroxidase-conjugated goat anti-mouse IgG antibody (Sigma) was diluted 1:3,000 in PBST containing 3 % BSA (Gibco) and added to each well. After incubation for 1 h, the wells were washed with PBST, and then 100 μL of the substrate solution was added. The substrate solution contained 0.4 % TMB and 0.02 % hydrogen peroxide in phosphate citrate buffer (pH 5.0). After incubation for 10 min, the reaction was terminated by the addition of 100 μL of 2-M H2SO4 solution and the A 450 was measured. Each sample was tested in triplicate. The COV used to assign the results was defined as the mean + 2SD of the absorbance of 120 normal samples.
Application of the new indirect ELISA
The PCR-based assay described above was used as the reference test. The newly developed ELISA based on the GST–CPAFm-specific mouse mAb was used to test 300 sputum pharyngeal swab samples. These samples were decomposed in 0.2-M NaHCO3 (pH 9.6) solution, as described previously [20], and then adsorbed onto enzyme immunoassay microtiter plates (Costar) for 2 h at 37 °C. The sensitivity, specificity, and concordance rate of the indirect ELISA were calculated from the test results. The differences between the two methods were measured with the χ2 test and p < 0.05 was considered significant. The incidence rates of patients infected with C. pneumoniae were analyzed.
Cross-reaction with C. trachomatis
The C. trachomatis D, F, and I type strains and 40 genital secretions from patients with acute C. trachomatis infection were tested with the new IIF assay and ELISA to detect C. pneumoniae antigen. A positive result implied that the test cross-reacted with C. trachomatis.
Results
Expression and purification of recombinant protein
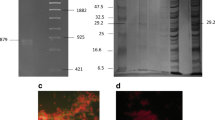
The recombinant plasmid constructed to express the target protein was confirmed with PCR and restriction enzyme analysis to contain the CPAFm gene. The specific fragment was observed with 1 % agarose gel electrophoresis. The size of the PCR amplification product was about 700 bp, consistent with the anticipated fragment size (Fig. 1a). A BLAST search demonstrated that the DNA sequence of the inserted fragment was identical to the target gene registered in GenBank.
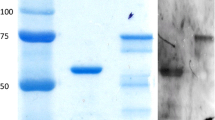
Expression and purification of recombinant protein. a 1.0 % agarose gel electrophoresis analysis of the PCR product: M 100-bp DNA marker; 1 PCR product (714 bp); 2 negative control. b Expression of GST–CPAFm at various induction temperatures: M protein marker; 1 induced E. coli BL21(DE3); 2 uninduced E. coli BL21(DE3) with pGEX6p-2/CPAFm; 3 induced E. coli BL21(DE3) with pGEX6p-2/CPAFm at 37 °C; 4 induced E. coli BL21(DE3) with pGEX6p-2/CPAFm at 24 °C. c SDS-PAGE analysis of purified GST–CPAFm: 1, 2 purified GST–CPAFm; M protein marker. d Immunoblotting analysis of recombinant GST–CPAFm protein: 1 induced E. coli BL21(DE3) cells with pGEX6p-2/CPAFm; 2 uninduced E. coli BL21(DE3) cells with pGEX6p-2/CPAFm; 3 purified recombinant protein GST–CPAFm
The analysis of E. coli BL21 cells containing the recombinant plasmid clearly showed a new protein band on a Coomassie Brilliant Blue-stained gel compared with the control cells (Fig. 1b). Its molecular weight was about 51.3 kDa (about 25 kDa for CPAFm and 26 kDa for GST) and was identical to the calculated molecular weight. The expression product formed inclusion bodies in E. coli at 37 °C and 24 °C. After purification, the expressed protein showed only a clear 51.3-kDa band, with no other band visible, and a purity of 95 % on 10 % SDS-PAGE (Fig. 1c). The protein concentration was 1.365 mg/mL, calculated with the A 280 UV absorbance method.
An immunoblotting assay demonstrated that the expressed recombinant GST–CPAFm protein reacted with human anti-C. pneumoniae antiserum (Fig. 1d). Only one specific immunoreactive band of CPAFm protein (molecular weight ~25 kDa) was clearly visible, but was not observed in the negative control.
Biological characteristics of mAbs
The four hybridomas were cloned three times with the limiting dilution method and designated 3F8, 7B9, 8C4, and 11B5. The titers of the ascites produced by these four hybridomas were 1:28,000, 1:16,000, 1:38,000, and 1:12,000, respectively. The isotypes of the mAbs secreted by the 3F8 and 7B9 strains were IgG2b and those of the other two were IgG1. The affinities of these four mAbs are shown in Table 1; 8C4 showed higher affinity than the other mAbs (Kaff = 4.98 × 108 M−1).
Specificity of GST–CPAFm mouse mAb tested with immunoblotting and IIF assays
The reaction specificity of each mAb was examined with IIF based on the C. pneumoniae type strain CWL029, and the assay results are shown in Table 2. Compared with the positive and negative controls, these mAbs reacted strongly with the endogenous CPAF antigen from the type strain. Although the other mAbs displayed good immunoreactivity, 8C4 showed the highest titer and the best reactivity, so it was selected to test the clinical specimens.
An immunoblotting analysis was used to confirm the specificity of the mAbs produced. HeLa cells infected with the C. pneumoniae type strain CWL029 were used as the antigen to be detected and each of the mAbs was used individually as the primary antibody. The mAbs excreted by the four hybridomas all combined with the endogenous 25-kDa CPAFm protein and the specific reaction bands are shown in Fig. 2. The internal control for the primary antibody was glyceraldehyde 3-phosphate dehydrogenase.
Clinical tests of the new IIF method
When mAb 8C4 was used to probe normal sputum pharyngeal swab samples as the negative controls, the cells in these samples were not labeled (Fig. 3a). In contrast, HeLa cells infected with the type strain were clearly fluorescent when treated with the same antibody preparation (Fig. 3b). When the same mAb was used to test the sputum pharyngeal swab samples from patients possibly infected with C. pneumoniae, the cells in these samples were also clearly fluorescent (Fig. 3c). The prevalence rates of infection in patients with respiratory disease who were possibly infected with C. pneumoniae were analyzed in two cities, Shanghai and Hengyang, in China. Three hundred specimens were tested for C. pneumoniae antigen simultaneously with PCR using commercial reagents and with the IIF test. The results are shown in Table 3. The sensitivity and specificity of the IIF test when compared with PCR were 88.89 % (48/54) and 90.24 % (222/246), respectively. When the differences between the two methods were measured with the χ2 test, they were not significantly different (p = 0.088). The prevalence rates for C. pneumoniae in the 300 patient specimens determined with IIF and PCR were 24 % (72/300) and 18 % (54/300), respectively. The concordance rate for the two methods in detecting C. pneumoniae antigen was 90.00 % [(48 + 222)/(72 + 228)].
IIF results with 8C4 mAb as the primary antibody (original magnification, ×400). a IIF staining of normal sputum pharyngeal swab samples. b IIF staining of HeLa cells infected with the C. pneumoniae type strain. c IIF staining of a sputum pharyngeal swab sample from a patient possibly infected with C. pneumoniae
Clinical application of the new ELISA
In this study, the COV for the ELISA based on the mAb directed against CPAFm (8C4) was calculated from 120 normal samples to be 0.316. The results of the indirect ELISA revealed that all absorbances were less than 0.300. C. pneumoniae antigen was detected simultaneously in 300 specimens with the commercial PCR reagents used and the new ELISA method based on mAb 8C4. The results are shown in Table 4. The sensitivity and specificity of the ELISA compared with PCR were 87.0 % (47/54) and 94.71 % (233/246), respectively. When the difference between the two methods was measured with the χ2 test, they were not significantly different (p =0.603). The prevalence rates for C. pneumoniae in the 300 patient specimens measured with the new indirect ELISA and PCR were 20 % (60/300) and 18 % (54/300), respectively. The concordance rate of the two methods in detecting C. pneumoniae antigen was 93.33 % [(47 + 233)/(60 + 240)].
Cross-reaction with C. trachomatis
The new IIF and ELISA methods for the detection of the endogenous CPAF antigen of C. pneumoniae based on mAb 8C4 were used to detect the C. trachomatis D, F, and I type strains and to test 40 genital secretion samples from C. trachomatis-infected patients. No result was positive, indicating that mAb 8C4 does not cross-react with C. trachomatis.
Discussion
The tests available for the serological diagnosis of C. pneumoniae infection are extensive and are frequently used. However, there is currently no perfectly satisfactory serological method for the diagnosis of C. pneumoniae infection. Detecting an antigen specific to the pathogen is a more direct and quicker method than serological diagnostic strategies to identify the cause of an infection. In general, culturing the pathogen or detecting an antigen of the live pathogen and its gene are used as direct evidence upon which to base the diagnosis of a human pathogenic infection. Therefore, the identification of new C. pneumoniae antigens should be useful and important for the development of highly sensitive and specific diagnostic methods for the detection of early C. pneumoniae infections. Based on the principle that an mAb directed against an antigen is highly specific, an mAb prepared against C. pneumoniae CPAF was used to diagnose infections caused by this pathogen to improve the specificity of the diagnostic method.
We successfully amplified the immunodominant region of the CPAF gene from C. pneumoniae and cloned it into the pGEX6p-2 vector. The recombinant protein GST–CPAFm expressed in induced E. coli BL21 cells and purified GST–CPAFm were identified with SDS-PAGE and immunoblotting assays. The results showed that GST–CPAFm reacted with C. pneumoniae IgG antisera from patients diagnosed with C. pneumoniae pneumonia, with excellent immunoreactivity.
BALB/c mice were immunized with the purified GST–CPAFm protein and four clones were generated that stably secreted mAbs. An indirect ELISA based on the purified GST–CPAFm protein indicated that each of the mAbs reacted specifically with the recombinant GST–CPAFm protein. We used an immunoblotting analysis to test the reaction specificity of the produced mAbs. The results showed that each of the mAbs specifically reacted with the 25-kDa endogenous CPAFm protein. The combined results of the indirect ELISA and immunoblotting analysis of the C. pneumoniae type strain demonstrated that these mAbs have excellent reaction specificity. A new indirect IIF assay based on the four mAbs was also developed and used to detect the C. pneumoniae type strain CWL029. The mAbs also reacted with the endogenous CPAFm protein from the C. pneumoniae type strain. Thus, the mAbs produced display specific immunoreactivity for C. pneumoniae CPAFm.
The new IIF assay was used to detect the endogenous CPAFm antigen of C. pneumoniae in 300 clinical sputum pharyngeal swab samples from patients diagnosed with C. pneumoniae pneumonia. The CPAFm-specific mouse mAb 8C4, which had the highest titer, was selected as the primary antibody. Compared with the reference PCR test, the sensitivity and specificity of the IIF test were 88.89 and 90.24 %, respectively. The differences between the two methods were not statistically significant. The prevalence rates of C. pneumoniae in the 300 patient specimens tested with IIF and PCR were 24 and 18 %, respectively. The agreement between the two methods in detecting the C. pneumoniae antigen was 90.00 %.
A new indirect ELISA based on CPAF-specific mouse mAb 8C4 was constructed and used to detect the endogenous CPAFm antigen of C. pneumoniae in 300 clinical sputum pharyngeal swab samples from patients diagnosed with C. pneumoniae pneumonia. The CPAFm-specific mouse mAb 8C4 was used as the primary antibody. Compared with the reference PCR test, the sensitivity and specificity of the ELISA were 89.36 and 92.89 %, respectively. The differences between the two methods were not statistically significant. The prevalence rates of C. pneumoniae in the 300 patient specimens tested with the ELISA and PCR were 20 and 15.67 %, respectively. The agreement of the two methods used to detect C. pneumoniae antigen was 92.33 %. The new IIF assay and ELISA based on mAb 8C4 were used to test the C. trachomatis D, F, and I type strains and 40 genital secretions from patients with acute C. trachomatis infection. The results implied that the new IIF assay and ELISA do not cross-react with other Chlamydia species when used to detect C. pneumoniae. Thus, we evaluated the performance of the mAb-based IIF assay and ELISA in the detection of the C. pneumoniae antigen in early pediatric pneumonia. We suggest that the novel mAbs produced here have excellent reaction specificity and may be useful in the development of screening tools for the early diagnosis of pediatric pneumonia.
Currently, there are few commercial IIF- or ELISA-based diagnostic kits to detect C. pneumoniae antigen in early bacterial infections. We have successfully prepared mAbs directed against a recombinant protein corresponding to the immunodominant region of CPAF from C. pneumoniae. Using these mAbs, we constructed a new IIF assay and indirect ELISA that can be used to detect the endogenous C. pneumoniae antigen in the early diagnosis of pediatric pneumonia, for both its prevention as well as clinical treatment. However, the process is a little time-consuming because CPAF-specific antibodies are produced during CPAF synthesis, which requires the replication of live chlamydial organisms in chlamydia-infected cells. Our findings also require confirmation with larger samples. In addition, our further research is to compare new IIF and ELISA tests against the “gold standard” MIF test.
References
She RC, Thurber A, Hymas WC, Stevenson J, Langer J, Litwin CM, Petti CA (2010) Limited utility of culture for Mycoplasma pneumoniae and Chlamydophila pneumoniae for diagnosis of respiratory tract infections. J Clin Microbiol 48:3380–3382
Thurman KA, Warner AK, Cowart KC, Benitez AJ, Winchell JM (2011) Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp. in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn Microbiol Infect Dis 70:1–9
Chu DJ, Guo SG, Pan CF, Wang J, Du Y, Lu XF, Yu ZY (2012) An experimental model for induction of lung cancer in rats by Chlamydia pneumoniae. Asian Pac J Cancer Prev 13:2819–2822
Luque A, Turu MM, Rovira N, Juan-Babot JO, Slevin M, Krupinski J (2012) Early atherosclerotic plaques show evidence of infection by Chlamydia pneumoniae. Front Biosci (Elite Ed) 4:2423–2432
Hilden J, Lind I, Kolmos HJ, Als-Nielsen B, Damgaard M, Hansen JF, Hansen S, Helø OH, Hildebrandt P, Jensen GB, Kastrup J, Kjøller E, Nielsen H, Petersen L, Jespersen CM, Gluud C; CLARICOR Trial Group (2010) Chlamydia pneumoniae IgG and IgA antibody titers and prognosis in patients with coronary heart disease: results from the CLARICOR trial. Diagn Microbiol Infect Dis 66:385–392
Gaona-Flores V, García-Elorriaga G, Valerio-Minero M, González-Veyrand E, Navarrete-Castro R, Palacios-Jiménez N, Del Rey-Pineda G, González-Bonilla C, Monasta L (2008) Anti-Chlamydophila pneumoniae antibodies as associated factor for carotid atherosclerosis in patients with AIDS. Curr HIV Res 6:267–271
Dowell SF, Peeling RW, Boman J, Carlone GM, Fields BS, Guarner J, Hammerschlag MR, Jackson LA, Kuo CC, Maass M, Messmer TO, Talkington DF, Tondella ML, Zaki SR; C. pneumoniae Workshop Participants (2001) Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada). Clin Infect Dis 33:492–503
Phoon MC, Yee GW, Koh WP, Chow VT (2011) Comparative seroepidemiologic analysis of Chlamydophila pneumoniae infection using microimmunofluorescence, enzyme immunoassay and neutralization test: implications for serodiagnosis. Indian J Microbiol 51:223–229
Pesonen E, Tiirola T, Andsberg E, Jauhiainen M, Paldanius M, Persson K, Saikku P, Sarna S, Ohlin H, Leinonen M (2009) Serum chlamydial lipopolysaccharide as a prognostic factor for a new cardiovascular event. Heart Lung 38:176–181
Sueur JM, Beaumont K, Cabioch T, Orfila J, Betsou F (2006) Diagnostic value of an ELISA using a recombinant 54-kDa species-specific protein from Chlamydia pneumoniae. Clin Microbiol Infect 12:470–477
Zhong G, Fan P, Ji H, Dong F, Huang Y (2001) Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J Exp Med 193:935–942
Sharma J, Bosnic AM, Piper JM, Zhong G (2004) Human antibody responses to a Chlamydia-secreted protease factor. Infect Immun 72:7164–7171
Dong F, Zhong Y, Arulanandam B, Zhong G (2005) Production of a proteolytically active protein, chlamydial protease/proteasome-like activity factor, by five different Chlamydia species. Infect Immun 73:1868–1872
Sharma J, Dong F, Pirbhai M, Zhong G (2005) Inhibition of proteolytic activity of a chlamydial proteasome/protease-like activity factor by antibodies from humans infected with Chlamydia trachomatis. Infect Immun 73:4414–4419
Zhang RY, Shen WD (2012) Monoclonal antibody expression in mammalian cells. Methods Mol Biol 907:341–358
Balakrishna K, Radhika M, Murali HS, Batra HV, Bawa AS (2012) Specific identification of pathogenic Yersinia enterocolitica by monoclonal antibodies generated against recombinant attachment invasion locus (rAil) protein. World J Microbiol Biotechnol 28:533–539
Zheng J, Wu Y, Liu J, Liu G, Chen C (2008) Early diagnosis using recombinant protein of immunodominant region gene of chlamydial protease-like activity factor from Chlamydophila pneumoniae. Wei Sheng Wu Xue Bao 48:520–525
Fazekas de St Groth S, Scheidegger D (1980) Production of monoclonal antibodies: strategy and tactics. J Immunol Methods 35:1–21
Beatty JD, Beatty BG, Vlahos WG (1987) Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J Immunol Methods 100:173–179
Benitez AJ, Thurman KA, Diaz MH, Conklin L, Kendig NE, Winchell JM (2012) Comparison of real-time PCR and a microimmunofluorescence serological assay for detection of Chlamydophila pneumoniae infection in an outbreak investigation. J Clin Microbiol 50:151–153
Acknowledgments
This work was supported by the National Natural Science Foundation (81372318), the 12th Five-Year Infectious Disease Research Project (2012ZX10001-003), the Medicine Bootstrap Class Project of the Shanghai Science and Technology Commission (134119a5300), the Shanghai Natural Science Foundation (13ZR1435000), the Shanghai Education Board Scientific Research Innovation Project (13YZ049), the Shanghai Health Bureau Scientific Research Project (20124309), and the Shanghai Public Health Clinical Center Research Fund (201309).
Conflict of interest
The authors declare that they have no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zheng, J., Ding, T., Chen, Z. et al. Preparation and evaluation of monoclonal antibodies against chlamydial protease-like activity factor to detect Chlamydia pneumoniae antigen in early pediatric pneumonia. Eur J Clin Microbiol Infect Dis 34, 1319–1326 (2015). https://doi.org/10.1007/s10096-015-2343-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-015-2343-8