Abstract
Objective
To investigate the impact of human serum albumin (HSA) levels on symptomatic cerebral vasospasm (SCVS) in patients with aneurysmal subarachnoid hemorrhage (aSAH).
Methods
We retrospectively reviewed the medical records. SCVS was defined as the development of a new neurological deterioration when the cause was considered to be ischemia attributable to vasospasm after other possible causes of worsening had been excluded. The aSAH patients were divided into two groups: those with SCVS (group 1) and those without SCVS (group 2). The HSA level data on the 1st, 2nd, and 3rd day after admission was collected. Multivariate logistical regression and receiver operating characteristic (ROC) analysis were performed to evaluate the ability of HSA level to predict the development of SCVS.
Results
A total of 270 patients were included in our study, of which 74 (27.4%) developed SCVS. The average and lowest HSA levels were lower in group 1 (P < 0.001). In univariate logistic regression, white blood cell count, neutrophil count, and average and lowest HSA levels were associated with SCVS. After adjustment for age, CT Fisher grade, Hunt-Hess grade, and WFNS grade, both the average and lowest HSA levels remained independent predictors of SCVS (P < 0.001). The CT Fisher grade was confirmed to be an independent predictor of SCVS across each model. ROC analysis revealed that the lowest HSA level was a better predictor for SCVS than average HSA level and CT Fisher grade.
Conclusion
Clinicians are encouraged to measure HSA levels for the first 3 days after admission to predict the occurrence of SCVS after aSAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Based on data generated from 2013 to 2019 in the China Stroke High-risk Population Screening and Intervention Program, stroke was the leading cause of death and years of life lost in China. Aneurysmal subarachnoid hemorrhage (aSAH) accounts for 4.4% of all strokes [1]. aSAH is a devastating cerebral vascular disease, with at least a quarter of patients dying, and approximately half of survivors are left with some persistent neurological deficit [2]. Due to the mature technology of clipping and endovascular coiling, the risk of rebleeding in patients with aSAH has been significantly reduced [3], but cerebral vasospasm (CVS) continues to be a major cause of clinical worsening in aSAH patients [4]. Throughout the literature, there are various definitions of CVS, using terms such as symptomatic CVS (SCVS), transcranial Doppler (TCD) CVS, and angiographic CVS. Approximately 20 to 40% of patients with aSAH experience SCVS, which is closely associated with delayed cerebral infarction, leading to disturbance of consciousness, cognitive impairment, permanent neurologic deficits, increased hospital costs, and poor 3-month outcomes [4, 5]. Up to 70% of aSAH patients may develop angiographic CVS, but the relationship between angiographic CVS and clinical symptoms is inconsistent [5,6,7]. The relationship between TCD CVS and clinical worsening is also unreliable [5,6,7]. Thus, SCVS is a more clinically meaningful definition than other definitions, and optimizing effective prediction and treatment of SCVS with subsequent improvement in outcome will certainly have a massive impact for patients with aSAH. Unfortunately, current prediction and treatment of ischemic complications caused by SCVS after aSAH are ineffective.
A substantial amount of biomarkers and risk factors have been conducted in an effort to predict and prevent the occurrence of CVS, and those include sex, age, hypertension, current smoking, cocaine use, leukocytosis, soluble adhesion molecules, lipid peroxides, cell proliferation and growth factors, endothelin 1, hypomagnesemia, genetic markers, WFNS grade, Hunt-Hess grade, Glasgow Coma Scale score, CT Fisher grade, thick cisternal clot on CT, size and location of aneurysm, and so on [8]. However, it is not easy to be popularized in clinical work to detect these biomarkers, and the effect of them on the development of CVS remains controversial. To optimize the outcome of SAH patients, it is necessary to seek a new biomarker for the prediction and prevention of CVS.
Although the pathophysiology of SCVS remains complex and is not completely understood, it is widely accepted that the local inflammatory response and oxidative stress play an important role in the process of SCVS development [9]. Since human serum albumin (HSA) is an essential protein with anti-inflammatory and antioxidant properties [10], HSA is suspected to play a role in the development of SCVS. Hypoproteinemia is known to be associated with increased morbidity and mortality in a variety of diseases. Furthermore, several preclinical and clinical studies have demonstrated the role of HSA as a neuroprotective treatment in aSAH [11, 12]. Intravenous infusion of HSA could reduce cerebral injury and improve neurological outcomes in the early stage of SAH in Sprague–Dawley rats [11]. The Albumin in Subarachnoid Hemorrhage (ALISAH) multicenter pilot clinical trial revealed that 1.25 g/kg/day HSA treatment was not only safe but also might produce a better functional outcome 3 months after aSAH [12]. However, the mechanism why HSA level influence the outcome of aSAH has not been fully studied. Previous studies have identified that patients with aSAH at higher risk of CVS were younger, and had a history of smoking, advanced SAH severity, poor hemodynamic status, and hypotension [13]. However, limited data are available on the impact of the HSA on the development of the SCVS. Therefore, the purpose of our study was to investigate the effect of HSA levels on the occurrence of SCVS after aSAH. Our hypothesis was that reduced HSA levels would be associated with an increased occurrence of SCVS in patients with aSAH.
Materials and methods
Study population
In this retrospective study, we used data from existing medical records of patients with a primary aSAH diagnosis who were admitted to multiple medical centers from September 2018 to June 2021. The multiple medical centers include the Department of Neurology and Neurosurgery in The First Affiliated Hospital of Anhui Medical University, the Department of Neurology in The Affiliated Huai’an Hospital of Xuzhou Medical University, and the Department of Neurology in The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University. The inclusion criteria were as follows: (1) the diagnosis of SAH met the diagnostic criteria of the 2015 edition of Chinese Guidelines for the Diagnosis and Treatment of Subarachnoid Hemorrhage [14]; and (2) the intracranial aneurysms were detected by head DSA or CTA. (3) Head CT was performed in time when neurological deterioration occurred. Patients were treated according to the standardized protocol provided by the guidelines for the management of aSAH [14]. The exclusion criteria were as follows: (1) treated with exogenous albumin administration; (2) with non-aneurysmal SAH, such as trauma, moyamoya disease, vasculitis, arteriovenous malformation rupture, and so on; (3) with dissecting aneurysms; (4) without identified source of bleeding using cerebral angiography; (5) rebleeding after initial bleeding; (6) lost to follow-up; and (8) HSA levels were not measured three times in the first 3 days after admission. Informed consent was obtained in all cases included in the present study and the protocol was approved by the local ethics committee of the hospital.
Data collection
We retrospectively collected data from existing medical records. Baseline demographics (age and sex), vascular risk factors (hypertension, diabetes mellitus, current smoking), other laboratory work-ups (HSA level, neutrophil count, lymphocyte count, white blood cell count), imaging data (rupture aneurysm size and location), and clinical and radiological severity (WFNS grade, Hunt-Hess grade, CT Fisher grade) were collected. The data on whether SCVS occurred were collected and reassessed by two certified neurologists based on medical records. The CT Fisher grade was split into 2 groups (grades I–II, grades III–IV), the Hunt-Hess grade was split into 2 groups (grades I–II, grades III–V), and the WFNS grade was divided into 2 groups (grades I–III and grades IV–V). The functional outcome was assessed 3 months after discharge using the GOS score obtained via telephone interviews. Good functional outcome was defined as a GOS score of 4–5, and poor functional outcome was defined as a GOS score of 1–3.
Definition of SCVS
SCVS is an ischemic consequence syndrome of CVS characterized by decreased levels of consciousness (a decrease of at least 2 points on the Glasgow Coma Scale (either on the total score or on one of its individual components [eye, motor on either side, verbal])) or focal neurological impairment (such as hemiparesis, aphasia, apraxia, hemianopia, or neglect). This is not apparent immediately after aneurysm occlusion and should last for at least 1 h, and cannot be attributed to other causes [15]. In our study, the diagnosis of SCVS was adjudicated by consensus of two certified neurologists based on the presence of angiographic CVS and neurological deterioration, when the cause was thought to be ischemia attributable to CVS after other possible causes of neurological deterioration (rebleeding, hydrocephalus, seizures, metabolic derangement, infection, excessive sedation, hypotension, hypoxia, fever, heart failure, and cerebral edema) had been excluded [5].
Measurement of HSA level
The HSA level data were collected from medical records. In all institutions, the registered nurses working in the hospital collected the specimens to send them to the laboratory for analysis. Then, the HSA specimens were measured by a BCP kit in accordance with the manufacturer’s instructions. The HSA level was measured on the 1st, 2nd, and 3rd day after admission, with an interval of 24 h each time. Three HSA predictor variables were calculated, including average HSA level, lowest HSA level, and highest HSA level.
Statistical analysis
The analysis was carried out on all patients included in the study. SPASS statistical software was used for data analysis. Variables were expressed as the mean ± SD or percentage of patients. Count data, including sex, hypertension history, diabetes history, current smoking, Hunt-Hess grade, CT Fisher grade, WFNS grade and GOS score, aneurysm location, and surgical approach, were compared by χ2 test. Measurement data, including age, average HSA level, lowest HSA level, highest HSA level, white blood cell count, lymphocyte count, and neutrophil count, were compared by t test. Multivariable binary logistical regression was performed to analyze the laboratory predictors of SCVS. We performed univariate logistic regression for each laboratory variable. All variables with P-values < 0.05 from univariate logistic regression analysis were entered into a multivariable logistic regression model, whereby each laboratory value was adjusted by covariates. The covariates entered in the multivariable logistical regression were age, CT Fisher grade, Hunt-Hess grade, and WFNS grade. The laboratory values entered in the multivariable logistical regression were white blood cell, neutrophil, and lowest and average HSA levels. Prior to formulation of the final regression model, we evaluated the covariables above for multicollinearity, which defined as variance inflation factor ≥5 or tolerance of <0.20, was assessed using weighted linear regression. Finally, analysis of the receiver operating characteristic (ROC) curves and the area under the ROC curves (AUC) was performed to evaluate the predictive value of HSA level and CT Fisher grade on the occurrence of SCVS. Two-tailed P-values of <0.05 were considered as statistically significant.
Results
Clinical data
Of 457 screened patients with SAH, 349 (76.4%) had an intracranial aneurysm as the confirmed source of hemorrhage. Of these, 270 (77.4%) were included in the study (Fig. 1). Seventy-nine patients with aSAH were excluded due to less than three HSA measurements in the first 3 days after admission (n = 56), exogenous albumin administration (n = 11), loss to follow-up (n = 8), and rebleeding (n = 4).
Patient characteristics are shown in Table 1. The aSAH patients were divided into two groups: those in whom SCVS occurred (group 1) and those in whom SCVS did not occur (group 2). Group 1 comprised 74 patients (43.2% male), and group 2 comprised 196 patients (30.6% male). SCVS occurred between 3 days and 2 weeks after aSAH in group 1. The average age of the patients was 62 years old in group 1 and 58 years old in group 2. The common location of aneurysms was the anterior communicating artery in group 1 (40 cases) and group 2 (106 cases). In terms of the aneurysm surgery method, aneurysm clipping was performed in 8.5% (23 cases), aneurysm coiling was performed in 90.4% (244 cases), and aneurysm surgical treatment was not performed in 1.1% (3 cases). Among group 1, 39 patients (52.7%) underwent DSA, 21 patients (28.4%) underwent CTA, and 14 patients (18.9%) underwent DSA and CTA.
Comparisons of demographic, clinical, laboratory, and radiological data in patients with aSAH according to the occurrence of SCVS are presented in Table 1. The univariate analysis did not identify any significant difference in sex (male 43.2% vs. male 30.6%, P = 0.051), history of hypertension (70.3% vs. 61.2%, P = 0.168), history of diabetes (13.5% vs. 11.2%, P = 0.604), current smoking (10.8% vs. 12.8%, P = 0.664), or lymphocyte count (1.79 ± 3.51 vs. 1.41 ± 2.49, P = 0.324) between group 1 and group 2. Patients in group 1 were likely to be older (62.32 ± 8.56 vs. 58.83 ± 11.53, P = 0.018) and to have a higher white blood cell count (12.89 ± 5.71 vs. 10.27 ± 4.13, P < 0.001) and neutrophil count (13.57 ± 15.20 vs. 8.96 ± 6.98, P = 0.001). Overall, Hunt-Hess grade, CT Fisher grade, and WFNS grade were significantly higher in group 1 than in group 2 (P < 0.001). Hunt-Hess grades III–V (63.5% vs. 21.9%, P < 0.001), CT Fisher grades III–IV (78.4% vs. 17.3%, P < 0.001), and WFNS grades IV–V (73.0% vs. 20.9%, P < 0.001) were more common in group 1 than in group 2.
Predictive values of HSA level and CT Fisher grade for SCVS in aSAH
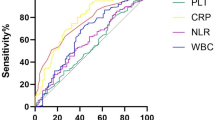
Comparisons of HSA levels between group 1 and group 2 in this study are presented in Table 1. The highest HSA level (38.96 ± 3.48 vs. 39.73 ± 3.18, P = 0.058) was numerically lower in group 1 than in group 2, but the difference was not statistically significant. Patients were significantly more likely to have lower average HSA levels (34.65 ± 2.50 vs. 37.39 ± 2.89, P < 0.001) and lowest HSA levels (30.20 ± 2.88 vs. 34.48 ± 3.15, P < 0.001) in group 1 than in group 2. Univariate logistic regression was performed to investigate the association between laboratory variables and SCVS. The white blood cell count, neutrophil count, and lowest and average HSA levels revealed the ability to predict development of SCVS (Table 2). After adjusting for baseline differences in age, CT Fisher grade, Hunt-Hess grade, and WFNS grade in multivariable logistic regression, both the lowest and average HSA levels predicted SCVS (Table 3). Lowest albumin had the lowest odds ratio (OR) of 0.641 (95% CI 0.550–0.747, P < 0.001, Table 3). Additionally, the CT Fisher grade was confirmed to be an independent predictor of SCVS across each model (P < 0.05, Table 3). Multicollinearity was evaluated between the independent variables and development of SCVS, and the VIF value was less than 5, while the tolerance value was more than 0.2, indicating that the covariables did not demonstrate multicollinearity. The predictive performance of HSA level and CT Fisher grade for the early prognostication of SCVS was evaluated using ROC analysis. The lowest HSA level showed modest ability to distinguish between patients with and without SCVS (AUC = 0.843, 95% CI 0.793–0.893, P <0.001), and those with an HSA level < 32.55 g/L were more likely to develop SCVS (Youden’s index = 0.561, sensitivity = 85.1%, specificity = 71.0%, Fig. 2). The average HSA level significantly differentiated between groups (AUC = 0.764, 95% CI 0.703–0.825, P < .0001), and those with average HSA level < 35.57 g/L were more likely to develop SCVS (Youden’s index = 0.402, sensitivity 66.2%, specificity 74.0%, Fig. 2). The CT Fisher grade demonstrated significant improvement in distinguishing between patients with or without SCVS (AUC = 0.796, 95% CI 0.736–0.855, P < .001). A CT Fisher grade > 2 was more likely to lead to the development of SCVS (Youden’s index = 0.509, sensitivity 70.3%, specificity 80.6%, Fig. 3). In addition, the predictive performance of the lowest HSA level for the occurrence of SCVS was better than that of the average HSA level and CT Fisher grade.
Association of the SCVS with functional outcome after aSAH
Comparisons of functional outcomes between group 1 and group 2 are presented in Table 1. Patients in group 1 were likely to have worse functional outcomes, as reflected by lower GOS after discharge (71.6% vs. 13.3%, P < 0.001).
Discussion
With the advancement of clipping and endovascular coiling, the risk of rebleeding in patients with aSAH is significantly reduced, CVS was considered to be the major complications of aSAH [3, 4], accounting for 20 to 40% of SAH cases [5]. In our study, the incidence of SCVS patients was 27.4%. CVS mostly occurred 3 days after aSAH, reached its peak 6 to 8 days later, and resolved 2 to 3 weeks later [16]. In our study, SCVS always occurred between 3 days and 2 weeks after aSAH, which was similar to previous research. SCVS is closely associated with delayed cerebral infarction after SAH and leads to higher rates of mortality and disability in SAH patients [4]. Consistent with previous studies, our study demonstrated that the functional outcome was likely to be worse in patients with SCVS. Therefore, SCVS might be a target for interventions to improve the outcome of patients with aSAH. First, reliable methods are needed to predict the occurrence of CVS early. Some abnormal laboratory biomarkers and risk factors have been assessed in an effort to predict the occurrence of CVS, and those include leukocytosis, soluble adhesion molecules, lipid peroxides, cell proliferation and growth factors, endothelin 1, hypomagnesemia, genetic markers, and so on, but the results are still inconsistent due to the different definitions of cerebral vasospasm. These biomarkers are not easy to popularize in clinical work, and few reports have demonstrated their effective predictive role [9]. Second, new treatment concepts of CVS are needed. At present, nimodipine is thought to act on cerebrovascular smooth muscle to prevent CVS, and it is the only FDA-approved medication to reduce CVS after aSAH. There is presently no available treatment other than nimodipine presently, and recent studies have found that simply blocking calcium ions entering vascular smooth muscle cells cannot completely prevent CVS [17]. There was a higher incidence of adverse events, complications, and clinical deterioration in patients with aSAH who received continuous intra-arterial infusion of nimodipine [18]. Therefore, many attempts have been made to predict and treat CVS early to optimize the outcome of SAH patients, but without success, so it is necessary to seek new easy-to-detect biomarkers for the prediction and treatment of CVS.
To the best of our knowledge, our study is the first to investigate the relationship between HSA levels and the occurrence of SCVS after aSAH. We found that patients in the SCVS group had lower average and lowest HSA levels. Further multivariate logistic regression analysis suggested that the average and lowest HSA level was significantly associated with the occurrence of SCVS after aSAH. In addition, this trial provides a biomarker for the occurrence of SCVS after aSAH, with lowest HSA level < 32.55 g/L and average HSA level < 35.57 g/L as a preliminary cutoff value. Below the HSA cutoff value, the probability of SCVS significantly increased. We hypothesized that patients with lowest HSA level < 32.55 g/L and average HSA level < 35.57 g/L during the first 3 days after admission were more likely to develop SCVS.
Clinically, the characteristic of aSAH is extravasation of blood into the spaces covering the central nervous system that are filled with cerebrospinal fluid; therefore, the etiology of CVS is thought to be associated with the spasmogenic and neuroinflammatory substances generated from the lysis of subarachnoid blood [19]. The following mechanism for the development of CVS is generally accepted: (1) A significant decrease in colloid osmotic pressure may induce CVS after SAH [20]. (2) aSAH causes white blood cell infiltration and neutrophil recruitment, which weaken microvascular perfusion and lead to the release of a large number of inflammatory mediators, thus inducing cerebral vasospasm [21]. In our study, the white blood cell count in the SCVS group was statistically significantly higher than that in the group without SCVS, suggesting that patients in the SCVS group exhibited higher inflammatory properties. In addition, oxidative stress is closely related to CVS, and inhibition of inflammation and antioxidants may significantly decrease the occurrence of CVS [16, 22, 23]. (3) CVS is caused by an imbalance between vasoconstrictor and vasodilator substances. A large number of vasoconstrictor substances are produced after SAH (thromboxane A2 [16], primary prostaglandins (PGs) [16], interleukin-6 [24], platelet-derived growth factor [25], endothelin [16], etc.), while vasodilator substances (nitrous oxide [16], prostacyclin [16], etc.) and potassium channel activity decreased [26], which contributed to cerebral vasomotor dysfunction and CVS. HSA is a negative acute reactive protein, and its multiple brain protective effects are similar to those of CVS. Based on these findings, we consider that the rationale for hypoalbuminemia-induced SCVS is based on the assumption discussed below: First, the role of HSA in the maintenance of colloid osmotic pressure. Patients with aSAH are prone to hypoalbuminemia due to systemic inflammation, pain, vomiting, disturbance of consciousness, and excessive consumption. A previous study demonstrated that the decreased level of HSA was accompanied by a decrease in colloid osmotic pressure, and the significant decrease in colloid osmotic pressure levels indicated the occurrence of CVS after aSAH [20]. Second, HSA has a role in limiting leakage from capillary beds by altering the nature and distribution of glycoproteins in the vessel wall during stress-induced capillary permeability [27]. Patients with aSAH suffer from severe acute stress, and the low level of HSA may reflect higher vascular permeability and a disrupted blood-brain barrier. Spasmogenic and inflammatory substances are more likely to extravasate, leading to SCVS. Third, HSA is the most important source of sulfhydryl groups, which can protect NO from rapid degradation, have an important effect on vasodilatory properties, and affect vascular NO signal transduction [28]; therefore, it is possible that HSA can regulate vascular tone in different vascular beds. Fourth, HSA has a role in inhibiting neutrophil aggregation, scavenging inflammatory mediators and oxidants, reducing the inflammatory response, and blocking the formation of pathological lipid peroxidation and free radicals, thus decreasing the occurrence of SCVS [10, 20, 29, 30]. On the other hand, the N-terminal sequence of albumin is chemically modified in ischemic conditions to generate ischemia-modified albumin (IMA), which has been shown in a meta-analysis to be elevated in patients with SAH [31]. IMA was negatively correlated with HSA levels, as confirmed by Van der Zee et al. [32]. Since IMA is thought to be the consequence of oxidative stress states, increased reactive oxygen species, and acidosis [33], we speculate that the decrease in HSA levels would lead to the elevation of IMA, which may contribute to SCVS.
In addition, CT Fisher grade and Hunt-Hess grade have been demonstrated to serve as valuable predictive factors for CVS [9]. In accordance with previous studies, our data also demonstrated that CT Fisher grade and Hunt-Hess grade were increased in the group with SCVS compared with the group without SCVS. Further multivariate logistic regression analysis and ROC analysis suggested that CT Fisher grade > 2 was independent predictors of SCVS after aSAH. According to our results, the predictive performance of the lowest HSA level for the occurrence of SCVS may be better than that of CT Fisher grade.
Hypoalbuminemia is an epiphenomenon related to the severity of the clinical picture in some patients, and the effects of HSA on disease prognosis are mainly based on its anti-inflammatory and antioxidative properties [34]. In our study, the incidence of hypoproteinemia during hospitalization was 67.4% in the aSAH group and 95.9% in the SCVS group. Hypoalbuminemia is associated with increased morbidity and mortality in a variety of diseases, and its predictive effects on diseases have been well established in previous studies [35, 36]. In our study, the patients with lowest HSA level < 32.55 g/L or average HSA level < 35.57 g/L may more likely to develop SCVS, thus the lowest and average HSA level can predict the probability of SCVS after aSAH. Further studies have suggested that hypoalbuminemia was not only common but also independently correlated with poor outcomes in aSAH patients [11]. In our study, SCVS was associated with poor functional outcome as reflected by a low GOS, similar to previous trials [11]. It has been reported that CVS is the main predictor of poor outcomes in patients with aSAH [37]. Based on these findings, we speculate that the lowest HSA can predict the occurrence of SCVS and help predict the poor functional outcome of aSAH.
This study has several limitations. First, due to the small number of samples, more variables, such as aneurysm shape, aneurysm size, and surgical treatment, could not be considered in the analysis. Second, this study was conducted in a single area, which might limit the generalizability of the results to other patient cohorts. However, the patients were selected from multiple centers via strict inclusion and exclusion criteria. Third, the follow-up period was 3 months, which is a relatively short-term follow-up study. Thus, it is necessary to perform long-term follow-up studies in the future.
Conclusion
In summary, we found that the lowest HSA level, average HSA level, and CT Fisher grade are predictive factors for SCVS in patients with aSAH. SCVS may be more likely to develop if the lowest HSA level < 32.55 g/L, average HSA level < 35.57 g/L or CT Fisher grade > 2, and the predictive performance of the lowest HSA level for the occurrence of SCVS may be better than that of average HSA level and CT Fisher grade. Therefore, the lowest HSA level can be used as a new, noninvasive, simple, low-cost, and feasible biomarker to predict the probability of SCVS after aSAH, and monitoring of HSA level is necessary in patients with aSAH. Clinicians are encouraged to measure HSA levels for the first 3 days after admission and calculate lowest HSA levels in patients with aSAH to help predict the occurrence of SCVS and take appropriate measures to prevent the occurrence of SCVS. In addition, since the HSA level is a factor that can be modified by albumin infusion, whether increasing the lowest HSA level by albumin infusion can affect the occurrence of SCVS and thus affect the functional outcome of SAH remains to be further investigated.
References
Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Wang Y, Feigin V (2017) Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation 135:759–771. https://doi.org/10.1161/circulationaha.116.025250
Connolly E, Rabinstein A, Carhuapoma J, Derdeyn C, Dion J, Higashida R, Hoh B, Kirkness C, Naidech A, Ogilvy C, Patel A, Thompson B, Vespa P (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43:1711–1737. https://doi.org/10.1161/STR.0b013e3182587839
Fu Q, Wang Y, Lin S, Yang Y (2021) Evaluation of efficacy and safety of endovascular coiling for patients with aneurysmal subarachnoid hemorrhage: a protocol for systematic review and meta-analysis. Medicine 100:e25728. https://doi.org/10.1097/md.0000000000025728
Matano F, Fujiki Y, Mizunari T, Koketsu K, Tamaki T, Murai Y, Yokota H, Morita A (2019) Serum glucose and potassium ratio as risk factors for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 28:1951–1957. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.03.041
Frontera J, Fernandez A, Schmidt J, Claassen J, Wartenberg K, Badjatia N, Connolly E, Mayer S (2009) Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke 40:1963–1968. https://doi.org/10.1161/strokeaha.108.544700
Sokolowski J, Chen C, Ding D, Buell T, Raper D, Ironside N, Taylor D, Starke R, Liu K (2018) Endovascular treatment for cerebral vasospasm following aneurysmal subarachnoid hemorrhage: predictors of outcome and retreatment. J Neurointerv Surg 10:367–374. https://doi.org/10.1136/neurintsurg-2017-013363
Vergouwen M, de Haan R, Vermeulen M, Roos Y (2010) Effect of statin treatment on vasospasm, delayed cerebral ischemia, and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke 41:e47–e52. https://doi.org/10.1161/strokeaha.109.556332
Haley EC Jr, Kassell NF, Torner JC (1992) The international cooperative study on the timing of aneurysm surgery. The North American experience. Stroke 23:205–214. https://doi.org/10.1161/01.str.23.2.205
Harrod C, Bendok B, Batjer H (2005) Prediction of cerebral vasospasm in patients presenting with aneurysmal subarachnoid hemorrhage: a review. Neurosurgery 56:633–654 discussion 633-654. https://doi.org/10.1227/01.neu.0000156644.45384.92
Lang J, Figueroa M, Chumley P, Aslan M, Hurt J, Tarpey M, Alvarez B, Radi R, Freeman B (2004) Albumin and hydroxyethyl starch modulate oxidative inflammatory injury to vascular endothelium. Anesthesiology 100:51–58. https://doi.org/10.1097/00000542-200401000-00012
Wang L, Li M, Xie Y, Xu L, Ye R, Liu X (2017) Preclinical efficacy of human albumin in subarachnoid hemorrhage. Neuroscience 344:255–264. https://doi.org/10.1016/j.neuroscience.2016.12.033
Suarez J, Martin R, Calvillo E, Dillon C, Bershad E, Macdonald R, Wong J, Harbaugh R (2012) The Albumin in Subarachnoid Hemorrhage (ALISAH) multicenter pilot clinical trial: safety and neurologic outcomes. Stroke 43:683–690. https://doi.org/10.1161/strokeaha.111.633958
Rumalla K, Lin M, Ding L, Gaddis M, Giannotta S, Attenello F, Mack W (2021) Risk factors for cerebral vasospasm in aneurysmal subarachnoid hemorrhage: a population-based study of 8346 patients. World Neurosurg 145:e233–e241. https://doi.org/10.1016/j.wneu.2020.10.008
Zhang J (2015) Guidelines for diagnosis and management of aneurysmal subarachnoid hemorrhage: top issues and prospective. Zhejiang Da Xue Xue Bao Yi Xue Ban 44:357–360. https://doi.org/10.3785/j.issn.1008-9292.2015.07.01
Vergouwen M, Vermeulen M, van Gijn J, Rinkel G, Wijdicks E, Muizelaar J, Mendelow A, Juvela S, Yonas H, Terbrugge K, Macdonald R, Diringer M, Broderick J, Dreier J, Roos Y (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41:2391–2395. https://doi.org/10.1161/strokeaha.110.589275
Ciurea A, Palade C, Voinescu D, Nica D (2013) Subarachnoid hemorrhage and cerebral vasospasm — literature review. J Med Life 6:120–125
Song Y, Qian S, Li Y, Liu J, Li Z, Jia X, Gao H, Zeng J (2019) Effectiveness and safety of nimodipine in preventing cerebral vasospasm after subarachnoid hemorrhage in children. Zhonghua Er Ke Za Zhi 57:338–343. https://doi.org/10.3760/cma.j.issn.0578-1310.2019.05.005
Kapapa T, König R, Mayer B, Braun M, Schmitz B, Müller S, Schick J, Wirtz C, Pala A (2021) Adverse events and complications in continuous intra-arterial nimodipine infusion therapy after aneurysmal subarachnoid hemorrhage. Front Neurol 12:812898. https://doi.org/10.3389/fneur.2021.812898
Provencio J, Vora N (2005) Subarachnoid hemorrhage and inflammation: bench to bedside and back. Semin Neurol 25:435–444. https://doi.org/10.1055/s-2005-923537
Ikeda K, Ikeda T, Suzuki H, Taniuchi H, Nagura M, Ooshima K (2003) Colloid osmotic pressure (COP) can be a good indicator of occurrence of vasospasm following subarachnoid hemorrhage (SAH). Med Sci Monit 9:CR43–CR47
Buce-Satoba I, Rozkalne D, Mamaja B, Krumina G, Ozolina A (2022) Leukocytosis and C-reactive protein may predict development of secondary cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Medicina (Kaunas) 58:323. https://doi.org/10.3390/medicina58020323
Tsai T, Lin S, Wu C, Tsai Y, Yang S, Lin C (2020) Mechanisms and therapeutic implications of RTA 408, an activator of Nrf2, in subarachnoid hemorrhage-induced delayed cerebral vasospasm and secondary brain injury. PLoS One 15:e0240122. https://doi.org/10.1371/journal.pone.0240122
Wu C, Tsai H, Su Y, Tsai C, Lu Y, Lin C (2022) 2-PMAP ameliorates cerebral vasospasm and brain injury after subarachnoid hemorrhage by regulating neuro-inflammation in rats. Cells 11:242. https://doi.org/10.3390/cells11020242
Kolias A, Sen J, Belli A (2009) Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: putative mechanisms and novel approaches. J Neurosci Res 87:1–11. https://doi.org/10.1002/jnr.21823
Croci D, Sivanrupan S, Wanderer S, Agnoletto G, Chiappini A, Grüter B, Andereggen L, Mariani L, Taussky P, Marbacher S (2022) Preclinical and clinical role of interleukin-6 in the development of delayed cerebral vasospasm and neuronal cell death after subarachnoid hemorrhage: towards a potential target therapy? Neurosurg Rev 45:395–403. https://doi.org/10.1007/s10143-021-01628-9
Ghali M, Srinivasan V, Johnson J, Kan P, Britz G (2018) Therapeutically targeting platelet-derived growth factor-mediated signaling underlying the pathogenesis of subarachnoid hemorrhage-related vasospasm. J Stroke Cerebrovasc Dis 27:2289–2295. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.02.017
Demling R (1986) Effect of plasma and interstitial protein content on tissue edema formation. Curr Stud Hematol Blood Transfus:36–52. https://doi.org/10.1159/000413163
Keaney J, Simon D, Stamler J, Jaraki O, Scharfstein J, Vita J, Loscalzo J (1993) NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J Clin Invest 91:1582–1589. https://doi.org/10.1172/jci116364
Chen J, Cheng K, Tsai Y, Hong Y, Howng S, Kwan A, Chen I, Wu B (2012) Potassium-channel openers KMUP-1 and pinacidil prevent subarachnoid hemorrhage-induced vasospasm by restoring the BKCa-channel activity. Shock 38:203–212. https://doi.org/10.1097/SHK.0b013e31825b2d82
Sheinenzon A, Shehadeh M, Michelis R, Shaoul E, Ronen O (2021) Serum albumin levels and inflammation. Int J Biol Macromol 184:857–862. https://doi.org/10.1016/j.ijbiomac.2021.06.140
Mangoni A, Zinellu A (2022) A systematic review and meta-analysis of serum concentrations of ischaemia-modified albumin in acute ischaemic stroke, intracerebral haemorrhage, and subarachnoid haemorrhage. Biomolecules 12:653. https://doi.org/10.3390/biom12050653
van der Zee P, Verberne H, van Straalen J, Sanders G, Van Eck-Smit B, de Winter R, Fischer J (2005) Ischemia-modified albumin measurements in symptom-limited exercise myocardial perfusion scintigraphy reflect serum albumin concentrations but not myocardial ischemia. Clin Chem 51:1744–1746. https://doi.org/10.1373/clinchem.2005.054635
Shevtsova A, Gordiienko I, Tkachenko V, Ushakova G (2021) Ischemia-modified albumin: origins and clinical implications. Dis Markers 2021:9945424. https://doi.org/10.1155/2021/9945424
Nicholson J, Wolmarans M, Park G (2000) The role of albumin in critical illness. Br J Anaesth 85:599–610. https://doi.org/10.1093/bja/85.4.599
Jin G, Li L, Cui S, Duan J, Wang H (2014) Persistent hypoalbuminemia is a predictor of outcome in cervical spinal cord injury. Spine J 14:1902–1908. https://doi.org/10.1016/j.spinee.2013.10.049
Seebacher V, Grimm C, Reinthaller A, Heinze G, Tempfer C, Hefler L, Polterauer S (2013) The value of serum albumin as a novel independent marker for prognosis in patients with endometrial cancer. Eur J Obstet Gynecol Reprod Biol 171:101–106. https://doi.org/10.1016/j.ejogrb.2013.07.044
Zimmermann J, Weller J, Grub S, Kebir S, Lehmann F, Vatter H, Schuss P, Güresir E, Müller M (2022) Arginase-1 released into CSF after aneurysmal subarachnoid hemorrhage decreases arginine/ornithine ratio: a novel prognostic biomarker. Transl Stroke Res 13:382–390. https://doi.org/10.1007/s12975-021-00944-y
Acknowledgements
I want to take this chance to thank my supervisor and colleague for helpful discussion on topics related to this work. We would like to thank the editor and anonymous reviewers for their helpful remarks.
Funding
This work was supported by the Research Foundation of Anhui Medical University (2019xkj149).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Informed consent
Informed consent was obtained in all cases in the present study.
Ethical approval
This research study was conducted retrospectively from data obtained for clinical purposes. The protocol was approved by the local ethics committee of the hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ling Chen, Yang Jin, and Ling Wang contributed to this work equally.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, ., Jin, Y., Wang, L. et al. Impact of human serum albumin level on symptomatic cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurol Sci 45, 213–222 (2024). https://doi.org/10.1007/s10072-023-07014-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07014-1