Abstract
Cerebrolysin therapy has the potential to significantly aid in the treatment of a wide variety of debilitating neurological diseases including ischemic strokes, neurodegenerative disorders, and traumatic brain injuries. Although Cerebrolysin is not approved for use in the USA, it is used clinically in over 50 countries worldwide. In this review, we focus on outlining the role that Cerebrolysin has in stimulating the molecular signaling pathways that are critical for neurological regeneration and support. An extensive evaluation of these signaling pathways reveals that Cerebrolysin has the potential to intervene in a diverse array of pathophysiological causes of neurological diseases. In the clinical setting, Cerebrolysin is generally safe for human use and has provided functional improvement when used as an adjunct treatment. However, our literature review revealed inconsistent results, as several clinical studies suggested that Cerebrolysin treatment has minor clinical relevance and did not have significant advantages over a placebo. In conclusion, we found that Cerebrolysin therapy can potentially play a major role in the treatment of many neurological diseases. Nevertheless, there remains much to be elucidated about the efficacy of this treatment for specific neurological conditions, and more robust clinical data is needed to reach a consensus and properly define the therapeutic role of Cerebrolysin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebrolysin (CBL) is a neurotrophic drug, made from a mixture of low molecular weight, porcine-derived peptides and free amino acids. The pharmaceutical preparation includes the peptide fragments: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), enkephalins, orexin, and P21. CBL is currently approved for use in more than 50 countries as a treatment for dementia and stroke; however, the Food and Drug Administration (FDA) has not approved its clinical use in the USA. In this review, we seek to provide a comprehensive analysis of relevant molecular mechanisms underlying the pharmacology of CBL. Additionally, our objective is to highlight the clinical outcomes and current limitations on effect analysis of CBL in the treatment of stroke, neurodegenerative disorders, and traumatic brain injury.
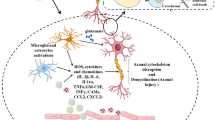
CBL targets the pathophysiological mechanisms involved in acute and chronic central nervous system disorders such as stroke, traumatic brain injury (TBI), and neurodegenerative disorders like Alzheimer’s disease (AD). Specifically, CBL exerts its neuroprotective effects by influencing multiple molecular targets, modulating substrates, enzymes, and receptors implicated in glutamatergic, GABAergic, and cholinergic transmission. By regulating the expression of caspases and other apoptotic and autophagy-related factors, it enhances neurogenesis and neurorestoration through the activity of the neurotrophic factor (NTF) and sonic hedgehog (Shh) signaling pathways [1]. CBL is a multimodal drug that works to provide neurotrophic support by mimicking the activity of NTFs, thereby protecting against excitotoxicity, oxidative stress, and pro-apoptotic enzymes, in addition to modulating the inflammatory response [1].
NTFs include three families of protein growth factors that function as signaling molecules to maintain, protect, and repair neuronal networks in the CNS. These endogenous polypeptides play an important role in neural regeneration, remyelination, and regulation of neuronal development. The pathogenesis of several neurodegenerative disorders has been linked to disruptions in the signaling cascades between NTFs and their receptors, and the therapeutic use of neurotrophic factors has shown tremendous potential in restoring neurological function [2]. Shh was originally identified as a morphogen involved in neural development during embryogenesis. However, Shh signaling has recently been implicated as an essential modulator of neurogenesis, anti-oxidation, anti-inflammation, and autophagy in adult neural tissues [3]. Shh plays an integral role in modulating the response of the adult brain to a wide variety of neurological damage such as ischemic stroke, TBI, and neurodegeneration. Shh also has a pronounced role in mitochondrial abundance, morphology, adenosine triphosphate (ATP) production, and resistance to oxidative neurotoxins (i.e., amyloid β, hydrogen peroxide, glutamate) in hippocampal neurons [4]. Notably, neurotrophic factors such as BDNF are intricately involved in Shh signaling. For example, BDNF has been shown to induce expression of Shh and provide neuroprotection against 3-nitropropionic acid (3-NP), an irreversible inhibitor of Complex II in the electron transport chain that is implicated in molecular mechanisms of cellular senescence in Huntington’s disease (HD) [5]. Furthermore, models of traumatic brain injury suggest that Shh signaling is upregulated following cortical injury and directly influences the proliferation of quiescent neural stem cells (NSCs) and neural progenitor cells (NPCs) in the hippocampus [6, 7].
Pharmacology
CBL is a medication mixture made with amino acids and neuropeptides that has been tested in various neurological conditions. It has been used in treatments to stimulate neurogenesis, support nerve cell function, and facilitate functional recovery and repair (Fig. 1). More specifically, CBL functions by activating the Shh signaling pathway which plays a role in the development and organization of organs. Shh activates the Gli complex, which is integral for developmental gene expression organizing regeneration and recovery. In the brain, CBL promotes oligodendrogenesis and neurogenesis by increasing Shh and its associated receptors (Patched and Smoothened) through mRNA modulation. Neural progenitor cells differentiate into oligodendrocyte progenitor cells to respond to brain injury [1].
CBL also mimics NTFs, which are signaling molecules that ensure the brain is functioning properly by protecting, maintaining, and regenerating the neuronal system. Studies have shown that CBL contains fragments that are structurally similar to NTFs, such as BDNF, NGF, and glial-derived neurotrophic factor (GDNF), which act on neural progenitor cells to promote neurogenesis [8]. CBL displays BDNF-like activity through excitation of the PI3K/Akt pathway which is important in cell growth, differentiation, and migration. Additionally, the PI3K/Akt pathway interacts with the Shh signaling pathway to regulate the proliferation and modeling of neural precursors [1, 9].
CBL has demonstrated protective effects against pathological cascades following neurodegenerative injury or disease by mediating the inflammatory response, lowering the accumulation of free radicals, and reducing pro-apoptotic enzymes [10]. CBL also increases expression of synaptic proteins by moderating amyloid precursor protein expression. It has advantageous effects on neuroplasticity by preserving the neuronal network, increasing synaptic density, and improving protein synthesis [11, 13].
Dosage recommendation and administration
Although CBL is currently not approved for clinical use in the USA, it is sometimes used to treat stroke, TBI, and cognitive impairment primarily in Europe, Asia, and South America [14, 15]. The recommended dosage of CBL ranges between 10 and 60 ml per day and varies by disease, severity, and onset of treatment (Table 1). CBL administered at this range should be diluted to at least 100 ml total volume with normal saline, Lactated Ringers, or 5% glucose solution and given as a slow intravenous (IV) infusion over a duration of at least 15 min. Per manufacturer guidelines, infusion solutions should not be mixed with neutral amino acid solutions, vitamins, or cardiovascular medicinal products [16]. Lower doses of up to 5 ml or 10 ml can be given as undiluted intramuscular or IV injections over a duration of 3 min respectively [16]. Rapid injection can lead to dizziness, diaphoresis, heat sensation, and rarely palpitations or arrhythmia. Contraindications to CBL therapy include history of seizures, especially epilepsy, and acute or severe renal failure [17]. Caution should be exercised in patients using lithium or antidepressants as some studies have shown that CBL may increase accumulation of lithium in brain tissues or enhance the effects of antidepressants, especially monoamine oxidase inhibitors (MAOIs) [18, 19].
A recent 2018 meta-analysis of nine ischemic stroke RTCs evaluated CBL clinical efficacy compared to placebo, defined as resolution of symptoms or change in National Institutes of Health Stroke Scale (NIHSS) greater than 4 points at 21 or 30 days after stroke. Treatment efficacy was demonstrated primarily with daily 30-ml CBL infusions for 10 or 21 days when initiated within 12 to 72 h of symptom onset [20]. Patients with higher baseline stroke severity, defined as a National Institutes of Health Stroke Scale (NIHSS) greater than 12, demonstrated greater magnitude improvement [24, 52]. However, some studies have also shown mixed evidence indicating no clinical benefit of CBL over a placebo in treating strokes [14, 53]. Use of CBL in TBI treatment remains controversial and further studies are needed. A 2018 meta-analysis demonstrated a dearth in RTCs investigating this relationship and that current knowledge primarily derives from cohort studies [31]. Additionally, significant heterogeneity exists amongst the studies regarding treatment dosage, duration, and measurement of functional outcome. Regimens for TBI employ dosages of 10 to 50 ml daily for durations ranging as little as 10 days to up to 12 months to achieve efficacy. This may be due in part to the variable standards used to evaluate functional recovery, including measurements with the Glasgow Outcome Scale (GOS), modified Rankin Scale (mRS), Mini-Mental State Examination (MMSE), and Cognitive Abilities Screening Instrument (CASI). Preliminary studies in animal models suggest a dose-dependent relationship on TBI recovery; however, no beneficial effects are seen if treatment is given 2 h after injury; Thereby suggesting that timely intervention is critical for treating TBI with CBL [15, 31, 33, 54]. Treatment of AD and vascular dementia with CBL differs from that of stroke and TBI primarily in timing and frequency of dosing. AD patients showed improvements in cognition (ADAS-Cog+ score), global clinical function (CIBIC+ score), and activities of daily living are seen with 30-ml doses administered five times a week for 4 weeks, with some protocols repeating the regimen after a treatment-free period of 8 to 12 weeks to further enhance effects [AD references]. Notably, these improvements are long-lasting and can be observed 6 months after discontinuing active treatment, thereby implying CBL’s capability to induce neuronal repair with long-term stability [55]. Studies investigating CBL in vascular dementia patients employ dosing schedules similar to those in AD patients, 10 to 30 ml CBL five times per week for 4 weeks [27, 43, 56]. Dosages ranged from 10 to 30 ml with this schedule; however, efficacy has also been shown with administering 20 ml daily for shorter 10 to 28-day courses [39, 42, 44].
Stroke
Rodent stroke model studies showing that CBL enhances neurogenesis and improves functional outcome after stroke prompted researchers to examine the drug’s clinical potential [9]. Clinical trials of greater than 1500 patients have established the safety and tolerability of CBL and shown it has clinical benefit in the setting of stroke [23, 27, 30]. In fact, a randomized-controlled trial (RCT) showed that this drug has clinical benefit in rehabilitating motor function post-stroke. In this trial, upper limb motor capacity was significantly increased in patients receiving IV CBL 72 h after stroke [27]. In a double-blind, placebo RCT, CBL showed a significant reduction of disability and mortality in the most severe stroke cases [24].
The results of meta-analyses support the safety of CBL; however, whether CBL improves functional outcomes remains inconclusive. According to these analyses, CBL likely has no beneficial effect on prevention of all-cause death or serious adverse effect and no significant efficacy on the neurological functional recovery in patients following acute ischemic stroke [14, 57]. Authors of one meta-analysis recommend that future studies examine the efficacy of CBL administered within 6 h post-stroke and maintained for a longer period [57]. However, in another meta-analysis of nine RCTs, CBL demonstrated a beneficial effect on early global neurologic deficits in patients with acute ischemic stroke and clinically relevant improvement of functional outcome in the moderate to severe stroke group [20].
Neurodegeneration
Out of the many types of neurodegenerative diseases, AD, Parkinson’s (PD), HD, and multiple sclerosis (MS) are the most commonly occurring forms [58]. Alterations in neurotrophin levels, particularly NGF and BDNF, have been implicated in neurodegenerative disorders, as well as psychiatric disorders, including depression and substance abuse, and thus have been proposed as therapeutic targets [59, 60].
CBL has demonstrated clinical efficacy in mild to moderate AD patients, where it has shown to reduce plasma tumor necrosis factor-alpha (TNF-α) concentrations and enhance serum BDNF levels [61]. A study published in 2020 by Alvarez et al. examined whether baseline vascular endothelial growth factor (VEGF) and changes in VEGF levels after treatment with CBL, donepezil, or a combined therapy were associated with cognitive and functional responses in AD patients. The results indicated that the combined therapy reverses increased levels of serum VEGF and has a significant treatment effect on cognition and functioning compared with donepezil monotherapy in advanced AD. In the presence of tauopathies, such as Pick’s disease, CBL has shown benefit in taupathy models by reducing the levels of aberrantly phosphorylated tau [61, 62]. Other studies have also demonstrated that CBL enhances the levels of insulin-like growth factor-1 (IGF-1) in the sera of AD patients and of diabetic rats, increases the maturation of nerve growth factor and the survival of cholinergic neurons in the brain of a transgenic mouse model of AD, and reverses the decrease of serum BDNF in a rat model of PD [63]. In PD brains, profound neuronal, glial, and axonal changes can be seen in substantia nigra pars compacta (SNpC) and striatum (STr) areas [64]. Normal CBL treatment has proven to markedly reduce neuronal changes in these brain areas in PD. However, most marked neuroprotection is an evident in PD brains following treatment with nanodelivery of CBL. Nanodelivery of CBL has shown to reduce myelin basic protein (MBP) degeneration and activation of astrocytes in SNpC and STr that is most pronounced than normal CBL treatment in PD. These observations suggest that nanodelivery of CBL is also able to reduce α-SNC levels near normal values in PD and thus is the superior method of delivery [64].
On the other hand, MS is characterized by an abnormal autoimmune response leading to the demyelination of the central nervous system (CNS) where therapeutic methods are aimed at inducing stimulation of remyelination processes with neurotrophic factors which potentially allows to reduce the residual neurological deficit. In a study published in 2016 by Khabirov et al., the efficacy and safety of CBL in the treatment of patients with MS in stage of relapse regression were evaluated. A high single dose of 20 ml of CBL was administered to a total of 40 patients, where a positive role in the stimulation of remyelination process in MS was reported and confirmed by comprehensive clinical and neurophysiological examinations. A RCT study published in Russia attributed the therapeutic response to the drug containing fragments of tubulin, actin, and myelin basic proteins, all of which are necessary to ensure non-specific trophic regenerated CNS myelin sheath [60, 65]. No additional data on CBL application in the scope of MS is reported in the literature which further limits the conclusions and indications in this particular pathology. Lastly, in the context of CBL as a therapeutic agent for HD, the scientific literature contains non-specific and scarce data.
Traumatic brain injury
Utilization of CBL therapy in TBI has been studied extensively in laboratory settings and has been shown to increase expression of neurotrophic factors to enhance axonal repair and regeneration mechanisms [66]. It was also observed that CBL therapy can play a role in microglial activation/neuroinflammation and inhibition of free-radical formation [66]. Clinical efficacy of CBL therapy is aimed at combating the pathologic features of TBI, including localized ischemia, difficulties with memory and cognition, intracranial hemorrhages, and axonal injuries [67]. Recommended dosing for IV CBL for TBI varies amongst the literature in both amount and duration, with dosages ranging from 10–50 ml/day over 5–30 days [31]. Timing of CBL administration after TBI also plays an important role in the efficacy of the therapy. Administration of CBL 5 min and 1 h after TBI showed improved sensory-motor functions, brain edema, and blood-brain barrier (BBB) leakage but no significant improvement in these parameters when CBL was administered more than 2 h after TBI [54].
Improved clinical outcomes of CBL therapy in TBI have been consistently observed, with the majority of literature being prospective and retrospective studies. A meta-analysis performed by Ghaffarpasand et al. highlighted numerous trials of CBL administration in patients with TBI ranging from mild-severe with statistically significant improvements in functional, cognitive, and electrical functions at appropriate dosages [31]. The largest retrospective multi-cohort study to date on CBL use in TBI published by Muresanu et al. demonstrated improved clinical outcomes, ranked by GOS and mRS disability score, in an experimental group of 615 patients receiving severity-dependent adjunctive CBL therapy [32]. There are no current clinical trials being performed investigating the sole use of CBL in TBI. However, a randomized, parallel-assigned quadruple masking study on the efficacy of combination treatment with amantadine and CBL in patients with TBI has been proposed but the data is not available [68]. An increase in both number and participants of these trials will further strengthen the validity of the aforementioned improvement in patients with TBI.
Conclusion
Basic science models have suggested that CBL has a pronounced effect on several neurotrophic molecular pathways. Notably, in vivo and in vitro models reveal that CBL stimulates neurological regeneration, neurological support and maintenance, neuroprotection, and neuroplasticity through a variety of the molecular mechanisms discussed in this review, emphasizing both Shh and NTF pathways. RCTs reveal that CBL is generally safe and well-tolerated in humans, although side effects of rapid injection have been noted. Furthermore, clinical studies on the efficacy of CBL have revealed inconsistent results. In this review, we highlighted clinical studies and meta-reviews that suggest CBL treatment may improve functionality in patients with ischemic stroke, neurodegenerative disorders, and TBI. These studies suggest that CBL is most effective for use in patients with severe neurological damage, if administered within a critical time window and in conjunction with traditional therapies. However, we also reported on several clinical studies that showed CBL has no significant benefit in enhancing recovery following neurological damage. Nevertheless, there is encouraging clinical and basic science data that suggest adjunct CBL therapy improves the biomarker profiles of several neurological diseases including AD, PD, and MS.
In conclusion, there remains a significant amount of research that must be done in order to fully understand the role of CBL in the clinical treatment of neurological disorders. Current data demonstrates significant heterogeneity in duration of therapy, initial and sustained dosage, combination therapeutics, and time frame of treatment initiation. Future high-quality RCTs are needed to reach consensus on exact dosing and treatment timelines and standardization of diagnostic criteria, in addition to clarifying which stages of disease (i.e., mild, moderate, severe) the drug would be most effective for in order to clarify clinical application and effect analysis. Additionally, future studies are needed to elucidate the role of CBL treatment in acute versus chronic neurological diseases, as well as long-term preventive trials specifically in the setting of AD. Especially in the USA, where CBL is not currently approved for use by the FDA, more robust clinical trials, with a greater number of participants, are needed to fully understand the potential clinical benefits of this treatment as a monotherapy and in combination with other therapeutics.
References
Zhang L, Chopp M, Meier DH, Winter S, Wang L, Szalad A, Lu M, Wei M, Cui Y, Zhang ZG (2013) Sonic hedgehog signaling pathway mediates cerebrolysin-improved neurological function after stroke. Stroke 44(7):1965–1972. https://doi.org/10.1161/STROKEAHA.111.000831
Razavi S, Nazem G, Mardani M, Esfandiari E, Esfahani S, Salehi H (2015) Neurotrophic factors and their effects in the treatment of multiple sclerosis. Adv Biomed Res 4(1). https://doi.org/10.4103/2277-9175.151570
Chen S-D, Yang J-L, Hwang W-C, Yang D-I (2018) Emerging roles of sonic hedgehog in adult neurological diseases: neurogenesis and beyond. Int J Mol Sci 19(8). https://doi.org/10.3390/ijms19082423
Yao PJ, Manor U, Petralia RS, Brose RD, Wu RT, Ott C, Wang YX, Charnoff A, Lippincott-Schwartz J, Mattson MP (2017) Sonic hedgehog pathway activation increases mitochondrial abundance and activity in hippocampal neurons. Mol Biol Cell 28(3):387–395. https://doi.org/10.1091/mbc.E16-07-0553
Wu CL, Chen SD, Hwang CS, Yang DI (2009) Sonic hedgehog mediates BDNF-induced neuroprotection against mitochondrial inhibitor 3-nitropropionic acid. Biochem Biophys Res Commun 385(1):112–117. https://doi.org/10.1016/j.bbrc.2009.04.145
Lai K, Kaspar BK, Gage FH, Schaffer DV (2003) Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci 6(1):21–27. https://doi.org/10.1038/nn983
Ahmed AI, Shtaya AB, Zaben MJ, Owens EV, Kiecker C, Gray WP (2012) Endogenous GFAP-positive neural stem/progenitor cells in the postnatal mouse cortex are activated following traumatic brain injury. J Neurotrauma 29(5):828–842. https://doi.org/10.1089/neu.2011.1923
Chen H, Tung YC, Li B, Iqbal K, Grundke-Iqbal I (2007) Trophic factors counteract elevated FGF-2-induced inhibition of adult neurogenesis. Neurobiol Aging 28(8):1148–1162. https://doi.org/10.1016/j.neurobiolaging.2006.05.036
Zhang C, Chopp M, Cui Y, Wang L, Zhang R, Zhang L, Lu M, Szalad A, Doppler E, Hitzl M, Zhang ZG (2010) Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J Neurosci Res 88(15):3275–3281. https://doi.org/10.1002/jnr.22495
Bliźniewska-Kowalska K, Łukasik M, Gałecki P (2019) Cerebrolysin – mechanism of action and application in psychiatry and neurology. Pharmacother Psychiatry Neurol 35(1):9–23. https://doi.org/10.33450/fpn.2019.03.002
Mallory M, Honer W, Hsu L, Johnson R, Rockenstein E, Masliah E (1999) In vitro synaptotrophic effects of Cerebrolysin in NT2N cells. Acta Neuropathol 97(5):437–446. https://doi.org/10.1007/s004010051012
Plosker GL, Gauthier S (2009) Cerebrolysin: a review of its use in dementia. Drugs Aging 26(11):893–915. https://doi.org/10.2165/11203320-000000000-00000
Plosker GL, Gauthier S (2009) Cerebrolysin. Drugs Aging 26(11):893–915. https://doi.org/10.2165/11203320-000000000-00000
Ziganshina LE, Abakumova T, Vernay L (2017) Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev 4(4):CD007026–CD007026. https://doi.org/10.1002/14651858.CD007026.pub5
Yanlu Z, Michael C, Zheng Gang Z, Yi Z, Li Z, Mei L, Talan Z, Stefan W, Hemma B, Asim M, Ye X (2018) Prospective, randomized, blinded, and placebo-controlled study of Cerebrolysin dose-response effects on long-term functional outcomes in a rat model of mild traumatic brain injury. J Neurosurg 129(5):1295–1304. https://doi.org/10.3171/2017.6.JNS171007
EVER NEURO PHARMA GMBH (2020) Cerebrolysin. https://www.cerebrolysin.com/en/cerebrolysin/administration/. Accessed Sep 20, 2020 2020
Onose G, Mureşanu DF, Ciurea AV, Daia Chendreanu C, Mihaescu AS, Mardare DC, Andone I, Spânu A, Popescu C, Dumitrescu A, Popescu M, Grigorean V, Ungur B, Marinescu F, Colibbeanu I, Onose L, Haras M, Sandu A, Spircu T (2009) Neuroprotective and consequent neurorehabilitative clinical outcomes, in patients treated with the pleiotropic drug cerebrolysin. J Med Life 2(4):350–360
Gromova OA, Torshin IY, Gogoleva IV, Pronin AV, Stelmashuk EV, Isaev NK, Genrikhs EE, Demidov VI, Volkov AY, Khaspekov GL, Alexandrova OP (2015) Pharmacokinetic and pharmacodynamic synergism between neuropeptides and lithium in the neurotrophic and neuroprotective action of cerebrolysin. Zh Nevrol Psikhiatr Im S S Korsakova 115(3):65–72. https://doi.org/10.17116/jnevro20151153165-72
Kalyn Ia B, Safarova TP, Sheshenin VC, Gavrilova SI (2014) Comparative efficacy and safety of antidepressant mono- and multimodal therapy in elderly patients with depression (a clinical experience in a psychogeriatric hospital). Zh Nevrol Psikhiatr Im S S Korsakova 114(6 Pt 2):20–29
Bornstein NM, Guekht A, Vester J, Heiss WD, Gusev E, Homberg V, Rahlfs VW, Bajenaru O, Popescu BO, Muresanu D (2018) Safety and efficacy of Cerebrolysin in early post-stroke recovery: a meta-analysis of nine randomized clinical trials. Neurol Sci 39(4):629–640. https://doi.org/10.1007/s10072-017-3214-0
Skvortsova V, Shamalov N, Stakhovskaya L (2005) Cerebrolysin in acute ischaemic stroke: results of randomised, double blind, placebo-controlled study. Cerebrovasc Dis 19:76
Shamalov NA, Stakhovskaia LV, Burenchev DV, Kichuk IV, Tvorogova TV, Botsina A, Smychkov AS, Kerbikov OB, Moessler H, Novak P, Skvortsova VI (2010) The effect of cerebrolysin in dosage 50 ml on the volume of lesion in ischemic stroke. Zh Nevrol Psikhiatr Im S S Korsakova 110(12 Pt 2):34–37
Gharagozli K, Harandi AA, Houshmand S, Akbari N, Muresanu DF, Vester J, Winter S, Moessler H (2017) Efficacy and safety of Cerebrolysin treatment in early recovery after acute ischemic stroke: a randomized, placebo-controlled, double-blinded, multicenter clinical trial. J Med Life 10(3):153–160
Heiss WD, Brainin M, Bornstein NM, Tuomilehto J, Hong Z, Cerebrolysin Acute Stroke Treatment in Asia I (2012) Cerebrolysin in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial. Stroke 43(3):630–636. https://doi.org/10.1161/STROKEAHA.111.628537
Lang W, Stadler CH, Poljakovic Z, Fleet D (2013) A prospective, randomized, placebo-controlled, double-blind trial about safety and efficacy of combined treatment with alteplase (rt-PA) and Cerebrolysin in acute ischaemic hemispheric stroke. Int J Stroke 8(2):95–104. https://doi.org/10.1111/j.1747-4949.2012.00901.x
Amiri-Nikpour MR, Nazarbaghi S, Ahmadi-Salmasi B, Mokari T, Tahamtan U, Rezaei Y (2014) Cerebrolysin effects on neurological outcomes and cerebral blood flow in acute ischemic stroke. Neuropsychiatr Dis Treat 10:2299–2306. https://doi.org/10.2147/NDT.S75304
Muresanu DF, Heiss WD, Hoemberg V, Bajenaru O, Popescu CD, Vester JC, Rahlfs VW, Doppler E, Meier D, Moessler H, Guekht A (2016) Cerebrolysin and recovery after stroke (CARS): a randomized, placebo-controlled, double-blind, multicenter trial. Stroke 47(1):151–159. https://doi.org/10.1161/strokeaha.115.009416
Guekht A, Heiss D, Gusev E, Vester J, Doppler E, Muresanu D (2015) Cerebrolysin and recovery after stroke (CARS 2): a randomized, placebo-controlled, double-blind, multicenter clinical study. J Neurol Sci 357. https://doi.org/10.1016/j.jns.2015.08.336
Xue LX, Zhang T, Zhao YW, Geng Z, Chen JJ, Chen H (2016) Efficacy and safety comparison of DL-3-n-butylphthalide and Cerebrolysin: effects on neurological and behavioral outcomes in acute ischemic stroke. Exp Ther Med 11(5):2015–2020. https://doi.org/10.3892/etm.2016.3139
Ladurner G, Kalvach P, Moessler H (2005) Neuroprotective treatment with cerebrolysin in patients with acute stroke: a randomised controlled trial. J Neural Transm (Vienna) 112(3):415–428. https://doi.org/10.1007/s00702-004-0248-2
Ghaffarpasand F, Torabi S, Rasti A, Niakan MH, Aghabaklou S, Pakzad F, Beheshtian MS, Tabrizi R (2019) Effects of cerebrolysin on functional outcome of patients with traumatic brain injury: a systematic review and meta-analysis. Neuropsychiatr Dis Treat 15:127–135. https://doi.org/10.2147/NDT.S186865
Muresanu DF, Ciurea AV, Gorgan RM, Gheorghita E, Florian SI, Stan H, Blaga A, Ianovici N, Iencean SM, Turliuc D, Davidescu HB, Mihalache C, Brehar FM, Mihaescu AS, Mardare DC, Anghelescu A, Chiparus C, Lapadat M, Pruna V, Mohan D, Costea C, Costea D, Palade C, Bucur N, Figueroa J, Alvarez A (2015) A retrospective, multi-center cohort study evaluating the severity- related effects of cerebrolysin treatment on clinical outcomes in traumatic brain injury. CNS Neurol Disord Drug Targets 14(5):587–599. https://doi.org/10.2174/1871527314666150430162531
Wong GK, Zhu XL, Poon WS (2005) Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl 95:59–60. https://doi.org/10.1007/3-211-32318-x_13
Khalili H, Niakan A, Ghaffarpasand F (2017) Effects of cerebrolysin on functional recovery in patients with severe disability after traumatic brain injury: a historical cohort study. Clin Neurol Neurosurg 152:34–38. https://doi.org/10.1016/j.clineuro.2016.11.011
Asghari M, Meshkini A, Salehpoor F, Agazadeh J, Shakeri M, Shokohi G, Ebrahimi N, Bazzazi A, Pourhajshokr N (2014) Investigation of the effect of cerebrolysin on patients with head trauma and diffuse axonal injury. Int J Curr Res Acad Rev 2:62–69
Alvarez XA, Sampedro C, Figueroa J, Tellado I, Gonzalez A, Garcia-Fantini M, Cacabelos R, Muresanu D, Moessler H (2008) Reductions in qEEG slowing over 1 year and after treatment with Cerebrolysin in patients with moderate-severe traumatic brain injury. J Neural Transm (Vienna) 115(5):683–692. https://doi.org/10.1007/s00702-008-0024-9
Cui S, Chen N, Yang M, Guo J, Zhou M, Zhu C, He L (2019) Cerebrolysin for vascular dementia. Cochrane Database Syst Rev 2019(11). https://doi.org/10.1002/14651858.CD008900.pub3
Guekht AB, Moessler H, Novak PH, Gusev EI, Cerebrolysin I (2011) Cerebrolysin in vascular dementia: improvement of clinical outcome in a randomized, double-blind, placebo-controlled multicenter trial. J Stroke Cerebrovasc Dis 20(4):310–318. https://doi.org/10.1016/j.jstrokecerebrovasdis.2010.01.012
Liang S, Li Z, Yan H, Pei X (2001) The efficacy of Cerebrolysin in multi-infarct dementia. Heilongjiang Med Pharm 24(5):75
Muresanu DF, Alvarez XA, Moessler H, Buia M, Stan A, Pintea D, Moldovan F, Popescu BO (2008) A pilot study to evaluate the effects of Cerebrolysin on cognition and qEEG in vascular dementia: cognitive improvement correlates with qEEG acceleration. J Neurol Sci 267(1-2):112–119. https://doi.org/10.1016/j.jns.2007.10.016
Muresanu DF, Alvarez XA, Moessler H, Novak PH, Stan A, Buzoianu A, Bajenaru O, Popescu BO (2010) Persistence of the effects of Cerebrolysin on cognition and qEEG slowing in vascular dementia patients: results of a 3-month extension study. J Neurol Sci 299(1-2):179–183. https://doi.org/10.1016/j.jns.2010.08.040
Vereshchagin NV, Nekrasova EM, Lebedeva NV, Suslina ZA, Solov'ev OI, Piradov MA, Altunina MN. Legkie formy mul'tiinfarktnoĭ dementsii: éffektivnost' tserebrolizina [Mild forms of multi-infarct dementia: effectiveness of cerebrolysin]. Sov Med. 1991;(11):6–8. Russian. PMID: 1767322
Xiao S, Heqin Y, Peifen Y, Luning W, Jianjun J, Xin M, Feng B, Jie L, Chunxie W, Zhihua D, Mingyi Z, Wenzhen X, Yinglu G, Cui M, Daping W, Binhua S (2000) Efficacy of FPF 1070 (Cerebrolysin) in patients with Alzheimer’s disease: a multicentre, randomised, double-blind, placebo-controlled trial. Clin Drug Investig 19(1):43–53
Zhang L-J, Pan X-H, Wang Y-H, Sheng H-T (2003) Cerebrolysis improves the intelligence of vascular dementia patients in clinic. Chin J Clin Rehab 7:3850–3851
Fragoso YD, Dantas DC (2002) Cerebrolysin for Alzheimer’s disease. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd003801
Gavrilova SI, Alvarez A (2020) Cerebrolysin in the therapy of mild cognitive impairment and dementia due to Alzheimer’s disease: 30 years of clinical use. Med Res Rev. https://doi.org/10.1002/med.21722
Ruther E, Ritter R, Apecechea M, Freytag S, Gmeinbauer R, Windisch M (2000) Sustained improvements in patients with dementia of Alzheimer’s type (DAT) 6 months after termination of Cerebrolysin therapy. J Neural Transm (Vienna) 107(7):815–829. https://doi.org/10.1007/s007020070061
Bae CY, Cho CY, Cho K, Hoon Oh B, Choi KG, Lee HS, Jung SP, Kim DH, Lee S, Choi GD, Cho H, Lee H (2000) A double-blind, placebo-controlled, multicenter study of Cerebrolysin for Alzheimer’s disease. J Am Geriatr Soc 48(12):1566–1571. https://doi.org/10.1111/j.1532-5415.2000.tb03865.x
Panisset M, Gauthier S, Moessler H, Windisch M, Cerebrolysin Study G (2002) Cerebrolysin in Alzheimer’s disease: a randomized, double-blind, placebo-controlled trial with a neurotrophic agent. J Neural Transm (Vienna) 109(7-8):1089–1104. https://doi.org/10.1007/s007020200092
Ruether E, Husmann R, Kinzler E, Diabl E, Klingler D, Spatt J, Ritter R, Schmidt R, Taneri Z, Winterer W, Koper D, Kasper S, Rainer M, Moessler H (2001) A 28-week, double-blind, placebo-controlled study with Cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol 16(5):253–263. https://doi.org/10.1097/00004850-200109000-00002
Alvarez XA, Cacabelos R, Sampedro C, Couceiro V, Aleixandre M, Vargas M, Linares C, Granizo E, Garcia-Fantini M, Baurecht W, Doppler E, Moessler H (2011) Combination treatment in Alzheimer’s disease: results of a randomized, controlled trial with cerebrolysin and donepezil. Curr Alzheimer Res 8(5):583–591. https://doi.org/10.2174/156720511796391863
Bornstein N, Poon WS (2012) Accelerated recovery from acute brain injuries: clinical efficacy of neurotrophic treatment in stroke and traumatic brain injuries. Drugs Today (Barc) 48(Suppl A):43–61. https://doi.org/10.1358/dot.2012.48(Suppl.A).1739723
Zhang D, Dong Y, Li Y, Chen J, Wang J, Hou L (2017) Efficacy and safety of Cerebrolysin for acute ischemic stroke: a meta-analysis of randomized controlled trials. Biomed Res Int 2017:4191670. https://doi.org/10.1155/2017/4191670
Sharma HS, Zimmermann-Meinzingen S, Johanson CE (2010) Cerebrolysin reduces blood-cerebrospinal fluid barrier permeability change, brain pathology, and functional deficits following traumatic brain injury in the rat. Ann N Y Acad Sci 1199:125–137. https://doi.org/10.1111/j.1749-6632.2009.05329.x
Windisch M, Gschanes A, Hutter-Paier B. Neurotrophic activities and therapeutic experience with a brain derived peptide preparation. J Neural Transm Suppl. 1998;53:289–98. https://doi.org/10.1007/978-3-7091-6467-9_25. PMID: 9700665
Guekht A, Vester J, Heiss W-D, Gusev E, Hoemberg V, Rahlfs VW, Bajenaru O, Popescu BO, Doppler E, Winter S, Moessler H, Muresanu D (2017) Safety and efficacy of Cerebrolysin in motor function recovery after stroke: a meta-analysis of the CARS trials. Neurol Sci 38(10):1761–1769. https://doi.org/10.1007/s10072-017-3037-z
Wang Z, Shi L, Xu S, Zhang J (2017) Cerebrolysin for functional recovery in patients with acute ischemic stroke: a meta-analysis of randomized controlled trials. Drug Des Devel Ther 11:1273–1282. https://doi.org/10.2147/DDDT.S124273
Hussain R, Zubair H, Pursell S, Shahab M (2018) Neurodegenerative diseases: regenerative mechanisms and novel therapeutic approaches. Brain Sci 8(9). https://doi.org/10.3390/brainsci8090177
Stepanichev M, Onufriev M, Aniol V, Freiman S, Brandstaetter H, Winter S, Lazareva N, Guekht A, Gulyaeva N (2017) Effects of cerebrolysin on nerve growth factor system in the aging rat brain. Restor Neurol Neurosci 35(6):571–581. https://doi.org/10.3233/rnn-170724
Chao Moses V, Rajagopal R, Lee Francis S (2006) Neurotrophin signalling in health and disease. Clin Sci 110(2):167–173. https://doi.org/10.1042/cs20050163
Alvarez XA, Alvarez I, Martinez A, Romero I, Benito C, Suarez I, Mourente S, Fantini M, Figueroa J, Aleixandre M, Linares C, Muresanu D, Winter S, Moessler H (2020) Serum VEGF predicts clinical improvement induced by Cerebrolysin plus donepezil in patients with advanced Alzheimer’s disease. Int J Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyaa046
Rockenstein E, Ubhi K, Mante M, Florio J, Adame A, Winter S, Brandstaetter H, Meier D, Masliah E (2015) Neuroprotective effects of Cerebrolysin in triple repeat Tau transgenic model of Pick’s disease and fronto-temporal tauopathies. BMC Neurosci 16(1). https://doi.org/10.1186/s12868-015-0218-7
Alvarez XA, Alvarez I, Iglesias O, Crespo I, Figueroa J, Aleixandre M, Linares C, Granizo E, Garcia-Fantini M, Marey J, Masliah E, Winter S, Muresanu D, Moessler H (2016) Synergistic increase of serum BDNF in Alzheimer patients treated with Cerebrolysin and donepezil: association with cognitive improvement in ApoE4 cases. Int J Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyw024
Ozkizilcik A, Sharma A, Lafuente JV, Muresanu DF, Castellani RJ, Nozari A, Tian ZR, Mössler H, Sharma HS (2019) Nanodelivery of cerebrolysin reduces pathophysiology of Parkinson’s disease. In: Nanoneuroprotection and nanoneurotoxicology. Progress in brain research, pp 201–246. https://doi.org/10.1016/bs.pbr.2019.03.014
Khabirov FA, Khaybullin TI, Granatov EV, Shakirzianova SR (2016) Effect of cerebrolysin on remyelination processes in multiple sclerosis patients in stage of relapse regression. Zh Nevrol Psikhiatr Im S S Korsakova 116(12). https://doi.org/10.17116/jnevro201611612148-53
Masliah E, Diez-Tejedor E (2012) The pharmacology of neurotrophic treatment with Cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disorders. Drugs Today (Barc) 48(Suppl A):3–24. https://doi.org/10.1358/dot.2012.48(Suppl.A).1739716
Kushner D (1998) Mild traumatic brain injury: toward understanding manifestations and treatment. Arch Intern Med 158(15):1617–1624. https://doi.org/10.1001/archinte.158.15.1617
Lee S, Lee HH, Lee Y, Lee J (2020) Additive effect of cerebrolysin and amantadine on disorders of consciousness secondary to acquired brain injury: a retrospective case-control study. J Rehabil Med 52(2):jrm00025. https://doi.org/10.2340/16501977-2654
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fiani, B., Covarrubias, C., Wong, A. et al. Cerebrolysin for stroke, neurodegeneration, and traumatic brain injury: review of the literature and outcomes. Neurol Sci 42, 1345–1353 (2021). https://doi.org/10.1007/s10072-021-05089-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05089-2