Abstract
Background
Fingolimod, natalizumab, and ocrelizumab are commonly used in the second-line treatment of relapsing-remitting multiple sclerosis (RRMS). However, these have only been compared in observational studies, not in controlled trials, with limited and inconclusive results being reported. A comparison of their effect on relapse and disability in a real-world setting is therefore needed.
Objectives
The objective of this study was to compare the efficacy of fingolimod, natalizumab, and ocrelizumab in reducing disease activity in RRMS.
Methods
This multicenter, retrospective observational study was carried out with prospectively collected data from 16 centers. All consecutive RRMS patients treated with fingolimod, natalizumab, and ocrelizumab were included. Data for relapses, Expanded Disability Status Scale (EDSS) scores, and brain magnetic resonance imaging (MRI) scans were collected. Patients were matched using propensity scores. Annualized relapse rates (ARR), time to first relapse, and disability accumulation were compared.
Results
Propensity score matching retained 736 patients in the fingolimod versus 370 in the natalizumab groups, 762 in the fingolimod versus 434 in the ocrelizumab groups, and 310 in the natalizumab versus 310 in the ocrelizumab groups for final analyses. Mean ARR decreased markedly from baseline after treatment in all three treatment groups. Mean on-treatment ARR was lower in natalizumab-treated patients (0.09, 95% confidence interval (CI), 0.07–0.12) than in those treated with fingolimod (0.17, 0.15–0.19, p<0.001), ocrelizumab (0.08, 0.06–0.11), and fingolimod (0.14, 0.12–0.16, p=0.001). No significant difference was observed in mean on-treatment ARR between patients treated with natalizumab (0.08, 0.06–0.11) and ocrelizumab (0.09, 0.07–0.12, p=0.54). Compared to fingolimod, the natalizumab and ocrelizumab groups exhibited a higher percentage of relapse-free patients and a lower percentage of MRI-active patients at year 1. No significance differences in disability accumulation were determined between the therapies.
Conclusion
Natalizumab and ocrelizumab exhibited similar effects on relapse control, and both were associated with better relapse control than fingolimod. The effects of the three therapies on disability outcomes were similar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The high-efficacy therapies fingolimod, natalizumab, and ocrelizumab are becoming increasingly widely used in relapsing-remitting multiple sclerosis (RRMS), either as first-line therapies or as a switch from other disease-modifying therapies. They have also been approved for RRMS on the basis of large phase III randomized clinical trials showing efficacy in reducing relapse rates, disability worsening, and magnetic resonance imaging (MRI) activity [1,2,3,4].
Fingolimod, natalizumab, and ocrelizumab have been available as treatments for RRMS since their approval by the Food and Drug Administration and European Medicines Agency. These drugs are generally prescribed for RRMS patients who have either experienced a suboptimal response or poor tolerability with previous therapies, or to treatment-naïve patients with highly active RRMS and an Expanded Disability Status Scale (EDSS) score less than 6.
Limited real-world studies have compared high-efficacy drugs. While the efficacy and safety profiles of fingolimod, natalizumab, and ocrelizumab have been established in clinical trials and real-world settings, limited data are available comparing their effectiveness. Observational studies comparing the real-world effectiveness of fingolimod and natalizumab have yielded conflicting results. Some studies have reported no difference in efficacy between fingolimod and natalizumab, while several studies have demonstrated that natalizumab exhibits superior effectiveness on relapse rates [5,6,7,8,9,10]. A recent study reported no difference in relapse and MRI activity between natalizumab and anti-CD20 (rituximab and ocrelizumab) after a fingolimod switch [11].
This study aimed to compare the effectiveness of fingolimod, natalizumab, and ocrelizumab in a real-world setting in Turkish patients with RRMS.
Patients and methods
This research was performed as an independent, multicenter, real-world observational study. Ethical committee approval was obtained from the Karadeniz Technical University Medical Faculty Ethical Committee. Each center received local regulation and local ethical committee approval for registry use for all multiple sclerosis (MS) patients. Written informed consent was required from all participating patients at each center.
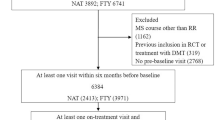
Data from all RRMS patients treated with fingolimod, natalizumab, and ocrelizumab between January 2014 and June 2021 who regularly attended the 16 tertiary MS centers were included retrospectively (Supplementary Table 1). Treatment selection was made by the treating neurologist.
Demographic, clinical, and MRI data were prospectively recorded as part of routine clinical practice. The data entry portal was “Imed software,” and quality assurance procedures were followed (Supplementary Table 2) [12]. A series of automated procedures were applied to identify any invalid or erroneous data entries.
The usual data entry practice was real time or a close approximation of real time at the time of clinical visits. Data for patients treated with fingolimod, natalizumab, and ocrelizumab were extracted in the form of an Excel file from each center and were then combined for analyses.
The inclusion criteria for analyses consisted of diagnosis with RRMS according to the 2017 Revised McDonald criteria, and continuous exposure to one of the study therapies for ≥ 3 months. Data for the following variables were required for all patients included: sex, age, date of first MS symptom, dates of clinical relapses, and disability score assessed by EDSS at commencement of treatment and at least two disability scores recorded after commencement of one of the study therapies. EDSS scores within 3 months prior to or 1 month after commencement of the study therapy were regarded as patients’ baseline EDSS.
Patients aged <18 years, treated with an immunosuppressant (cladribine, alemtuzumab, mitoxantrone, or cyclophosphamide) within the previous year, or with a progressive MS course, were excluded from the study.
The study follow-up duration was defined as the time between the start of therapy and the last available EDSS entry visit. Disability was assessed using EDSS by Neurostatus-certified MS specialists. Any EDSS score recorded within 30 days of a previous relapse was excluded.
Available MRI information reported by treating neurologists was also collected. MRI scans of the brain acquired within 3 months prior to the initiation of the study therapy were regarded as baseline MRI. MRI scans acquired at the 12th month after the initiation of therapy (± 60 days) were defined as year 1 MRI.
Study endpoints
The primary study outcomes were annualized relapse rate (ARR) and time to first relapse. The secondary endpoints were time to disability progression and MRI activity at year 1.
A relapse was defined as any new neurological symptom or exacerbation of existing symptoms persisting for at least 24 h in the absence of concurrent illness or fever, and occurring at least 30 days after a previous relapse [13]. Disability progression was defined as a 1.5-point increase (if the baseline EDSS score was 0), a 1.0-point increase (if the baseline EDSS score was <5.5), or a 0.5-point increase (if the baseline EDSS score was ≥5.5) confirmed at 6-month intervals [14].
In order to minimize unstandardized MRI results, MRI data had to be available in ≥25% of patients in the centers included. Categorized baseline T2 and Gd-enhancing (Gd+) lesion numbers (unavailable, 1–8, ≥9 T2 lesions and absent or ≥1 Gd+ lesion) were analyzed. Percentages of patients with brain MRI activity (defined as the presence of ⩾1 new/enlarging T2 lesion compared to previous brain MRI scans and/or the presence of ⩾1 Gd+ lesion) at year 1 were calculated.
Statistical analyses
Matching and statistical analyses were conducted using R (version 4.0) [15]. The patients included in the study were matched in terms of their propensity scores in three separate matched analyses of fingolimod versus natalizumab, fingolimod versus ocrelizumab, or natalizumab versus ocrelizumab-treated groups using MatchIt package [16]. The propensity score was based on a multivariable logistic regression model. Independent variables consisted of age, age at onset, gender, disease duration, baseline EDSS, and number of relapses in the year before treatment. Patients were matched without replacement in a 2:1 ratio (fingolimod versus natalizumab, and fingolimod versus ocrelizumab) or a 1:1 ratio (natalizumab versus ocrelizumab) using nearest neighbor matching within a caliper of 0.2 standard deviations of the propensity score. The distribution of propensity scores is shown in Supplementary Figure 1.
Patients who switched or discontinued treatment were censored. Only matched pairs were included in this analysis. Patients’ baseline characteristics were evaluated in order to identify possible statistical differences among the two treatment groups. Mean, standard deviation, median, min, max, or interquartile range (IQR) values were reported for continuous variables, depending on the statistical distribution.
In order to investigate statistical relevance, the t test was used for continuous normally distributed variables and the χ2 test for categorical data. On-treatment ARRs were compared with a marginal weighted negative binomial regression model with additional year 1 and year 2 ARR comparisons using the t test.
The proportion of patients without relapse or without an increase in EDSS was evaluated using the Cox marginal proportional hazards model. The proportional hazards assumption was examined visually and by testing Schoenfeld’s residuals. Kaplan–Meier survival estimate curves were used to visualize time-to-event outcomes.
Where no statistically significant differences were observed, analytical power was quantified as the minimum detectable effect at 1-β=0.8 using 200 simulations. Significance was set at p< 0.05.
We performed three sensitivity analyses to assess the robustness of our primary outcome results. First, pairwise censoring was applied to the analyses of time to first relapse to determine common on-treatment follow-up times in order to mitigate attrition bias and the effect of differential treatment persistence [17].
Second, the analyses were repeated after the baseline covariates were adjusted with propensity score weighting method using overlap weight (PSOW) with the R-WeightIt package to reduce the probability of an inadequate covariate balance. An PSOW-adjusted negative binomial model was used to compare ARRs, with the relapse count as the dependent variable.
Third, we excluded patients who had received one of the two medications as a previous therapy and reran the analysis using the newly generated propensity matched groups.
Results
A total of 6243 patients were assessed for eligibility, 3601 treated with fingolimod, 839 with natalizumab, and 1803 with ocrelizumab. A total of 2790 patients (1955 in the fingolimod group, 376 in the natalizumab group, and 459 in the ocrelizumab group) met the inclusion criteria and were included in the propensity matching analyses (Fig. 1). The baseline characteristics of the three treatment groups differed before matching (Supplementary Table 3). Patients treated with ocrelizumab tended to be older, with a longer duration of MS, and with higher EDSS scores compared to fingolimod and natalizumab, while natalizumab-treated patients exhibited more relapses and more MRI activity during the previous year.
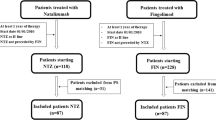
The numbers of patients retained after the propensity score matching procedure in the matched cohorts were 736 in the fingolimod versus 370 in the natalizumab groups, 762 in the fingolimod versus 434 in the ocrelizumab groups, and 310 in the natalizumab versus 310 in the ocrelizumab groups. The matched patients’ baseline demographic and clinical characteristics are shown in Table 1.
Fingolimod versus natalizumab
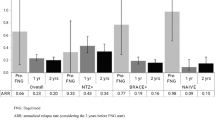
After matching, the patients treated with fingolimod were similar in age (40.1±9.7 vs. 39.7±10.2 p=0.55) and had similar baseline ARR (mean, 95% confidence interval (CI); 1.07, 0.99–1.14 vs. 1.06, 0.96–1.15, respectively, p=0.87) and baseline EDSS (3.1±1.5 vs. 3.1±1.4, respectively, p=0.9) values to those treated with natalizumab. ARR values for fingolimod and natalizumab in the year before treatment initiation and in the first year and second years are shown in Fig. 2a. ARR decreased markedly after treatment in both the fingolimod and natalizumab groups. Mean on-treatment ARR was lower in natalizumab-treated patients (mean, 95% CI; 0.09, 0.07–0.12) than in those treated with fingolimod (mean, 95% CI; 0.17, 0.15–0.19, p<0.001) (Fig. 2a).
Annualized relapse rates (mean and 95% CI of the mean) for fingolimod and natalizumab (a), for fingolimod and ocrelizumab (b), and for natalizumab and ocrelizumab (c) in the year before the start of treatment and in the first and in second years of treatment. Years 0–2 represent overall ARR. p values are from t test for year 1 and year 2, and the negative binomial regression model for years 0–2
The proportion of relapse-free patients was higher in the natalizumab group than in the fingolimod group (hazard ratio (HR) 0.35, 95% CI; 0.24–0.49, p < 0.001) (Fig. 3a).
No differences were observed between fingolimod and natalizumab in terms of rates of confirmed disability accumulation (HR 0.78, 95% CI 0.52–1.16, p=0.21) (Fig. 4a).
The proportion of brain MRI activity-free patients at year 1 was higher among patients treated with natalizumab (availability 50.4%, MRI inactive 91.5%) than in the fingolimod group (availability 47.9%, MRI inactive=74.8%, p<0.001).
Fingolimod versus ocrelizumab
After matching, patients treated with fingolimod were similar in age (42.7 ± 9.6 vs. 43.7 ± 10.6, respectively, p=0.07) and had similar baseline ARR (mean, 95% CI; 0.66, 0.60–0.70, vs. 0.62, 0.55–0.69, respectively, p=0.43) and baseline EDSS (3.35±1.41 vs. 3.49±1.45, p=0.09) to those treated with ocrelizumab. ARRs for fingolimod and ocrelizumab in the year before treatment initiation and in the first year and second years are shown in Fig. 2b.
ARR values decreased markedly after treatment in both the fingolimod and ocrelizumab groups. Mean on-treatment ARR was lower in ocrelizumab-treated patients (mean, 95% CI; 0.08, 0.06–0.11) than in those treated with fingolimod (mean, 95% CI; 0.14, 0.12–0.16, p=0.001) (Fig. 2b).
The proportion of relapse-free patients was higher among patients treated with ocrelizumab than in the fingolimod group (HR 0.32, 95% CI 0.21–0.47, p=0.001; Fig. 3b).
No differences were observed between fingolimod and ocrelizumab in terms of rates of confirmed disability accumulation (HR 1.1, 95% CI 0.75–1.62, p=0.62, Fig. 4b).
The proportion of brain MRI activity-free patients at year 1 was higher among patients treated with ocrelizumab (availability 47.7%, MRI inactive 88.4%) than in the fingolimod group (availability 41.2%, MRI inactive=74.8%, p=0.001).
Natalizumab versus ocrelizumab
After matching, patients treated with natalizumab were similar in age (40.9 ± 10.4 vs. 42.4 ± 10.9, respectively, p=0.14) and had similar baseline ARR (mean, 95% CI; 0.84, 0.76–0.92, vs. 0.84, 0.76–0.92, respectively, p=0.9) and baseline EDSS (3.22±1.48 vs. 3.40±1.50, respectively p=0.14) values to those treated with ocrelizumab. ARR values for natalizumab and ocrelizumab in the year before treatment initiation and in the first year and second years are shown in Fig. 2c.
ARR decreased markedly after treatment in both the natalizumab and ocrelizumab groups. No difference was observed in mean on-treatment ARR between the natalizumab (mean, 95% CI; 0.08, 0.06–0.11) and ocrelizumab (mean, 95% CI; 0.09, 0.07–0.12, p=0.54) groups (Fig. 2c).
The proportion of relapse-free patients was similar between the natalizumab and ocrelizumab groups (hazard ratio (HR) 1.53, 95% CI 0.84–2.77, p=0.16; Fig. 3c).
No differences were observed between natalizumab and ocrelizumab in terms of rates of confirmed disability accumulation (HR 0.98, 95% CI 0.56–1.72, p=0.91, Fig. 4c).
The proportion of brain MRI activity-free patients at year 1 was similar between the natalizumab (availability 46.1%, MRI inactive 92%) and ocrelizumab (availability 40.6%, MRI inactive 93.3%, p=0.78) groups.
Secondary analyses
In order to test for attrition bias, on-treatment follow-up was censored at the shorter of the two individual follow-up periods for each matched fingolimod-natalizumab, natalizumab-ocrelizumab, and fingolimod-ocrelizumab patient pair (pairwise censoring). Consistent with the primary analysis, similar results were obtained in terms of time to first relapse outcomes (Supplementary Figure 2).
In PSOW-adjusted treatment groups, natalizumab and ocrelizumab exhibited similar effects on relapse control, and both were associated with a statistically significant reduced ARR and significantly reduced hazard of the first relapse when compared to fingolimod (Supplementary Table 4 and Supplementary Figure 3a, b, c).
Clinical characteristics of matched patients after excluding patients who had received one of the two medications as a previous therapy are shown in Supplementary Table 5.
Consistent with the primary analysis, similar results were obtained in terms of ARR and time to first relapse outcomes except for a loss of statistical significance in year 1 relapse rate comparison between fingolimod versus natalizumab and fingolimod versus ocrelizumab groups due to the likely insufficient power with small sample size (Supplementary Table 6 and Fig. 4a, b, c).
Discussion
This propensity score matching analysis compared the efficacy of three commonly used high-efficacy therapies in patients with RRMS. Patients were mostly switchers from first-line therapies. Before matching, the natalizumab group was more active than the fingolimod and ocrelizumab groups. Natalizumab therapy may be initiated preferentially in more highly active disease due to its rapid control of disease activity.
In the matched populations, all three high-efficacy drugs, fingolimod, natalizumab, and ocrelizumab, significantly reduced relapse activity. While the relapse reducing effects of natalizumab and ocrelizumab were similar, natalizumab and ocrelizumab exhibited a greater effect on relapse than fingolimod. All three therapies exhibited comparable effects on disability accumulation during the initial 2.5 years.
The on-treatment ARRs observed in this study for the fingolimod (0.14), natalizumab (0.10), and ocrelizumab (0.08) groups were slightly lower than those reported in the pivotal trials (0.16–0.20, 0.20–0.24, and 0.16, respectively) [2, 3, 18, 19].
The magnitude of the effects on relapse activity may be partly due to lower pre-treatment disease activity than in the pivotal trials. Our pre-treatment relapse rates were also lower than those in pivotal trials. Pre-treatment disease activity and age are well-known factors affecting on-treatment relapse activity as well as modulators for medication efficacy.
While some previous observational studies comparing fingolimod and natalizumab in MS have reported no differences in terms of effectiveness, most recent studies have reported that natalizumab was superior in reducing relapse activity [5, 6, 8,9,10, 20,21,22]. Heterogeneity of baseline characteristics may explain the discrepant results.
In the present study, natalizumab-treated patients exhibited more inflammation at the start of natalizumab therapy than those treated with fingolimod and ocrelizumab, which may have affected the comparison results, despite multivariate analysis. The propensity score model is unable to adjust for unknown confounders, such as physicians’ treatment decisions.
Similar to the results of the present research, a recent French observational study reported a similar effect on relapse and disability outcomes for natalizumab and anti-CD20 therapies in highly active RRMS patients after fingolimod withdrawal [11]. Swedish studies have reported a similar effectiveness of rituximab compared to natalizumab [23,24,25]. Two network meta-analyses comparing ocrelizumab and other treatments have shown that ocrelizumab exhibits superior efficacy to first-line therapies and to fingolimod [26, 27]. Alping et al. showed that rituximab was superior to fingolimod in the prevention of relapses within 1.5 years [23]. In a more recent study, Bigaut et al. described as ocrelizumab superior to fingolimod after natalizumab cessation [28].
The main strengths of this study are that it involved direct comparisons of the three high-efficacy therapies from 16 academic MS centers with a prospectively defined observational plan.
The main limitation of this study is its observational retrospective design. Although data entry typically occurs in real time or nearly real time, some variables such as relapse-related information may be susceptible to detection and reporting bias. However, the follow-up protocols used in this study were largely comparable, and such bias may therefore be expected to be minimal.
Another limitation was the relatively short follow-up duration. Fingolimod-treated patients had longer follow-up times than those treated with natalizumab and ocrelizumab. However, similar results were replicated on time to relapse after applying pairwise censoring as a sensitivity analysis to control attrition bias. Detecting significant changes in EDSS during this short period is challenging. Findings for disability accumulation therefore require confirmation over a longer observation period.
We were also unable to compare our radiological data to those of pivotal studies due to the absence of systematic acquisition of radiological outcomes. Consistent acquisition of quantitative MRI data in the context of retrospective multicenter studies still represents a significant challenge. The MRI data in the present study were reported by treating neurologists and were therefore subject to inter-scanner, inter-protocol, and inter-rater error. However, the lack of a formalized definition of MRI follow-up was not expected to introduce a systematic bias, as the same physician reported MRI in the three patient groups, and categorized results were subjected to analysis.
Most of the patients in the present study were switchers from other types of therapy. Numbers of naïve patients were relatively low. Analyses could not be applied to naïve patients, nor to subjects with low disease activity. Therefore, although the results of this study suggest that natalizumab and ocrelizumab are superior to fingolimod in terms of relapse rates and MRI activity, the same assumption cannot be extended to patients with less disease activity.
Rebound activity in patients switching from fingolimod or natalizumab may be related to early relapse activity. However, low relapse rates with natalizumab and ocrelizumab imply that rebound activity did not cause an obvious increase in relapse activity. Low numbers of patients switched from natalizumab in fingolimod cohorts (3.3% and 3.4%).
Although all three drugs share similar indications, their availability differs since ocrelizumab only entered the market after 2018. Neurologists may be oriented toward to selecting ocrelizumab in case of a higher risk of development of a progressive form of the disease. Several patients were treated with fingolimod and natalizumab before the availability of ocrelizumab. However, disease activity was the main reason for initiating therapy in all three groups.
In conclusion, this real-world observational study using propensity score-based matching suggests that natalizumab and ocrelizumab exhibited similar effectiveness in reducing relapse activity. Natalizumab and ocrelizumab were superior to fingolimod in reducing relapse activity. All three drugs exhibited similar effects on disability accumulation in a short period.
Although randomized clinical trials are the mainstay for evaluating drug efficacy and safety, the analysis of observational data provides practical evidence. Efficacy is not the only criterion to be considered in the selection of therapies for an individual patient. Observed statistical significances in reducing relapse activity of natalizumab and ocrelizumab comparing to fingolimod are from small differences. Statistical significance may not clinically significant for an individual patient. Treatment decisions need to be made based on complex evaluations and must include patient preference, adherence, treatment safety, family planning, and cost.
Given the uncertainty and variability associated with treatment effects between DMTs, real-world data, preferably from longitudinal follow-up studies, will provide additional information with which to assess long-term effectiveness.
References
O'Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP et al (2011) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 365(14):1293–1303
Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P et al (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 362(5):387–401
Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B et al (2017) Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 376(3):221–234
Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH et al (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 354(9):899–910
Baroncini D, Ghezzi A, Annovazzi PO, Colombo B, Martinelli V, Minonzio G et al (2016) Natalizumab versus fingolimod in patients with relapsing-remitting multiple sclerosis non-responding to first-line injectable therapies. Mult Scler. 22(10):1315–1326
Frisell T, Forsberg L, Nordin N, Kiesel C, Alfredsson L, Askling J et al (2016) Comparative analysis of first-year fingolimod and natalizumab drug discontinuation among Swedish patients with multiple sclerosis. Mult Scler. 22(1):85–93
Kalincik T, Horakova D, Spelman T, Jokubaitis V, Trojano M, Lugaresi A et al (2015) Switch to natalizumab versus fingolimod in active relapsing-remitting multiple sclerosis. Ann Neurol. 77(3):425–435
Koch-Henriksen N, Magyari M, Sellebjerg F, Soelberg SP (2017) A comparison of multiple sclerosis clinical disease activity between patients treated with natalizumab and fingolimod. Mult Scler. 23(2):234–241
Lorscheider J, Benkert P, Lienert C, Hänni P, Derfuss T, Kuhle J et al (2018) Comparative analysis of natalizumab versus fingolimod as second-line treatment in relapsing-remitting multiple sclerosis. Mult Scler. 24(6):777–785
Sharmin S, Lefort M, Andersen JB, Leray E, Horakova D, Havrdova EK et al (2021) Natalizumab versus fingolimod in patients with relapsing-remitting multiple sclerosis: a subgroup analysis from three international cohorts. CNS Drugs. 35(11):1217–1232
Rollot F, Couturier J, Casey R, Wiertlewski S, Debouverie M, Pelletier J et al (2022) Comparative effectiveness of natalizumab versus anti-CD20 in highly active relapsing-remitting multiple sclerosis after fingolimod withdrawal. Neurother 19(2):476–490. https://doi.org/10.1007/s13311-022-01202-1
Kalincik T, Kuhle J, Pucci E, Rojas JI, Tsolaki M, Sirbu CA et al (2017) Data quality evaluation for observational multiple sclerosis registries. Mult Scler. 23(5):647–655
Havrdova E, Galetta S, Stefoski D, Comi G (2010) Freedom from disease activity in multiple sclerosis. Neurology. 74(Suppl 3):S3–S7
Kalincik T, Cutter G, Spelman T, Jokubaitis V, Havrdova E, Horakova D et al (2015) Defining reliable disability outcomes in multiple sclerosis. Brain. 138(Pt 11):3287–3298
Team RC (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Zhang Z (2017) Propensity score method: a non-parametric technique to reduce model dependence. Ann Transl Med. 5(1):7
Kalincik T, Butzkueven H (2016) Observational data: understanding the real MS world. Mult Scler. 22(13):1642–1648
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 362(5):402–415
La Mantia L, Tramacere I, Firwana B, Pacchetti I, Palumbo R, Filippini G (2016) Fingolimod for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev. 4:Cd009371
Butzkueven H, Licata S, Jeffery D, Arnold DL, Filippi M, Geurts JJ et al (2020) Natalizumab versus fingolimod for patients with active relapsing-remitting multiple sclerosis: results from REVEAL, a prospective, randomised head-to-head study. BMJ Open. 10(10):e038861
Iaffaldano P, Lucisano G, Pozzilli C, Brescia Morra V, Ghezzi A, Millefiorini E et al (2015) Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain. 138(Pt 11):3275–3286
Vollmer BL, Nair KV, Sillau S, Corboy JR, Vollmer T, Alvarez E (2019) Natalizumab versus fingolimod and dimethyl fumarate in multiple sclerosis treatment. Ann Clin Transl Neurol. 6(2):252–262
Alping P, Frisell T, Novakova L, Islam-Jakobsson P, Salzer J, Björck A et al (2016) Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 79(6):950–958
Boremalm M, Juto A, Axelsson M, Novakova L, Frisell T, Svenningsson A et al (2019) Natalizumab, rituximab and fingolimod as escalation therapy in multiple sclerosis. Eur J Neurol. 26(8):1060–1067
Granqvist M, Boremalm M, Poorghobad A, Svenningsson A, Salzer J, Frisell T et al (2018) Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 75(3):320–327
Li H, Hu F, Zhang Y, Li K (2020) Comparative efficacy and acceptability of disease-modifying therapies in patients with relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. J Neurol. 267(12):3489–3498
McCool R, Wilson K, Arber M, Fleetwood K, Toupin S, Thom H et al (2019) Systematic review and network meta-analysis comparing ocrelizumab with other treatments for relapsing multiple sclerosis. Mult Scler Relat Disord. 29:55–61
Bigaut K, Kremer L, Fabacher T, Ahle G, Goudot M, Fleury M et al (2022) Ocrelizumab versus fingolimod after natalizumab cessation in multiple sclerosis: an observational study. J Neurol. 1-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Ethical committee approval was obtained from the Karadeniz Technical University Medical Faculty Ethical Committee. Each center received local regulation and local ethical committee approval for registry use for all multiple sclerosis (MS) patients. Written informed consent was required from all participating patients at each center.
Conflict of interest
Cavit Boz received conference travel support from Biogen, Novartis, Bayer-Schering, Merck, and Teva, and has participated in clinical trials by Sanofi Aventis, Roche, and Novartis. Murat Terzi received travel grants from Novartis, Bayer-Schering, Merck, and Teva, and has participated in clinical trials by Sanofi Aventis, Roche, and Novartis. Serkan Ozakbas did not declare any competing interests. Rana Karabudak did not declare any competing interests related to this study. Serhan Sevim did not declare any competing interests. Recai Turkoglu did not declare any competing interests. Aysun Soysal did not declare any competing interests. Belgin Petek Balcı did not declare any competing interests. Hüsnü Efendi did not declare any competing interests. Ömer Faruk Turan did not declare any competing interests. Nur Yüceyar did not declare any competing interests. Mehmet Fatih Yetkin did not declare any competing interests. Serap Zengin Karahan did not declare any competing interests. Meltem Demirkıran did not declare any competing interests. Sibel Guler did not declare any competing interests. Kadriye Agan did not declare any competing interests. Nefati Kıylıoğlu received conference travel support from Novartis, Bayer-Schering, Merck, and Teva. Cavid Baba did not declare any competing interests. Asli Tuncer did not declare any competing interests. Mesrure Köseoğlu did not declare any competing interests. Recai Turkoglu did not declare any competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figure 1.
Propensity score distribution in the fingolimod and natalizumab cohort (Figure 1a), in the fingolimod and ocrelizumab cohort (Figure 1b), and in the natalizumab and ocrelizumab cohort (Figure 1c) before and after matching (DOCX 93 kb)
Supplementary Figure 2.
Annualized relapse rates (mean and 95% CI of the mean) after pair-wised censoring (ZIP 30 kb)
Supplementary Figure 3.
Proportions of patients free from relapses after overlap weighted adjustments for fingolimod and natalizumab (Supplementary Figure 3a), for fingolimod and ocrelizumab (Supplementary Figure 3b), and for natalizumab and ocrelizumab (Supplementary Figure 3c) (DOCX 74 kb)
Supplementary Figure 4.
Proportions of patients free from relapses after ignoring previous same dmd for fingolimod and natalizumab (Supplementary Figure 4a), for fingolimod and ocrelizumab (Supplementary Figure 4b), and for natalizumab and ocrelizumab (Supplementary Figure 4c). (DOCX 70 kb)
Supplementary Table 1.
Patient distributions by centers (DOCX 20 kb)
Supplementary Table 2.
Data quality procedures (DOCX 81 kb)
Supplementary Table 3.
Baseline demographic and clinical characteristics of the unmatched patients. (DOCX 95 kb)
Supplementary Table 4.
Sensitivity analysis of relapse outcomes in overlap weighted adjusted treatment groups. (DOCX 12 kb)
Supplementary Table 5.
Clinical characteristics of matched patients after excluding patients who had received one of the two medications as a previous therapy. (DOCX 14 kb)
Supplementary Table 6.
Relapse outcomes in propensity matched treatment groups after excluding patients who had received one of the two medications as a previous therapy. (DOCX 13 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boz, C., Ozakbas, S., Terzi, M. et al. The comparative effectiveness of fingolimod, natalizumab, and ocrelizumab in relapsing-remitting multiple sclerosis. Neurol Sci 44, 2121–2129 (2023). https://doi.org/10.1007/s10072-023-06608-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06608-z