Abstract
Objective
Wernicke encephalopathy (WE) is a neuropsychiatric syndrome caused by thiamine deficiency. Despite its low sensitivity, brain magnetic resonance imaging (MRI) is the most useful diagnostic technique. Our aim was to investigate whether the timing of the imaging study, and thiamine replacement can influence brain MRI findings in these patients.
Methods
Retrospective observational study of hospitalized patients between January/2008 and December/2020 with a clinical diagnosis of WE. Data from clinical presentation, diagnostic features, therapeutic approach, and outcomes were collected.
Results
We identified 41 patients (55 ± 13.3 years) with WE. Brain MRI was performed in 36 patients, and one third had T2/FLAIR hyperintensities suggestive of WE. We found an association between a history of poor diet and periventricular hyperintensities (p = 0.023), especially on the ventral surface of the thalamus and the periaqueductal region. It was found that the odds of having a typical imaging of WE decreased by 5.3% for each additional unit (100 mg) of thiamine administered (p = 0.046) (95% CI [0.89, 0.99]). On the other hand, the number of days from clinical presentation was not found to be a viable predictor (p = 0.254) (95% CI [0.88, 1.03]) Recovery was positively correlated with the total dose of thiamine received until discharge (p = 0.020).
Conclusions
MRI hyperintensities seem to be dependent on the timing of thiamine correction and, particularly, on the thiamine dosage prescribed at admission. Nevertheless, thiamine replacement should not be delayed, as its timely prescription is associated with a better prognosis at discharge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wernicke encephalopathy (WE) is a neuropsychiatric syndrome caused by thiamine deficiency. Although the diagnosis is primarily clinical, the classic triad of symptoms (altered mental state, oculomotor abnormalities, and cerebellar dysfunction) is seldom present, which is why most diagnoses were only made postmortem [1]. Twenty-five years ago, Caine et al. tried to improve antemortem identification of patients with WE [2], by adding impaired diet intake to clinical criteria. Considering this, complementary exams are an essential tool to support the diagnosis. Brain magnetic resonance imaging (MRI) is useful to identify abnormal intensities in locations with clinical correlation: mammillary bodies and thalamus (altered mental state), periaqueductal grey matter (oculomotor abnormalities), and the floor of the fourth ventricle (cerebellar disfunction). Nevertheless, MRI studies have high specificity but low sensitivity [3]. Thiamine blood levels should also be measured [3], and, in ambiguous cases, transketolase (an enzyme involved in the carbohydrate catabolism which requiring thiamine as a cofactor) activity can also be determined [4], but this is not widely available. Administration of high doses of intravenous thiamine, followed by normal diet, is indicated whenever WE is suspected [3].
We hypothesized that MRI sensitivity is influenced by the timing of imaging acquisition and thiamine replacement until its completion. Therefore, the aim of this work was to review clinical and imaging features of a cohort of patients with clinically diagnosed WE in order to investigate whether thiamine posology interferes both with diagnosis and outcome.
Methods
Cohort
Clinical records of consecutive patients discharged with a diagnosis of WE from a tertiary hospital were retrospectively reviewed from January 2008 to December 2020. Only patients with a neurology consultation while in hospital and had at least two Caine criteria: (1) dietary deficiencies — a body mass index lower than two standard deviation below normal or a record of grossly impaired dietary intake (2) oculomotor abnormalities — ophthalmoplegia, nystagmus or gaze palsy, (3) cerebellar dysfunction — dysmetria, dysdiadokokinesia or impaired heel-shin testing, or (4) altered mental state — disorientation, amnesia, delirium, or coma were included. Data from clinical presentation, diagnostic features, therapeutic approach, and outcomes was collected.
To search for risk factors, alcoholism was defined as more than 280-g alcohol/week for men and 170-g alcohol/week for women and impaired diet intake as a history of fasting, greatly reduced dietary intake or severe vomiting. Psychiatric disorders were only considered if previously diagnosed by a doctor.
MRI data
Imaging data were collected on a clinically approved Magnetom Avanto 1.5 T (Siemens, Erlangen, Germany) MRI scanner at the Hospital of Braga, using a Siemens 12-channel receiver-only head coil. Brain MRI scans were reviewed by two neuroradiologists who searched for (1) T2-weighted/fluid attenuation inversion recovery (FLAIR) hyperintensities on mammillary bodies, fornix, thalamus, hypothalamus, periaqueductal grey matter, mesencephalon, cerebellum, and cerebral cortex, (2) mammillary bodies atrophy, (3) cerebellar atrophy, and (4) cortical atrophy. They also searched for contrast enhanced lesions whenever gadolinium-based contrast sequences were performed.
Statistical analysis
Statistical analysis was performed using SPSSv26 (IBM®). Normality was verified by the Kolmogorov–Smirnov test. Descriptive statistics were performed for clinical characterization. Continuous variables presented as means ± standard deviations or median (interquartile range) in case of abnormal distribution. Categorical variables are presented as absolute (relative) frequencies. Between groups, continuous variables were compared with two samples t test or Mann–Whitney and categorical variables with Pearson chi-square test or Fisher exact test.
Firstly, patients were divided into two groups: alcoholics and non-alcoholics, according to the definition above. Both clinical presentation, risk factors, and MRI results were compared between groups. Secondly, we searched for statistical differences between patients with typical WE lesions on MRI and those without, considering MRI acquisition delay, total dose of thiamine prescribed, clinical presentation, and risk factors. Logistic regression was used to analyze the relationship between the total dose of thiamine and the number of days from clinical onset on the probability of having typical WE MRI abnormalities. Kappa statistic was used to evaluate inter-observer agreement on MRI lesions. Differences were considered significant if two-tailed p value was less than 0.05. The data supporting the findings of this study are available upon reasonable request.
Results
Clinical presentation
Forty-one admitted patients met at least two Caine criteria of WE (41.5% with two criteria, 43.9% with three criteria, and 14.6% with four criteria). Ataxia was the most common symptom on admission (Table 1). Less than half (39.0%) presented the classical triad of WE. There was a median delay of 7 [1–15] days between symptoms onset and clinical diagnosis. Most patients (73.2%) were admitted in the Neurology department with a median length of stay of 10 [8–15] days.
Three quarters of patients had a history of alcoholism. Of these, six (19.3%) had concomitant painful peripheral neuropathy, four (12.9%) had liver steatosis, three (9.7%) had alcoholic steatohepatitis two (6%) had confirmed liver cirrhosis. In the remainder, either there was no liver disease (52%), or there was a lack of information (19.3%). There was no difference between groups in age, gender, or clinical presentation, except for disorientation which was more common in alcoholic patients (p = 0.001) and emesis which was present in all non-alcoholic patients (p < 0.001).
MRI results
Thirty-six (87.8%) patients underwent brain MRI. Only one scan was provided per patient in the acute phase, except in two cases. The median average interval between the symptom’s onset and the image acquisition was 10 [6–21] days. There was good intra-observer agreement on hyperintensities identification (κ = 0.68, p < 0.001).
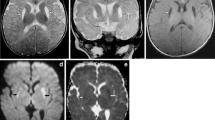
MRI of a middle-aged man with a previous history of alcoholism and a clinical diagnosis of WE. Coronal (A and B) and axial (C and D) FLAIR images (9000/150/1800 ms [TR/TE/TI]) show pathological hyperintensities in mammillary bodies and the periventricular region of the third ventricle (A), medial thalami (B and D), hypothalamus, midbrain tectum, and periaqueductal grey matter (C) and anterior pillars of fornix (D)
Twenty-two (53.7%) patients had an abnormal MRI (Table 2), and twelve (33%) had typical WE abnormalities (example in Fig. 1): T2-weighted/FLAIR hyperintensities on mammillary bodies (n = 9), ventral surface of thalamus (n = 7), hypothalamus (n = 4), and periaqueductal grey matter (n = 7). The remaining patients presented mammillary bodies atrophy, cortical and subcortical atrophy, cerebellar atrophy, or small vessel disease. Periaqueductal hyperintensities were more common in non-alcoholic patients (p = 0.030), and brain atrophy was only present on alcoholic patients. There were no other statistical significant differences in MRI findings between groups.
A history of poor diet was associated with an abnormal MRI (p = 0.023). Both deficient diet, emesis and a history of gastrointestinal disease were correlated with hyperintensities on the ventral surface of the thalamus (p = 0.024; p = 0.032) and the periaqueductal region (p = 0.026; p = 0.004; p = 0.029). Hyperintensities on the mammillary bodies were correlated with amnesia and confabulation (p = 0.046). It was found that the odds of having a typical imaging of WE decreased by 5.3% for each additional unit (100 mg) of thiamine administered (p = 0.046) (95% CI [0.89, 0.99]). On the other hand, the number of days from clinical presentation was not found to be a viable predictor (p = 0.254) (95% CI [0.88, 1.03]) and was not statistically significant associated (r2 = 0.027, p = 0.384) with the dose of thiamine (Fig. 2).
Therapeutic approach and outcome
On admission, half of patients were started on high dose thiamine (at least 200 mg, three times a day), and one-third were prescribed with 500 mg three times a day. Half (51.2%) of patients fully recovered and most of the remaining (78.6%) presented a partial recovery at the time of discharge. The median time from thiamine prescription to partial recovery was 6 days. Full recovery tended to be associated with a high dose of thiamine administered on admission (p = 0.055) and with total thiamine dose received at discharge (t(27.127) = -2.462, p = 0.020). Two patients died due to postoperative complications.
Three patients relapsed: two male who kept drinking alcohol and relapsed 1 week and 1 month later respectively, and a woman who underwent a gastric sleeve surgery and relapsed 2 months later after withdrawal of oral thiamine. Eleven patients (57.9%) with a previous history of alcoholism were abstinent on follow-up. Four patients with typical MRI lesions repeated brain MRI at 6, 9, 10, and 12 months respectively follow-up: the hyperintensities were gone in three patients (Fig. 3). Hyperintensity on the ventral surface of thalamus and periaqueductal grey matter were still present in a patient with a gastrointestinal disease who had stopped thiamine supplementation.
MRI of a middle-aged non-alcoholic woman with a history of severe vomiting for three days followed by ataxia and diplopia. Axial FLAIR images show pathological hyperintensities in periaqueductal grey matter (A) and medial thalami (B). After 6-month, follow-up MRI showed regression of the lesions (C and D)
Discussion
In this study, we showed that the sensitivity of MRI findings in WE is influenced both by the patient’s diet and thiamine replacement.
Caine criteria identified two times more patients than the classic triad of Wernicke, a finding that is consistent with other studies [2, 5, 6]. The main difference is the consideration of dietary intake impairment as a main criterion, allowing the inclusion of patients with onset of symptoms in the setting of gastrointestinal disease or surgery. By being less restrictive, Caine criteria promote an early correction of thiamine in a larger number of patients, without losing specificity [2]. Nevertheless, there was almost a 1-week delay on diagnosis that should not be disregarded, considering the risk of permanent brain damage, which can cause Korsakoff syndrome (severe anterograde and retrograde amnesia and confabulation) or death. This delay might be due to misdiagnosis with alcoholic delirium, negative history of alcohol intake, atypical presentation, and clinician’s unfamiliarity with the new criteria.
Thiamine is needed by the cell membranes to sustain osmotic gradients and is involved in carbohydrates metabolism. It is stored in body tissues predominantly as thiamine diphosphate and is an essential cofactor for several enzymes in the tricarboxylic acid cycle and pentose phosphate pathways [7]. Cellular energy depletion leads to toxic intermediate metabolic products accumulation, such as lactic acid, and reduction of intracellular pH which promotes oxidative stress and cytotoxic edema. Vasogenic edema is also present as a consequence of blood–brain barrier impairment.
Insufficient levels of this vitamin have a greater impact in areas of high carbohydrate metabolic consumption, such as the periventricular regions (ventral thalami, mammillary bodies, periaqueductal grey matter, tectal plate of the midbrain, and the cerebellum) (Harper, 1997), which accounts for the clinical presentation. Our results are consistent with this hypothesis by showing an association between a poor diet or a gastrointestinal disease and periventricular lesions. Even though chronic alcohol abuse remains the main risk factor for WE, gastrointestinal disorders, parenteral nutrition, or chemotherapy have increasingly been reported as predisposing factors [8]. Bearing this, a reasonable speculation is that the risk in alcoholic patients may be more related with impaired diet intake than with direct alcohol toxicity per se. In fact, we found no relevant clinical differences between alcoholic and non-alcoholic patients and a higher frequency of mammillary bodies atrophy in alcoholic patients, which is suggestive of previous episodes of WE [7, 9]. The histopathological abnormalities in the mammillary bodies of WE patients have previously been reviewed [10].
MRI stood out as a useful tool in clinical investigation of WE, by revealing T2-weighted/FLAIR signal hyperintensities in the areas abovementioned. Regardless, the effectiveness of MRI remains lower than desired as typical abnormalities are only observed in about one-third of patients in our cohort as in previous ones [5]. Thus, a normal MRI does not exclude the diagnosis [3]. On clinical practice, thiamine is frequently initiated empirically on admission, considering its low price and exemption from complications [3]. We hypothesized that thiamine replacement could rearrange the osmotic gradients before MRI acquisition and contribute to the disappearance of lesions. Previously, a decrease on T2-weighted and FLAIR signals has been shown in conjunction with the resolution of clinical symptoms, in follow-up studies (Zhong, Jin and Fei, 2005; [9]. Our data confirmed this assumption by showing that the odds of presenting with MRI typical abnormalities decreased with additional units of thiamine replacement at the time of acquisition and that clinical duration did not contribute to the same model. Notwithstanding, MRI should still be performed as it is an essential imaging tool for differential diagnosis. On the other hand, thiamine prescription should not be delayed to increase MRI sensitivity, as clinical recovery seems to be associated both with a high thiamine dose administered on admission and with total prescribed dose of thiamine.
We are conscious that the retrospective and unicentric design, the wide temporal range and the small sample size are limitations of our study. Also, the MRI protocol was variable between patients and not every patient performed gadolinium-based contrast sequences. Nevertheless, the characteristics of our cohort were very similar to others reported in literature, including clinical presentation and diagnostic features [4, 5]. We chose to confine our study to patients observed by a neurologist, because the remaining lacked information on neurological examination. However, we also found MRI abnormalities suggestive of WE in patients admitted in the psychiatric department who were not observed by a neurologist and therefore were excluded. The fact that the measurement of thiamine or transketolase blood levels was not available at our center is another limitation to consider.
Conclusions
MRI sensitivity correlates with an inadequate dietary intake and depends on the total dose of the thiamine at the time of acquisition. Therefore, MRI should be performed as soon as possible, but without delaying thiamine replacement.
Data availability
The data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Harper C (1983) The incidence of Wernicke’s encephalopathy in Australia–a neuropathological study of 131 cases. J Neurol Neurosurg Psychiatry 46(7):593–598. https://doi.org/10.1136/jnnp.46.7.593
Caine D et al (1997) Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry 62(1):51–60. https://doi.org/10.1136/jnnp.62.1.51
Galvin R et al (2010) EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol 17(12):1408–1418. https://doi.org/10.1111/j.1468-1331.2010.03153.x
Sinha S et al (2019) Wernicke encephalopathy—clinical pearls. Mayo Clin Proc 94(6):1065–1072. https://doi.org/10.1016/j.mayocp.2019.02.018
Chamorro AJ et al (2017) Differences between alcoholic and nonalcoholic patients with Wernicke encephalopathy: a multicenter observational study. Mayo Clin Proc 92(6):899–907. https://doi.org/10.1016/j.mayocp.2017.02.019
Harper CG, Giles M (1986) Clinical signs in the Wernicke-Korsakoff complex : retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry 49(4):341–345. https://doi.org/10.1136/jnnp.49.4.341
Chandrakumar A, Bhardwaj A, T’Jong GW (2019) Review of thiamine deficiency disorders: Wernicke encephalopathy and Korsakoff psychosis. J Basic Clin Physiol Pharmacol 30(2):153–162. https://doi.org/10.1515/jbcpp-2018-0075
Oudman E, Wijnia JW, Oey MJ, van Dam M, Postma A (2021) Wernicke-Korsakoff syndrome despite no alcohol abuse: a summary of systematic reports. J Neurol Sci 426:117482
Manzo, G. et al. (2014) ‘MR imaging findings in alcoholic and nonalcoholic acute Wernicke’s encephalopathy: a review’. BioMed Res Int. https://doi.org/10.1155/2014/503596
Arts NJ, Pitel AL, Kessels RP (2021) The contribution of mamillary body damage to Wernicke’s encephalopathy and Korsakoff’s syndrome. Handb Clin Neurol 180:455–475. https://doi.org/10.1016/B978-0-12-820107-7.00029-X
Harper C, B. R. (1997) ‘Nutritional and metabolic disorders’, in Greenfield’s Neuropatholog. 6th ed. London, UK, pp. 601–52.
Zhong C, Jin L, Fei G (2005) ‘MR imaging of nonalcoholic Wernicke encephalopathy: a follow-up study AJNR. Am J Neuroradiol 26(9):2301–2305
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Approval for this study was obtained from the local Ethics Committee (REF 74/2018).
Consent to participate and consent for publication
Because of the retrospective observational nature of the study, patient informed consent was waived by the local ethics committee.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, A.R., Almeida-Xavier, S., Lopes, M. et al. Is there a time window for MRI in Wernicke encephalopathy — a decade of experience from a tertiary hospital. Neurol Sci 44, 703–708 (2023). https://doi.org/10.1007/s10072-022-06477-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06477-y