Abstract
Background
Neuromyelitis optica spectrum disorders (NMOSD) are autoantibody-mediated inflammatory diseases of the central nervous system predominantly targeting optic nerves and the spinal cord. Two distinct phenotypes are recognized based on the presence of serum aquaporin-4 (AQP4–IgG) antibodies. However, contrasting clinical course patterns have been identified between AQP4-IgG-positive and AQP4-IgG-negative patients.
Aims
This study aimed to present demographic and clinical characteristics of patients with NMOSD in Slovakia and to evaluate the significance of differences between AQP4-IgG-seropositive and AQP4-IgG-seronegative patients.
Methods
We performed a longitudinal multi-centric retrospective study and analysed the clinical and demographic characteristics of a cohort of 63 Slovak NMOSD patients.
Results
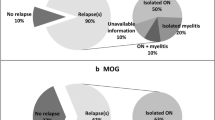
Eighty-six percent of patients were women, and ninety-four patients were Caucasian. The median age at diagnosis was 37 years. The most frequent initial manifestations were optic neuritis (47.6% of patients) and transverse myelitis (39.7% of patients). The median EDSS score deteriorated from the initial 3.0 to 4.0 at the last follow-up. Sixty-eight percent of patients were AQP4-IgG positive; 10% of patients were MOG-IgG positive; 27% of patients had no NMOSD-specific antibodies detected. There was a higher prevalence of autoimmune thyroiditis among AQP4-IgG-positive patients (25.6%) compared to AQP4-IgG-negative patients (0%) (p = 0.01).
Conclusion
This study provides a detailed overview of the clinical and demographic characteristics of NMOSD based on a retrospective analysis of a Slovak cohort of 63 NMOSD patients and extends information provided by similar recently published studies. The most important finding is that there is a high prevalence of autoimmune thyroiditis among AQP4-IgG-negative patients (25%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuromyelitis optica spectrum disorders (NMOSD) are female-predominant chronic autoantibody-mediated inflammatory diseases of the central nervous system mainly targeting optic nerves and the spinal cord [1, 2]. Reported prevalence rates only rarely exceed five cases per 100,000 inhabitants and are particularly high in Caucasian populations (Mori et al., 2018). In Slovakia, the prevalence is 1.42 cases per 100,000 inhabitants [3]. A diagnosis of NMOSD is based on the 2015 International Panel for NMOSD diagnosis (IPND) criteria (derived from the earlier 2006 NMO Criteria) [4], and the algorithm recognizes two distinct phenotypes based on the presence of serum antibodies that target the water channel aquaporin-4 (AQP4–IgG) [2, 5, 6]. The pathogenesis of NMOSD is believed to be mediated by the humoral immune system, and AQP4-IgG antibodies demonstrate a direct pathogenic role [7]. Furthermore, a small subset of NMOSD patients has recently been identified as having anti-myelin oligodendrocyte glycoprotein antibodies (MOG-IgG) [8, 9], which also demonstrate a direct pathogenic role [9]. Hallmark features of NMOSD are acute attacks of optic neuritis or transverse myelitis with a typically relapsing course, but clinical presentation is not limited to these only and may also include symptomatology of the central nervous system involvement outside the optic nerves and spinal cord (area postrema, brainstem, diencephalon or cerebrum) [2]. However, contrasting clinical course patterns have been identified between AQP4-IgG-positive and AQP4-IgG-negative patients [10, 11]. This study aimed to present the demographic and clinical characteristics of patients with NMOSD in Slovakia and to evaluate the significance of differences between AQP4-IgG-seropositive and AQP4-IgG-seronegative patients.
Methods
We performed a longitudinal multi-centric retrospective study of a cohort of patients treated for NMOSD from 1 January 2006 through 20 June 2022 in all 13 Slovak centres for multiple sclerosis and related disorders.
Local investigators completed case report forms (CRF) with on-call assistance from the study coordinator and collected detailed demographic and clinical information as follows: (1) gender, (2) race (self-reported by patients), (3) age at disease onset, (4) clinical manifestation at onset, (5) antibody status (both AQP4-IgG and MOG-IgG), (6) brain and spinal cord MRI findings, (7) results of cerebrospinal fluid workup, (8) disease course (monophasic or relapsing + the total number of relapses), (9) disability profile (score on the Expanded Disability Status Scale, EDSS) [12] both at the beginning of the disease and at the last follow-up, (10) history of comorbidities, (11) and treatment history. Diagnosis of comorbidities was based on the sole discretion of the respective specialist.
The exclusion criteria were as follows: (1) the 2015 IPND diagnostic criteria were not met; (2) missing data required for verifying the diagnosis of NMOSD; (3) duplicate records, or (4) the refusal to provide informed consent.
All serum samples were tested uniformly in two laboratories (Bratislava and Kosice, Slovakia) for the presence of antibodies by the CBS (cell-based assay) method using the NMOSD Screen. Both laboratories use the same tests. Anti-AQP4-IIFT was used for AQP4-IgG detection (antibody detection in living cells using HEK-293 cells stably transfected with the M23 isoform of AQP4). Anti-MOG-IIFT was used for MOG-IgG detection (CBA with live HEK-293 cells transfected with a plasmid containing full-length human MOG). Both antibody types were evaluated by indirect immunofluorescence assay (anti-AQP4-IIFT). This assay has a sensitivity of 80% for AQP4-IgG and 95% for MOG-IgG and a specificity of 100% for AQP4-IgG and 84.1% for MOG-IgG (Euroimmun, Germany). In Slovakia, this method of AQP4-IgG and MOG-IgG detection became available in late 2013.
The study protocol is in accordance with the 1964 Declaration of Helsinki, its later amendments, and with the principles of good clinical practice. The study protocol was approved by the Ethics Committee of BLINDED FOR THE PURPOSE OF THE REVIEW, on May 21, 2020 (approval code 2020/EK/05035). All authors had access to the study data and have reviewed and approved the final manuscript.
Statistical analyses were carried out using RStudio (version 1.2.1335). Continuous variables are presented as medians and interquartile ranges (IQR). Categorical variables are presented as absolute counts and percentages (%). The pair-wise deletion was used when values were “missing-at-random”. The significance of differences was tested using Mann–Whitney tests, Chi-square tests or Fisher’s exact tests (as appropriate). All tests were performed at a significance level of p ≤ 0.05.
Results
Initially, 71 cases were reported from neurological centres. Eight patients met the exclusion criteria; thus, 63 NMOSD patients were included in the final analysis, 33 of whom (52.4%) also met the diagnostic criteria for NMO. Sixty-one patients (93.8%) were Caucasian, 2 patients were Roma (3.2%), 54 (85.7%) patients were women, and the female-to-male ratio was 6:1.
The median age at diagnosis was 37 years (IQR 22), and the median disease duration was 6 years (IQR 8). Optic neuritis was the initial manifestation in 30 patients (47.6%) and transverse myelitis in 25 patients (39.7%). In this cohort, patients usually suffered from the relapsing form of the disease (n = 51, 81%) with an annual attack rate of one. Fifty-two patients (82.5%) experienced optic neuritis during the course of the disease at least once, and 49 patients (77.8%) experienced at least one attack of transverse myelitis. Median EDSS score deteriorated from the initial 3.0 (IQR 1.75) to 4.0 at the last follow-up (IQR 2.5). One patient (1.6%) died during the follow-up period. Further characteristics are presented in Table 1.
Fifty-three patients underwent a CSF examination, and oligoclonal IgG bands were found in 15 of them (28.3%). Magnetic resonance imaging (MRI) was repeatedly performed during acute attacks and revealed T2-hyperintense lesions in the following regions: optic nerve/optic chiasm (34.1% of patients), brain stem (17.1% of patients), diencephalon (4.9% of patients) and non-MS/atypical cerebral lesions (46.3% of patients). Normal brain MRI findings were reported in 4.9% of patients. Similarly, spinal cord MRI revealed longitudinal extensive transverse myelitis (LETM; 63.4% of patients), non-LETM lesions (9.8% of patients), and normal findings were present in 26.8% of patients.
Twenty-four patients (38.1%) had a history of comorbidities, and 24 patients suffered from a concomitant autoimmune disease (38.1% of all NMOSD patients) (Table 2).
During an acute attack, 35 patients (60.3%) were treated with intravenous methylprednisolone (IVMP), 20 patients (34.5%) with a combination of IVMP and plasma exchange (PE), and 2 patients (3.4%) with a combination of IVMP and intravenous immunoglobulins (IVIG). We were unable to retrospectively identify the way that 5 patients (7.9%) had been managed during acute attacks. All patients received long-term maintenance treatment (most commonly prednisone and other immunosuppressive treatment alone or in combination, including rituximab).
In the Slovak cohort of NMOSD patients, 43 patients (68.3%) were AQP4-IgG positive, and 20 (31.7%) were AQP4-IgG negative. Six patients (9.5%) were MOG-IgG positive and 3 of them showed “double seropositivity” (both AQP4 and MOG antibodies were present). In 17 patients (27%), no “NMOSD-specific” autoantibodies were detected. There were several differences in the demographic and clinical characteristics between the two AQP4-IgG subgroups: (1, 2) The disease duration was longer and the age at the last follow-up was higher in seropositive patients (p = 0.04; Table 1). (3) Male seronegative patients were younger than male seropositive patients (p = 0.02; Table 1), and while there was no difference in the frequency of comorbidities in general, (4) autoimmune comorbidities alone were more frequent among male AQP4-IgG-negative patients compared to male AQP4-IgG-positive patients (p = 0.05; Table 2). Most importantly, (5) there was a higher prevalence of autoimmune thyroiditis among AQP4-IgG-positive patients (25.6%) compared to AQP4-IgG-negative patients (0%) (p = 0.01, Table 2).
There was no difference between the two AQP4-IgG subgroups in other disease characteristics, such as the initial manifestation, clinical course and number of attacks, or in the EDSS scores at the onset of the disease or at the last follow-up examination.
Discussion
In this study, we present the demographic and clinical characteristics of patients with NMOSD in Slovakia and evaluate the significance of differences between AQP4-IgG-seropositive and AQP4-IgG-seronegative patients. We analysed a cohort of 63 patients with a median age of 37 years (IQR 22) at disease onset. Eighty-six percent of patients were women, and the median duration of the disease was six years (IQR 8). Except for the Brazilian patients being younger, all of these demographic characteristics are similar to those reported for Brazilian, Chinese and Spanish studies [10, 13, 14].
Optic neuritis was the initial manifestation in 47.6% and transverse myelitis in 39.7% of all patients. These numbers are similar to those reported from a Chinese cohort [14], although the proportion of patients with the initial brain stem involvement was lower in our cohort (27.6% vs 3.2%). Eighty-one percent of patients suffered from a relapsing course of the disease, which is higher than the proportion reported from the Brazilian cohort (72%) [10] and similar to the Algerian (85%) [15], Chinese (87.6%) [14] and Spanish cohorts (89%) [13]. Considering these comparisons, we believe that our cohort’s characteristics did not substantially deviate from those mentioned above.
We also analysed the differences between AQP4-IgG-positive (68.3%) and AQP4-IgG-negative patients (31.7%). We found that the disease duration in AQP4-IgG-positive patients was longer and that they were older at the last follow-up examination compared to AQP4-IgG-negative patients. This difference probably results from the fact that IPND criteria (which recognize the existence of AQP4-IgG-negative NMOSD patients) has been around since 2015 only, and this study retrospectively examines a time frame that extends beyond that year.
The most important finding of this study is the high prevalence of autoimmune thyroiditis (AIT) in AQP4-IgG-positive patients (25% compared to 0% in AQP4-IgG-negative patients) and in patients with NMOSD in general (17.5%). Despite the fact that Wang et al. observed a significantly higher prevalence of anti-thyroid antibodies (ATAbs) among NMOSD patients compared to a healthy control population [16], a higher prevalence of actual autoimmune thyroiditis has thus far gone unnoticed [10, 14]. This finding may be of importance, because thyroid disease/thyroid dysfunction may have some bearing on the clinical course and disease activity of NMOSD itself [17], although similar hypotheses were not confirmed in other autoimmune diseases, such as primary biliary cholangitis or inflammatory bowel diseases [18, 19].
Jarius et al. followed patients up for a similar time as in our study (58 months vs 72 months) and came out during the last follow-up with a median EDSS score of five, which is slightly higher than that from our cohort (the median was four). Similar to our results, seropositive and seronegative patients did not differ significantly with regard to EDSS score at the last follow-up or with regard to the annual attack rate [11]. However, seropositive patients were more likely to suffer from the relapsing course and less likely to have simultaneous optic neuritis and myelitis; we were unable to confirm the differences [11].
We acknowledge the limitations of this study. First, despite a relatively large cohort, there are small numbers of cases in certain groups of patients. However, because NMOSD is a rare disease, this could not be avoided, and it does not preclude the results from being used later in systematic reviews and meta-analyses. Second, we could not provide a more detailed description of MRI findings, because only a description rather than the original images was provided in certain cases (typically the original MR images from earlier years were missing). Thirdly, it is important to note that obtaining detailed treatment histories was not feasible due to the extended timeframe and the absence of electronic health records in Slovakia during that period. Consequently, any conclusions drawn regarding the effects of individual treatments would lack validity.
Conclusion
This study provides a detailed overview of the clinical and demographic characteristics of NMOSD based on a retrospective analysis of a Slovak cohort of 63 NMOSD patients and extends information provided by similar recently published studies. The most important finding is that there is a high prevalence of autoimmune thyroiditis among AQP4-IgG-negative patients.
Data availability
The data supporting this study will be made available by the corresponding author upon a reasonable request.
References
Wingerchuk DM (2009) Neuromyelitis optica: effect of gender. J Neurol Sci 286(1–2):18–23
Wingerchuk DM et al (2015) International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurol 85(2):177–189
Szilasiová J et al (2022) Incidence and prevalence of neuromyelitis optica spectrum disorders in Slovakia. Neurol Res 44(1):38–46
Wingerchuk DM et al (2006) Revised diagnostic criteria for neuromyelitis optica. Neurol 66(10):1485–1489
Jarius S et al (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation 13(1):1–16
Weinshenker BG, Wingerchuk DM (2017) Neuromyelitis spectrum disorders. In Mayo Clinic Proceedings. Elsevier
Bennett JL et al (2009) Intrathecal pathogenic anti–aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol 66(5):617–629
Mader S et al (2011) Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation 8(1):1–14
Saadoun S et al (2014) Neuromyelitis optica MOG-IgG causes reversible lesions in mouse brain. Acta Neuropathol Commun 2(1):1–9
Fragoso YD et al (2019) Clinical characteristics of 153 Brazilian patients with neuromyelitis optica spectrum disorder (NMOSD). Mult Scler Relat Disord 27:392–396
Jarius S et al (2012) Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation 9(1):1–17
Kurtzke JFJN (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurol 33(11):1444–1444
Sepúlveda M et al (2016) Neuromyelitis optica spectrum disorders: comparison according to the phenotype and serostatus. Neurol Neuroimmunol Neuroinflamm 3(3):e225
ZhangBao J et al (2017) The clinical characteristics of AQP4 antibody positive NMO/SD in a large cohort of Chinese Han patients. J Neuroimmunl 302:49–55
Bouzar M et al (2017) Neuromyelitis optica spectrum disorders with antibodies to myelin oligodendrocyte glycoprotein or aquaporin-4: clinical and paraclinical characteristics in Algerian patients. J Neurol Sci 381:240–244
Wang X et al (2016) Anti-thyroid antibodies and thyroid function in neuromyelitis optica spectrum disorders. J Neurol Sci 366:3–7
Cho EB et al (2016) Low T3 syndrome in neuromyelitis optica spectrum disorder: Associations with disease activity and disability. J Neurol Sci 370:214–218
Floreani A et al (2017) Thyroid dysfunction in primary biliary cholangitis: a comparative study at two European centers. Am J Gastroenterol 112(1):114–119
Shizuma T (2016) Concomitant thyroid disorders and inflammatory bowel disease: a literature review. BioMed Res Int 2016:1
Acknowledgements
The authors are grateful to the study participants and would like to thank the members of the Slovak Neurological Society for their support and the MS centres who assisted with data collection. We would like to thank Dr. Andrea Cimprichová, Dr. František Jurčaga, Dr. Edita Kahancová, Dr. Ladislav Gurčík, Dr. Eleonóra Klímová, Dr. Georgi Krastev, Dr. Andrea Megová, Dr. Jozef Michalík, Dr. Ľubica Snopeková, Dr. Zuzana Števková, Dr. Vladimír Donáth for data collection. We would like to thank Dr. Marcela Papovňaková from the Medirex laboratory for her assistance and support.
Author information
Authors and Affiliations
Contributions
JS, JG, AC, MF, VH, MK, SK, and PM conceived and designed the study. JS, JG and PM conducted the analyses. JS prepared the initial draft and JG, PM, MF and MK critically reviewed the manuscript. All authors approved the final draft.
Corresponding author
Ethics declarations
Ethical approval
The study protocol is in accordance with the 1964 Declaration of Helsinki, its later amendments, and with the principles of good clinical practice. The study protocol was approved by the Ethics Committee of Louis Pasteur University Hospital in Kosice, Slovakia, on May 21, 2020 (approval code 2020/EK/05035). All patients signed an informed consent. All authors had access to the study data and have reviewed and approved the final manuscript.
Conflict of interest
JS has received research support from Biogen and Roche and is a board member and receives fees for scientific consulting from Merck, Biogen, Roche and Novartis; she has received travel sponsorship from Novartis, Biogen, Merck, and Teva and has received speaker honoraria from Roche, Biogen, Novartis, and Teva.
JG has no competing interests.
PM has no competing interests.
AC has received speaker honoraria from Biogen, Merck, Teva, and Novartis and is a board member and receives fees for scientific consulting from Novartis, and Merck; she has received travel sponsorship from Biogen and Teva, and is an investigator in clinical trials sponsored by Biogen, Novartis and Merck.
MF has received speaker honoraria and travel sponsorship from Biogen, Novartis, Merck, Roche, and Teva.
VH has received travel sponsorship from Merck, Biogen, Roche and Novartis and has received speaker honoraria from Merck and Biogen.
EK has received travel sponsorship from Merck Serono, and Biogen, and has received speaker honoraria from Biogen, Merck, and Novartis; she has received scientific consulting fees from Merck, Biogen and Novartis.
MK has received travel sponsorship from Novartis, Roche, Biogen, Merck, and Teva, and has received speaker honoraria from Roche, Biogen, Novartis, and Teva; he has received research support from Biogen and scientific consulting fees from Merck, Biogen and Novartis.
SK has received travel sponsorship from Roche, Biogen, Teva and Sanofi Genzyme, and has received speaker honoraria from Roche, Biogen Idec, Novartis, Teva and Sanofi Genzyme; she has received scientific consulting fees from Merck, Biogen, Novartis and Roche.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Szilasiová, J., Gazda, J., Mikula, P. et al. Clinical and demographic characteristics of patients with NMOSD: a longitudinal retrospective analysis of a Slovak cohort of 63 patients. Neurol Sci 45, 693–698 (2024). https://doi.org/10.1007/s10072-023-07050-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07050-x