Abstract
Introduction
Nusinersen was effective in improving motor function and survival in infantile and childhood-onset spinal muscular atrophy (SMA), and the value of real-world experiences in adult SMA patients increase gradually. Here, we present our clinical experience in adult SMA patients treated with nusinersen according to CHERISH study.
Material and methods
Thirty-two SMA patients treated with nusinersen were included in the study.
Results
Median age at nusinersen initiation was 33.5 (20.0–60.0) years and 23 of SMA patients were male. Six (18.8%) patients had SMA type 2, and 26 (81.2%) had SMA type 3. Median follow-up period of patients under nusinersen treatment was 17 months (9–21). Twenty-three patients improved by at least 3 Hammersmith Functional Motor Scale Expanded (HFMSE) points after loading doses. There was significant HFMSE score increase in type 3 patients at each time point, whereas type 2 patients seem to benefit from nusinersen loading doses, subsequently stayed stable. Motor improvement was positively correlated with baseline HFMSE scores in patients whose baseline HFMSE scores were ≤47. There was a correlation between the changes in Amyotrophic Lateral Sclerosis Functional Rating Scale Revised (ALSFRS-R) score and HFMSE scores. Ambulatory patients who could not show clinically meaningful increase in HFMSE scores improved at least 30 m by 6-min walk test (6MWT).
Conclusion
Overall, 78% of patients have responded to treatment according to HFMSE or 6MWT. ALSFRS-R and 6MWT may be alternative tools to monitor nusinersen effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal muscular atrophy (SMA) represents a group of inherited, progressive neuromuscular disorders characterized by the degeneration of anterior horn cells of the spinal cord and brainstem motor nuclei [1]. Homozygote deletions in exon 7 and/or exon 8 of SMN1, survival motor neuron 1 gene, mapped in 5q11.2–13.3 are responsible for 5q autosomal recessive SMA which is the most common SMA mutation accounting for over 95% of SMA cases [2, 3]. SMN1 mutations cause deficiency of SMN protein leading to motor neuron dysfunction and progressive muscle atrophy [4]. SMN protein may also be produced by SMN2 gene which is nearly identical except a substitution at base 840 in exon 7. This alteration results in alternative splicing with exclusion of exon 7 and a malfunctioning protein with short half-life [5]. However, this low amount of protein is sufficient to modulate disease severity and determine distinct SMA types by copy number of SMN2 [6].
Nusinersen, which is an antisense-oligonucleotide (ASO) derivative, modifies the SMN2 pre-mRNA splicing process and increases SMN protein synthesis [7,8,9]. The drug was approved by Turkish National Ministry of Health in February 2019 for adults with SMA types 2 or 3 with the dosage schedule according to CHERISH study [10]. Here, we present our clinical experience with adult SMA patients treated with nusinersen.
Material and methods
This study was approved by local and National Ethics Committees (KA-21040 and 21-AKD-94). Written informed consent was obtained from all participants, according to the Helsinki declaration.
Patients
Patients with SMA type 2 and type 3 receiving nusinersen were included in the study. Inclusion criteria were defined by National Ministry of Health, as follow: (1) confirmed molecular diagnosis for SMA associated with 5q and detection of at least two copy numbers of SMN2; (2) nusinersen therapy started at age > 18 years; (3) no evidence of intracranial infection and oral intake problems (no need of any additional assistive devices for nutrition); (4) no history of brain or spinal cord disease that may interfere with lumbar puncture procedures or cerebrospinal fluid circulation; (5) no need for invasive or non-invasive respiratory support; (6) available clinical data at baseline (t0), 9th month (t1), and 15th month (t2); and (7) fulfillment of four loading doses of nusinersen in accordance with CHERISH study protocol (on days 0, 29, 85, and 274).
Dosing
All patients recruited to this study received loading doses of 12 mg nusinersen at baseline (t0), day 29, day 85, and day 274 in accordance with CHERISH study protocol due to the national policies [10]. First maintenance treatment was administered at 15th month in accordance with CHERISH study protocol and subsequent administrations were scheduled at 4-month intervals in patients who were classified as “responders,” which will be defined in the “Clinical evaluation” section.
Intrathecal administration
Intrathecal injections were performed by using the standard lumbar puncture procedure in 29 patients (90.6%). CT-guided intrathecal administration was performed in two patients; reservoir was used in one patient whom standard lumbar puncture could not be done because of scoliosis.
Clinical evaluation
Demographic data, patient characteristics, and motor function were evaluated, and safety data were collected at baseline (t0), 9th month (t1), and 15th month (t2). SMA type 3 patients were divided into two groups as “sitters” and “walkers.” The walkers were patients who were able to walk at least 30 m with or without support. SMA type 3 patients who cannot walk this distance were classified as sitters.
Outcome assessments were rated by trained evaluators. Primary outcome measurements were determined as (1) Hammersmith Functional Motor Scale Expanded (HFMSE) score at t0, t1, and t2 [11]; (2) Medical Research Council sum score (MRC-SS) at t0 and t2 [12]; (3) Amyotrophic Lateral Sclerosis Functional Rating Scale Revised (ALSFRS-R) score at t0 and t2 [13]; and (4) 6-min walk test (6MWT) distance for ambulatory patients (SMA type 3 walkers) at t0 and t2 [14].
Responders were defined as patients who improved from baseline by at least 3 HFMSE points [15] or 30 m in 6MWT distance at t2 [16], and these changes were further defined as “clinically meaningful improvement.” Patients who demonstrated clinical meaningful improvement continued to nusinersen maintenance treatment. Motor functions were evaluated after first (19th months, t3) and second maintenance dose (23rd months, t4) by HFMSE in a subgroup consisted of responder patients who continued maintenance dosing.
Safety
Safety data was evaluated at each time point (t0, t1, and t2) by (1) clinical adverse events defined as post-lumbar puncture headache and injection site pain and (2) laboratory findings including complete blood count, liver enzyme level, blood creatine and urea nitrogen, spot urine protein and creatinine levels, prothrombin time, and international normalized ratio (INR). Additionally, random protein to creatinine ratio, which is strongly associated with 24-h total protein excretion, was analyzed for early prediction of nephrotoxicity effect of nusinersen at each time point. Each abnormal finding was evaluated according to their severity and relationship with nusinersen.
Statistical analysis
The data was analyzed by SPSS version 23 software package program (IBM Corporation, USA) and graphics were designed by GraphPad Prism version 9.3.1 (GraphPad Prism Software Inc., San Diego, CA). The normality of data distribution was determined by using the Kolmogorov–Smirnov test. Patient characteristics were presented using descriptive statistics. Wilcoxon signed-rank test was used to compare two related samples and matched samples, while Mann–Whitney U test was used for independent samples. The Friedman test was used to analyze three or more repeated measurements. If the difference is statistically significant, a post hoc paired comparison was implemented. Chi-square test and Fisher’s exact test were used to determine if there are non-random associations between categorical variables. Fisher-Freeman-Halton test was used to analyze the mean of multiple groups in pairs to determine if they differed significantly from each other. When statistically significant results were reached, post hoc analyses were applied to predict which groups caused the difference. Spearman’s correlation was used to measure the strength and direction of monotonic association between variables. The significance level was set at p < 0.05.
Results
Patient characteristics
Forty-one SMA patients receiving nusinersen in our center were evaluated. Seven patients who could not complete four loading doses due to lumbar puncture difficulties and two patients who were dosed according to ENDEAR study were excluded. Finally, 32 SMA patients treated with nusinersen were included in the study.
The median age at nusinersen initiation was 33.5 (20–60) years, and 23 of SMA patients were male. The median age of disease onset (t0) was 4.5 (0.6–19) years. Six (18.8%) patients had SMA type 2 whereas 26 (81.2%) had SMA type 3. Among SMA type 3 patients, 16 (61.5%) were classified as sitters and the rest were able to walk walkers. Median follow-up period of patients on nusinersen treatment was 23 months (range 15–23). Patient characteristics, SMN2 copy number, and baseline functional assessments of the patients are summarized in Table 1.
Functional assessments
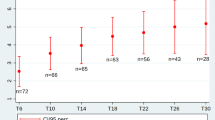
The median HFMSE score was 33.5 (range 2–65) at t0, 36.5 (range 4–66) at t1, and 38 (range 4–66) at t2. There was a statistically significant difference in HFMSE scores depending on time points (χ2(2) = 57.4, p < 0.001). HFMSE score in whole group analysis increased from baseline by a median of 3 points (0–10) at t1 (p < 0.001) and 4 points (0–11) at t2 (p < 0.001). Median HFMSE score of all study groups at each time point was demonstrated in Fig. 1.
HFMSE changes were statistically significant compared to baseline in all study groups: a median of 2 points (0–3) increases in SMA type 2 (p = 0.041), a median of 4 points (1–11) in sitters (p < 0.001), and a median of 6 points (1–11) increase in walkers (p = 0.005) was observed at t2. Although the difference between HFMSE scores at t1 and t2 was statistically significant in SMA type 3 groups sitters and walkers, it was not statistically significant in SMA type 2 patients (Table 2 and Fig. 2).
Correlation analysis did not disclose any correlation between the change in HFMSE score and patient age (r = − 0.29, p = 0.95). However, a moderate positive correlation was observed between HFMSE scores at baseline and improvement in HFMSE score at t2 in patients with overall HFMSE score lower than 47 at baseline (r: + 0.43, p = 0.03).
All patients treated with nusinersen were also assessed by ALSFRS-R and MRC-SS at t0 and t2 (Fig. 3). The median ALSFRS-R score was 36 (28–45) at t0 and 37 (28–47) at t2. ALSFRS-R score in whole group analysis increased from baseline by a median of 1 point (0–4) at t2 (p < 0.001). Additionally, MRC-SS in whole group analysis increased from baseline with a range between 0 and 2 at t2 (p = 0.001). At t2, SMA type 3 walkers had the highest score increase in all assessments as the median increase was 6 points by HFMSE, 1.5 points by ALSFRS-R, and 1 point by MRC-SS. However, both ALSFRS-R score and MRC-SS did not show any statistically significant difference in SMA type 2 patients comparing to baseline (p = 0.31 and p > 0.99, respectively). In the whole group analysis, there was a positive correlation between the change of ALSFRS-R and HFMSE scores from baseline to t2 (r: + 0.48, p = 0.005), but no correlation was observed between MRC-SS and HFMSE score changes (r: + 0.24, p = 0.17) (Table 2).
All ambulatory patients (n = 10, SMA type 3 walkers) were also assessed by 6MWT at t0 and t2. Median 6MWT distance at t0 was 377 (60–523) and 410 (69–558) at t2 (p = 0.008).
Responders
Patients who improved from baseline at least 3 HFMSE points or 30 m by 6MWT at t2 were defined as responders. Based on HFMSE criteria 23 patients responded to nusinersen treatment. Besides, two ambulatory SMA type 3 patients who have high baseline scores (baseline scores were 65 and 63, respectively) were accepted as “responder” due to 30-m increase in 6MWT distance despite the lack of 3-point increase in HFMSE score. As a result, 78% (n = 25) of all SMA patients showed clinically meaningful improvement by HFMSE or 6MWT after loading doses of nusinersen and considered as responders to treatment. The maintenance treatment was continued in responders except for one patient who rejected the treatment due to lumbar puncture difficulties. Demographic, genetic, or clinical characteristics affecting response to treatment were examined (Online Resource 1). In our cohort, SMA type 3 patients responded better to nusinersen treatment (p = 0.012), and in post hoc analyses, it was observed that the difference between SMA type 2 and type 3 was significant due to SMA type 3 sitters.
Subgroup evaluation
HFMSE scores were also evaluated at t3 and t4 in 22 patients with SMA type 3 who were defined as responders and continued to maintenance treatment. One patient who received nusinersen by conventional lumbar puncture and improved from baseline by 6 HFMSE points at t2 rejected the maintenance treatment due to invasive procedure. No significant change was observed by HFMSE score after t3; therefore, it was concluded that the patients remained stable after this time point (Online Resource 2).
Safety
The most common side effects related to procedure were injection site pain and post-lumbar puncture headache which were reported at least once in 53.1% (n = 17) and 43.75% (n = 14) of patients, respectively. Headache intensity was mild as less than 4 according to visual analog scale score, and mostly resolved within 1–3 days. Only one SMA type 2 patient (16.6%) required hospitalization and intravenous hydration due to severe headache after the second dose. Additionally, 32.2% of the patients (n = 10) showed proteinuria at least once. Proteinuria was detected in seven patients at t3, and in three patients at t4. Proteinuria was mild (150–500 mg/g creatinine) in all cases and did not require discontinuation of the treatment.
Discussion
Nusinersen has been found to be effective in improving motor function and survival in infantile and childhood-onset SMA, but at the time of approval, nusinersen trials did not include adults [17, 18]. After FDA approval which enabled the use of the treatment in all SMA patients, multiple real-life outcome data and retrospective observational studies were published [15, 19]. However, the lack of placebo controlled clinical trials in adult SMA patients increases the value of real-world experiences day by day. In addition, current functional assessment tools are insufficient to measure the outcome in adult SMA patients whose baseline functional levels are very heterogenous [20]. Besides, adult SMA patients experience chronic musculoskeletal complications and age-specific comorbidities due to long disease duration [21]. All these conditions complicate the follow-up of adult SMA patients and support the need of objective criteria for clinicians to evaluate and monitor treatment response.
Considering all these disadvantages, a few real-world experiences and some multicenter observational studies continue to support the efficacy of nusinersen in adult SMA patients [22,23,24,25,26,27]. This current study differs from previous studies in terms of dosage schedule which was done according to CHERISH study due to national policy at the time of study. However, our observational study also showed comparable safety and efficacy with CHERISH dosage scheme in adult SMA patients.
Main finding of the study was the statistically significant increase of HFMSE score in SMA type 3 patients receiving nusinersen. Our cohort did not demonstrate any significantly difference in terms of treatment response between SMA type 3 sitters and walkers. Additionally, improvement in motor function, which is assessed by HFMSE at 9th and 15th months, was positively correlated with baseline HMFSE scores in patients with baseline HMFSE scores less than 47 points. This finding supports that the increase in HFMSE score is correlated with the lower severity of the disease at baseline, as demonstrated previously [25]. However, differing from Hagenacker et al.’s cohort [25], positive correlation was only seen in patients with a baseline score less than 47, and this may be due to the heterogeneity of our cohort and due to the patients with a higher baseline score comparing to other cohorts. This finding emphasizes the necessity of alternative outcome measures in the follow-up of patients with higher HFMSE scores. Thus, we also assessed the motor functions by ALSFRS-R and MRC-SS in adult SMA patients at t0 and t2 to increase the reliability of the study and found similar significant improvements. There was a positive correlation between the increase of ALSFRS-R and HFMSE score changes, but not between MRC-SS and HFMSE score changes in SMA 3 patients, whereas none of the scores show any statistically significant difference in SMA type 2 patients compared to baseline. Therefore, unlikely to MRC-SS, ALSFRS-R may be an alternative tool to demonstrate clinically meaningful motor improvements in SMA type 3 patients whose HFMSE scores are higher.
SMA type 3 walkers were demonstrated median change of 6 points between t0 and t2 with HFMSE, which is very surprising considering these are highly performing patients with a median of 50 points at t0. On the other hand, baseline functional scores of the SMA type 3 walker group showed heterogeneous features. There were patients with a nearly reached overall HFMSE score, as well as patients with a baseline score of 38 or 39. Benefit was more pronounced in patients with lower baseline scores among type 3 walkers (patients with a baseline score range between 38 and 50). Although higher baseline HFMSE points indicate better clinical response from nusinersen therapy, in patients who are close to the overall score of HFMSE, it is hard to demonstrate powerful increase with HFMSE score. One of SMA type 3 walkers showed 10 HFMSE point increase at t1; this patient gained only one point increase between t1 and t2. The early effect of nusinersen in this patient did not affect the primary outcome, and the patient showed motor stabilization during follow-up. In our cohort, only three patients showed an increase in HFMSE score more than 10 points. All these patients had SMA type 3; male at the age ranged from 24 to 27 years. Although the number is low, this finding is worth to mention due to different baseline functional levels as two patients were sitter, and one was walker. However, we did not find any correlation between the age at treatment initiation and treatment response. We think that with the analysis of longer and larger studies, specific characteristics of patients who take the best advantages from nusinersen will come out.
In our patients with SMA type 2, only two of the six showed clinically meaningful increase in HFMSE score. On the other hand, we found statistically significant changes in SMA type 2 patients both at t1 and t2 by HFMSE score, in comparison to baseline (t0). However, there was no statically significant difference between t1 and t2 scores. It can be concluded that SMA type 2 patients may benefit from nusinersen in early period and in the advanced period they may maintain a stabilization of motor functions. This finding was also supported by ALSFRS-R score and MRC-SS. While the small size of our SMA type 2 group does not allow the exact results to be related to treatment response, it supports existing data [28]. On the follow-up, the stabilization of the patients can be considered as a positive effect of nusinersen.
Overall, 78% of the patients have responded to the treatment and demonstrated clinically meaningful increase in HFMSE score or 6MWT distance. Besides, subgroup analysis of responder SMA type 3 patients showed motor improvement until 19th month, then functional motor stabilization at the following visit (23rd month) which can also be considered as a positive effect of nusinersen.
In our cohort, there were two ambulatory patients with baseline HFMSE scores above 60. Even though they could not show clinically meaningful increase in HFMSE scores after loading doses, an increase of more than 30 m was observed in 6MWT distance. In these particular groups of patients, other functional assessment tools such as 6MWT are needed to measure outcome [22, 25, 27, 29] and 6MWT seems to be more useful to evaluate treatment response in patients who had almost reached the maximum HFMSE score.
This study verifies the safety and efficacy of intrathecal nusinersen. However, our study has some limitations. Due to observational design of the study, we have some missing assessments such as RULM (revised upper limb module) and pulmonary functional tests. However, differing from other cohorts, we included the analyses of MRC-SS and ALSFRS-R to find alternative outcome measurements for adult SMA patients. The other limitation is small sample size of the cohort, which is as being of a single-center experience.
Conclusions
Despite small sample size of SMA type 2 and lack of other functional assessment tools, our study confirms the safety and efficacy in adult SMA patients. The noticeable difference of our study from the literature is the dosage schedule done according to CHERISH study. However, the difference of dosage schedule did not change the outcome in adult SMA patients. Therefore, assessment tools other than HFMSE score may provide alternative ways to evaluate clinical response in adult SMA patients who cannot be monitored by HFMSE score or cannot demonstrate clinically meaningful increase by HFMSE score.
Data Availability
Data are available for any authorized researcher upon formal request to the corresponding author.
References
Werdnig G (1971) Two early infantile hereditary cases of progressive muscular atrophy simulating dystrophy, but on a neural basis. 1891. Arch Neurol 25:276–278. https://doi.org/10.1001/archneur.1971.00490030102014
Brzustowicz LM, Lehner T, Castilla LH, Penchaszadeh GK, Wilhelmsen KC, Daniels R, Davies KE, Leppert M, Ziter F, Wood D, Dubowitz V, Zerres K, Hausmanowa-Petrusewicz I, Ott J, Munsat TL, Gilliam TC (1990) Genetic mapping of chronic childhood-onset spinal muscular atrophy to chromosome 5q1 1.2–13.3. Nature 344:540–541. https://doi.org/10.1038/344540a0
Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M et al (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80:155–165. https://doi.org/10.1016/0092-8674(95)90460-3
Vitte J, Fassier C, Tiziano FD, Dalard C, Soave S, Roblot N, Brahe C, Saugier-Veber P, Bonnefont JP, Melki J (2007) Refined characterization of the expression and stability of the SMN gene products. Am J Pathol 171:1269–1280. https://doi.org/10.2353/ajpath.2007.070399
Wirth B (2000) An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum Mutat 15:228–237. https://doi.org/10.1002/(sici)1098-1004(200003)15:3%3c228::Aid-humu3%3e3.0.Co;2-9
Gavrilov DK, Shi X, Das K, Gilliam TC, Wang CH (1998) Differential SMN2 expression associated with SMA severity. Nat Genet 20:230–231. https://doi.org/10.1038/3030
Chiriboga CA, Swoboda KJ, Darras BT, Iannaccone ST, Montes J, De Vivo DC, Norris DA, Bennett CF, Bishop KM (2016) Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology 86:890–897. https://doi.org/10.1212/wnl.0000000000002445
Hua Y, Sahashi K, Hung G, Rigo F, Passini MA, Bennett CF, Krainer AR (2010) Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev 24:1634–1644. https://doi.org/10.1101/gad.1941310
Passini MA, Bu J, Richards AM, Kinnecom C, Sardi SP, Stanek LM, Hua Y, Rigo F, Matson J, Hung G, Kaye EM, Shihabuddin LS, Krainer AR, Bennett CF, Cheng SH (2011) Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci Transl Med 3:72ra18. https://doi.org/10.1126/scitranslmed.3001777
Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, Iannaccone ST, Kirschner J, Kuntz NL, Saito K, Shieh PB, Tulinius M, Mazzone ES, Montes J, Bishop KM, Yang Q, Foster R, Gheuens S, Bennett CF, Farwell W, Schneider E, De Vivo DC, Finkel RS (2018) Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med 378:625–635. https://doi.org/10.1056/NEJMoa1710504
Glanzman AM, O’Hagen JM, McDermott MP, Martens WB, Flickinger J, Riley S, Quigley J, Montes J, Dunaway S, Deng L, Chung WK, Tawil R, Darras BT, De Vivo DC, Kaufmann P, Finkel RS (2011) Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J Child Neurol 26:1499–1507. https://doi.org/10.1177/0883073811420294
Kleyweg RP, van der Meché FG, Schmitz PI (1991) Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 14:1103–1109. https://doi.org/10.1002/mus.880141111
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169:13–21. https://doi.org/10.1016/s0022-510x(99)00210-5
Montes J, McDermott MP, Martens WB, Dunaway S, Glanzman AM, Riley S, Quigley J, Montgomery MJ, Sproule D, Tawil R, Chung WK, Darras BT, De Vivo DC, Kaufmann P, Finkel RS (2010) Six-Minute Walk Test demonstrates motor fatigue in spinal muscular atrophy. Neurology 74:833–838. https://doi.org/10.1212/WNL.0b013e3181d3e308
Pera MC, Coratti G, Forcina N, Mazzone ES, Scoto M, Montes J, Pasternak A, Mayhew A, Messina S, Sframeli M, Main M, Lofra RM, Duong T, Ramsey D, Dunaway S, Salazar R, Fanelli L, Civitello M, de Sanctis R, Antonaci L, Lapenta L, Lucibello S, Pane M, Day J, Darras BT, De Vivo DC, Muntoni F, Finkel R, Mercuri E (2017) Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy. BMC Neurol 17:39. https://doi.org/10.1186/s12883-017-0790-9
Dunaway Young S, Montes J, Kramer SS, Marra J, Salazar R, Cruz R, Chiriboga CA, Garber CE, De Vivo DC (2016) Six-minute walk test is reliable and valid in spinal muscular atrophy. Muscle Nerve 54:836–842. https://doi.org/10.1002/mus.25120
Finkel RS, Chiriboga CA, Vajsar J, Day JW, Montes J, De Vivo DC, Yamashita M, Rigo F, Hung G, Schneider E, Norris DA, Xia S, Bennett CF, Bishop KM (2016) Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 388:3017–3026. https://doi.org/10.1016/s0140-6736(16)31408-8
Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, Chiriboga CA, Saito K, Servais L, Tizzano E, Topaloglu H, Tulinius M, Montes J, Glanzman AM, Bishop K, Zhong ZJ, Gheuens S, Bennett CF, Schneider E, Farwell W, De Vivo DC (2017) Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med 377:1723–1732. https://doi.org/10.1056/NEJMoa1702752
Mercuri E, Finkel R, Montes J, Mazzone ES, Sormani MP, Main M, Ramsey D, Mayhew A, Glanzman AM, Dunaway S, Salazar R, Pasternak A, Quigley J, Pane M, Pera MC, Scoto M, Messina S, Sframeli M, Vita GL, D’Amico A, van den Hauwe M, Sivo S, Goemans N, Kaufmann P, Darras BT, Bertini E, Muntoni F, De Vivo DC (2016) Patterns of disease progression in type 2 and 3 SMA: implications for clinical trials. Neuromuscul Disord 26:126–131. https://doi.org/10.1016/j.nmd.2015.10.006
Mercuri E, Sansone V (2020) Nusinersen in adults with spinal muscular atrophy: new challenges. Lancet Neurol 19:283–284. https://doi.org/10.1016/s1474-4422(20)30068-5
Finkel RS, Mercuri E, Meyer OH, Simonds AK, Schroth MK, Graham RJ, Kirschner J, Iannaccone ST, Crawford TO, Woods S, Muntoni F, Wirth B, Montes J, Main M, Mazzone ES, Vitale M, Snyder B, Quijano-Roy S, Bertini E, Davis RH, Qian Y, Sejersen T (2018) Diagnosis and management of spinal muscular atrophy: part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord 28:197–207. https://doi.org/10.1016/j.nmd.2017.11.004
Walter MC, Wenninger S, Thiele S, Stauber J, Hiebeler M, Greckl E, Stahl K, Pechmann A, Lochmüller H, Kirschner J, Schoser B (2019) Safety and treatment effects of nusinersen in longstanding adult 5q-SMA type 3 — a prospective observational study. J Neuromuscul Dis 6:453–465. https://doi.org/10.3233/jnd-190416
Veerapandiyan A, Eichinger K, Guntrum D, Kwon J, Baker L, Collins E, Ciafaloni E (2020) Nusinersen for older patients with spinal muscular atrophy: a real-world clinical setting experience. Muscle Nerve 61:222–226. https://doi.org/10.1002/mus.26769
Jochmann E, Steinbach R, Jochmann T, Chung HY, Rödiger A, Neumann R, Mayer TE, Kirchhof K, Loudovici-Krug D, Smolenski UC, Witte OW, Grosskreutz J (2020) Experiences from treating seven adult 5q spinal muscular atrophy patients with nusinersen. Ther Adv Neurol Disord 13:1756286420907803. https://doi.org/10.1177/1756286420907803
Hagenacker T, Wurster CD, Günther R, Schreiber-Katz O, Osmanovic A, Petri S, Weiler M, Ziegler A, Kuttler J, Koch JC, Schneider I, Wunderlich G, Schloss N, Lehmann HC, Cordts I, Deschauer M, Lingor P, Kamm C, Stolte B, Pietruck L, Totzeck A, Kizina K, Mönninghoff C, von Velsen O, Ose C, Reichmann H, Forsting M, Pechmann A, Kirschner J, Ludolph AC, Hermann A, Kleinschnitz C (2020) Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol 19:317–325. https://doi.org/10.1016/s1474-4422(20)30037-5
Yeo CJJ, Simeone SD, Townsend EL, Zhang RZ, Swoboda KJ (2020) Prospective cohort study of nusinersen treatment in adults with spinal muscular atrophy. J Neuromuscul Dis 7:257–268. https://doi.org/10.3233/jnd-190453
Maggi L, Bello L, Bonanno S, Govoni A, Caponnetto C, Passamano L, Grandis M, Trojsi F, Cerri F, Ferraro M, Bozzoni V, Caumo L, Piras R, Tanel R, Saccani E, Meneri M, Vacchiano V, Ricci G, Soraru G, D’Errico E, Tramacere I, Bortolani S, Pavesi G, Zanin R, Silvestrini M, Politano L, Schenone A, Previtali SC, Berardinelli A, Turri M, Verriello L, Coccia M, Mantegazza R, Liguori R, Filosto M, Marrosu G, Siciliano G, Simone IL, Mongini T, Comi G, Pegoraro E (2020) Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J Neurol Neurosurg Psychiatry 91:1166–1174. https://doi.org/10.1136/jnnp-2020-323822
Coratti G, Cutrona C, Pera MC, Bovis F, Ponzano M, Chieppa F, Antonaci L, Sansone V, Finkel R, Pane M, Mercuri E (2021) Motor function in type 2 and 3 SMA patients treated with nusinersen: a critical review and meta-analysis. Orphanet J Rare Dis 16:430. https://doi.org/10.1186/s13023-021-02065-z
De Wel B, Goosens V, Sobota A, Van Camp E, Geukens E, Van Kerschaver G, Jagut M, Claes K, Claeys KG (2021) Nusinersen treatment significantly improves hand grip strength, hand motor function and MRC sum scores in adult patients with spinal muscular atrophy types 3 and 4. J Neurol 268:923–935. https://doi.org/10.1007/s00415-020-10223-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by local and National Ethics Committees (KA-21040 and 21-AKD-94). Written informed consent was obtained from all participants, according to the Helsinki declaration.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arslan, D., Inan, B., Kilinc, M. et al. Nusinersen for adults with spinal muscular atrophy. Neurol Sci 44, 2393–2400 (2023). https://doi.org/10.1007/s10072-023-06698-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06698-9