Abstract
Migraine sciences have witnessed tremendous advances in recent years. Pre-clinical and clinical experimental models have contributed significantly to provide useful insights into the brain structures that mediate migraine attacks. These models have contributed to elucidate the role of neurotransmission pathways and to identify the role of important molecules within the complex network involved in migraine pathogenesis. The contribution and efforts of several research groups from all over the world has ultimately lead to the generation of novel therapeutic approaches, specifically targeted for the prevention of migraine attacks, the monoclonal antibodies directed against calcitonin gene-related peptide or its receptor. These drugs have been validated in randomized placebo-controlled trials and are now ready to improve the lives of a large multitude of migraine sufferers. Others are in the pipeline and will soon be available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Complexity of migraine
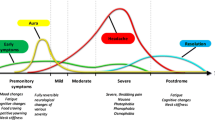
Migraine is a chronic neurological condition that manifests with recurrent attacks of severe cranial pain associated to sensorial (photo, phono, and osmophobia) and gastrointestinal (nausea, repeated vomiting) symptoms. These recurrent attacks reflect the involvement of a complex set of brain structures: cortical, subcortical, and brainstem areas that regulate autonomic, affective, cognitive, and sensory functions. Clinical and neurophysiological studies point to an important role for abnormal nociceptive processing, altered habituation of somatosensory stimuli, central sensitization, and changes in cerebrovascular and immune functions [1].
Migraine can be viewed as the more or less frequent recurrence of a brain network abnormality, where several nodes of which—cortical and subcortical (hypothalamus, thalamus, trigeminocervical complex, hypothalamus, and thalamus) are central to migraine pathophysiology.
Genetics plays an important role in the preparation of migraine terrain; however, until now no convincing evidence has suggested specific genetic alterations with large effect sizes, even though a multitude of genomic loci have been associated to migraine, to configure a condition of predisposition to a generalized neuronal excitability [2, 3].
Although migraine subjects function normally interictally, migraine brain differs from non migraine brain in many aspects also interictally. This is supported by several functional neuroimaging studies as well as by neurophysiological investigations. Functional neuroimaging studies have shown that interictally the migraine brain has stronger activation in pain-facilitating regions and hypoactivation in pain-inhibiting regions [4]. It seems that the migraine brain is also morphologically different from the normal brain, as suggested by the reduced gray matter volume in pain processing areas, such as the anterior cingulate cortex, amygdala, insula, operculum, and the frontal, temporal, and precentral gyri, and the increased gray matter volume in the caudate nuclei bilaterally in migraineurs with high-frequency attacks as compared with patients with low frequency [5, 6]. Neurophysiological studies have suggested that the migraine brain is poorly wired in terms of the protective mechanism represented by the habituation to repeated stimuli [7].
The extreme complexity of migraine pathophysiology—and the diffuse involvement of brain and neruvascular systems—has been partly unveiled through the adoption of experimental models. These are not capable of mimicking the entire array of structures involved anatomically and functionally, but still have allowed to identify and understand several components of migraine, thus leading to tremendous advances in the knowledge of migraine neurobiology. One for all is represented by the recent availability of the first target therapy for the prevention of migraine: the monoclonal antibodies targeting calcitonin gene-related peptide (CGRP) [8].
Several experimental models of migraine pain have been proposed and applied to investigate the mechanisms underlying headache pathophysiology. Most of them are focused on trigeminal sensory processing. Human models, associated to advanced imaging techniques, are useful to identify potential biomarkers and to improve the treatment. Advantages and drawbacks of each model should be considered to evaluate the predictive feature of antimigraine activity in the clinical setting, information about tolerability profile of drugs, neuro-anatomy of the structures (neuronal and vascular) involved in migraine, and non-pain associated symptoms. Here, we briefly discuss the preclinical and clinical research impact in the migraine field via a description of the relevant experimental models used, their utility in clinical setting, and therapeutic targets individuation.
Animal models of migraine
Plain vascular models: in vitro and in vivo
Within in vitro studies on blood vessels, vascular segments are mounted in organ baths and contraction or relaxation is measured isometrically to determine the potency (pEC50) and efficacy (Emax) of a potential antimigraine agent. In vitro models [9,10,11,12] (Table 1) provide the possibility of studying drug–receptor interactions and detailed pharmacological analysis that can be performed on multiple vessels segments in parallel.
In vivo vascular models are based on the involvement of vascular and neuronal components involved in disease and, although not including all migraine pathophysiological features, they contributed to the development of new antimigraine drugs [13,14,15,16,17,18,19,20,21,22] (Table 2).
Neurovascular models: in vitro and vivo
Neurogenic inflammation (vasodilatation and plasma protein extravasation) within cephalic tissues has been suggested as a potential mechanism of migraine pathogenesis. Numerous clinically effective abortive antimigraine drugs, such as ergots and triptans, have been shown to reduce the release of neuropeptides, such as CGRP and Substance P, and thus to inhibit neurogenic plasma protein extravasation [23].
In vitro neurovascular models
A trigeminal ganglion-skull cavity preparation, hemi-skull Preparation, has been developed to preserve some degree of trigeminal/meningeal interface and used as neurovascular model in vitro. In the hemi-skull preparation the nervus spinosus (branch of the V3 branch of the trigeminal nerve) is cleaned to remove the dura mater and prepared for placement into the recording system. This nerve innervates a region of the medial meningeal artery, a key structure for migraine pain [24]. This model overcomes several limitations of the in vivo models and allows to directly record the activity in nociceptive terminals under conditions of adequate drug concentration control and without application of concomitant anesthesia.
In vivo neurovascular models
Stimulation of the trigeminal ganglion and plasma protein extravasation
The trigeminal ganglion of anesthetized animals can be electrically stimulated using bipolar electrodes inserted stereotactically. Trigeminal ganglion neurons are activated with low-frequency ( ̴ 5-Hz) stimulation [25, 26], which induces plasma protein extravasation in the dura mater detected by radiolabeled albumin given intravenously prior to stimulation [27]. Protein leakage also occurs in extracranial tissues [28]. The model induces tissue-specific changes in the meninges and cerebral activation by c-Fos protein expression, a phenomenon that is inhibited by antimigraine drugs [29]. Stimulation of the trigeminal ganglion, however, failed to induce uniform activation of the brainstem nuclei related to migraine, although it activated the descending pain modulatory system [30]. Other studies have used the prolonged stimulation of the trigeminal ganglion (approximately 30 min) to induce morphological changes, whereas a shorter stimulation (3–5 min) causes the peripheral release of CGRP, a phenomenon that was inhibited by triptans and dihydroergotamine [31]. The repetitive electrical stimulation of the trigeminal ganglion, an experimental paradigm simulating migraine chronification [32], causes allodynia. Additionally, with this model it was possible to detect a direct link between pituitary adenylate cyclase-activating polypeptide (PACAP, another vasoactive neuropeptide) and the kynurenine system during trigeminal vascular system activation [33].
It is from the late 1980s the demonstration that a specific 5-HT receptor subtype mediates sumatriptan effects in the trigeminal ganglion stimulation model [34] and results obtained with the electrical stimulation of the trigeminal ganglion using diverse 5-HT agonists and antagonists showed that sumatriptan effect were most evident by 5-HT1B/D activation [31]. It must be noted that the drawbacks of this model are the generation of inflammatory responses both locally and in the dura mater due to the insertion of electrodes into the brain parenchyma.
Stimulation of the superior sagittal sinus
Electrical stimulation of meningeal nerve terminals, innervating the superior sagittal sinus (SSS) [35], the transverse sinus [36], or middle meningeal arteries [37], have also been used as preclinical model of migraine. The SSS is one of the sources of cephalic pain; indeed, its stimulation in humans causes pain referred to the head. SSS stimulation has been performed in rats, cats, and nonhuman primates to evaluate mechanisms underlying cephalic pain [38]. Data gathered with this model showed central actions of antimigraine drugs such as ergotamine and sumatriptan, but also of acetylsalicylic acid on transmission of trigeminal nociceptive input in the brainstem [39]. SSS stimulation has been used to investigate the effect of a variety of drugs on c-Fos protein expression [40], a neuronal marker of activation. For instance, a significant reduction of SSS stimulation-evoked c-Fos expression after MK-801 administration was reported in rat, indicating a role for glutamate in neurotransmission within the trigeminocervical complex [41].
Electrical and chemical stimulation of the meningeal nerve terminals
Electrical stimulation of the dura mater with stimuli that are able to activate thin myelinated and unmyelinated nerve fibers increases the meningeal blood flow in the rat. This change is attenuated by 5-HT1 receptor agonists and abolished by a CGRP receptor antagonist [42, 43].
Direct stimulation of primary sensory neurons supplying meninges has been obtained through infusion of irritant substances (capsaicin, carrageenan) on the meninges via microcannulas located into the cisterna magna of anesthetized rats, mice, or guinea pigs [44, 45]. These substances induce c-Fos protein expression within both sides of the trigeminal nucleus caudalis (TNC) 2 h after the administration. The limitation of this model is the lack of intra-animal control, since lateralization of c-Fos protein expression within the TNC cannot be explored and may be associated with possible damage caused by capsaicin [46].
Local application or infusion of inflammatory or algesic substances onto the dura mater or chemical stimulation of the dural receptive fields in rat causes hypersensitivity to mechanical and thermal stimulation together with direct activation of the trigeminal ganglion [47, 48]. The application of an inflammatory soup to the dura mater alters additional neural networks indirectly related to the primary nociceptive pathways via the spinal cord to the thalamus and cortex [49]. This inflammatory solution elicits both facial and plantar allodynia that can be reversed by sumatriptan and CGRP8–37 [50].
In mice, dural administration of allyl-isothiocyanate (a TRPA1 agonist), low pH, interleukin-6, or inflammatory soup lead to cephalic/extracephalic allodynia, that can be reversed by systemic injection of sumatriptan [51]. Repetitive infusion of inflammatory soup (histamine, bradykinin, serotonin, prostaglandin E2) induces a chronic periorbital hypersensitivity in rat that continues for up to 3 weeks. This condition is reversed by propranolol treatment via blocking the chronic sensitization of descending pain controls and preventing the central sensitization within the trigeminocervical complex [52]. Moreover, the repeated dura mater inflammatory soup infusion causes depression and anxiety like behaviors [53]. Migraine-like pathological changes and abnormal behaviors in conscious rhesus monkeys have been also reported after chronic inflammatory soup infusion. Higher expression levels of c-Fos, nNOS, and CGRP were found in various brain structures, including the trigeminal nucleus caudalis (TNC), thalamus, hypothalamus, midbrain, pons, and other areas involved in pain perception after infusion [54]. In selecting the present model, the researcher must consider that upstream events leading to trigeminal activation are bypassed and the chemical cocktail utilized requires cautious control to prevent supramaximal stimulation.
Nitric oxide donors
Nitric oxide (NO) donors, including nitroglycerin (NTG), have appeared as the most noticeable exogenous algogenic substances to date in the field of migraine research [55]. The animal and human models based on NTG administration have proved over the years to be reliable tools for investigating migraine mechanisms and have provided a wealth of data [56].
Systemic NTG via intraperitoneal administration (10 mg/kg) or intravenous infusion (4 μg/kg/min, for 20 min) in rodents induces neuronal activation in several brain nuclei, such as TNC, that are relevant for migraine pain [57], as well as behavioral nocifensive response. Such effects can be abolished by anti-migraine drugs [58, 59]. NTG-induced changes in central and/or peripheral neurotransmission are associated with trigeminal and spinal hyperalgesic state [60], which have been reported also in migraine subjects [61]. The abovementioned model, with a single NTG administration, is used to mimic the episodic migraine condition; whereas a repeated and intermittent treatment with NTG (10 mg/kg or 5 mg/Kg i.p.) experimentally reproduces the condition of chronic migraine. Within this experimental setting of chronic migraine, NTG induces sustained mechanical hyperalgesia, light hypersensitivity, and hypoactivity [62].
The ability of the NTG model to act at both the neuronal and vascular compartments has provided a relevant amount of evidences for better understanding of the pathophysiology of migraine attacks, in which NO and other mediators play a pivotal role (for extensive information see for review Demartini et al. [56]). Although highly attractive, several variables need to be carefully controlled when adopting the NTG based animal model for the study of the trigeminovascular system: (1) NTG dose and route of administration; (2) NTG vehicle; (3) choice of the time of observation, and (4) differences in rodent species.
Another NO donor worth mention is sodium nitroprusside (SNP). When administered intraperitoneally (4 mg/Kg) it induces in rat neuronal activation limited to the areas involved in the neuroendocrine control [63]. By contrast, when infused slowly over 2 h it causes a persistent increase in the neuronal activity of the TNC, which can be inhibited by a high dose of the CGRP antagonist olcegepant (900 μg/kg, i.v) [64].
Others algogenic substances
CGRP has been shown to trigger photophobia, periorbital hypersensitivity, and spontaneous pain behaviors in rodents [65,66,67]. Intravenous CGRP facilitated vibrissal responses, which seemed to suggest that CGRP-induced vasodilation activating the primary afferent meningeal nociceptors [68]. However, this observation was not supported by electrophysiological studies [69] when CGRP was administered via dural infusion. Whereas CGRP failed to increase c-Fos and Zif268 (neuronal pain markers) expression in the TNC of rat [70]. It must be noted that within this model, different results related to pain sensitization (hyperalgesia and allodynia) are obtained depending on the route of CGRP administration.
Pituitary adenylate cyclase-activating peptide (PACAP) is a neuropeptide implicated in a wide range of functions, such as nociception and primary headaches. PACAP infusion in rodents causes a delayed sensitization of trigeminovascular nociceptive processing [71]; subcutaneous PACAP injection in the periorbital area (10 μl/site) elicits periorbital mechanical sensitivity, which was attenuated by PACAP receptor antagonist, PACAP6-38. Moreover, PAPAP induces vasodilation in vivo suggesting that this effect might be mediated through degranulation of mast cells. This latter issue is confirmed by in vitro observations [72].
Models of cortical spreading depression
Cortical spreading depression (CSD) is supposed to mimic the underlying mechanism of migraine aura. CSD can be induced in animals by either chemical (superfusion of potassium chloride solution), pinprick, or electrical stimulation over cortex surface. CSD results in an increase in the extracellular (K+) and intracellular (Na + and Ca2+) ions and neurotransmitter (glutamate). CSD provokes the expression of c-fos protein-like immunoreactivity within neurons of the TNC via trigemino-vascular mechanisms, and it is a useful tool for testing current and novel prophylactic drugs (see Costa et al. [73] for review).
CSD induction showed similar transcriptomic profiles with and without drug treatment in cortex, involving genes related to hormone stimulus, apoptosis, synaptic transmission, and interleukin signaling. Glutamate NMDA receptors, voltage-dependent sodium channels, and CGRP play roles in CSD agents [74].
Human models
In vitro human models
Besides the animal in vitro models, also specimens of human origin have been used in the attempt to improve the understanding of antimigraine drug-induced effects. For instance, CGRP has been widely studied by using in vitro human models; the development and characterization of CGRP receptor antagonists, such as olcegepant, were performed on human and also other species arteries [75,76,77,78]. Table 3 summarizes in vitro human models.
In vivo human models of migraine
The animal models of migraine are critical to understanding the pathobiology of migraine and the factors involved such as the trigeminovascular system, CSD, central and peripheral nociception, localization of specific receptors, and key mediators. On the other hand, the human models can be used to test whether endogenous signaling molecules or other putative trigger factors may induce migraine-like attacks and are therefore suitable for investigating the mechanisms underlying spontaneous migraine attacks.
The first human migraine model was developed using the oral administration [79, 80] or i.v. infusion of NTG [81] to provoke migraine attacks without aura in migraine patients. To date, other human models are currently used, e.g., intravenous infusion of CGRP [82] and PACAP38 [83]; however, differently from the NTG model, for these models the comparative analysis with their pre-clinical counterparts is still limited due to the scarce use in animal experimental models.
NTG human model
Infusion or sublingual administration of NTG causes a biphasic response in migraineurs with an immediate, non-specific headache followed, hours later, by a migraine-like attack [79,80,81], with intensity and characteristics that are reminiscent of the patient’s own spontaneous attacks without aura. The reliability of the NTG model resides in its ability to reproduce headache attacks with features that are reminiscent of the spontaneous migraine attack. For instance, NTG in migraine patients induces premonitory symptoms together with the activation of specific brain areas (see for review Demartini et al. [56]).
CGRP human model
Intravenous CGRP infusion triggers delayed migraine-like attacks in patients with migraine without aura. Like NTG, CGRP is able to induce both an immediate and a delayed headache, although bearing generally milder and less frequently specific migraine features (see Ashina et al. [82] for review). Infusion of CGRP in healthy volunteers showed that the CGRP-induced headache was prevented by pretreatment with the olcegepant, but not abolished by sumatriptan [84].
Other vasoactive peptides
Besides CGRP, also, the vasoactive peptides VIP and PACAP may trigger migraine attacks in humans. Systemic administration of VIP induced mild headaches for a short time in healthy volunteers. Although VIP is able to induce a marked dilation of cranial arteries, it does not trigger a migraine-like attack in migraine sufferers [85], while PACAP38 infusion does [86].
Informative differences and similarities of the mostly used human models, NTG and CGRP, with pre-clinical models are illustrated in Table 4.
Other ongoing stories
The wealth of scientific output gathered with the experimental models described above has greatly contributed to identify potential therapeutic targets for migraine, paving the way, for instance, to the development of monoclonal antibodies targeting CGRP. In this frame, other potential therapeutic molecules are currently under evaluation and will hopefully lead to additional advances in migraine science. In Table 5 we show some potential targets/pathways for both acute and prophylactic treatments of migraine.
Another interesting story that is going on regard the role of acute medication overuse in the transformation of episodic migraine into chronic migraine. The long-lasting ongoing debate has not yet clarified how acute medications can intercept the neurobiology of migraine to negatively affect the disease outcome. A series of recent data provided by different groups using animal models of medication overuse clearly points to the capability of some acute medications to induce a condition of hyperalgesia and will therefore contribute to disentangle this critical issue [116, 117].
Conclusions
Experimental models of migraine have yielded significant insights into brain structures that mediate migraine symptoms and the recurrence of attacks. These models have contributed to elucidate the role of small peptides as neurotransmitters within this network, thus fostering the generation of novel therapeutic approaches that have been validated by randomized placebo-controlled trials and will hopefully provide a substantial improvement to the lives of a large multitude of migraine sufferers.
References
Andreou AP, Edvinsson L (2019) Mechanisms of migraine as a chronic evolutive condition. J Headache Pain 20(1):117
Gormley P, Anttila V, Winsvold BS et al (2016) Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet 48(8):856–866
de Boer I, van den Maagdenberg AMJM, Terwindt GM (2019) Advance in genetics of migraine. Curr Opin Neurol 32(3):413–421
Mainero C, Boshyan J, Hadjikhani N (2011) Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 70(5):838–845
Da Silva AF, Granziera C, Tuch DS, Snyder J, Vincent M, Hadjikhani N (2007) Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport. 18(4):301–305
Maleki N, Becerra L, Nutile L, Pendse G, Brawn J, Bigal M, Burstein R, Borsook D (2011) Migraine attacks the basal ganglia. Mol Pain 7:71
Coppola G, Di Lorenzo C, Schoenen J, Pierelli F (2013) Habituation and sensitization in primary headaches. J Headache Pain 14(1):65
Dodick DW (2019) CGRP ligand and receptor monoclonal antibodies for migraine prevention: evidence review and clinical implications. Cephalalgia 2019 39:445–458
Mehrotra S, Gupta S, Garrelds IM, Villalón CM, Saxena PR, Bogers AJ, Maassenvandenbrink A (2006) Effects of current and prospective antimigraine drugs on the porcine isolated meningeal artery. Naunyn Schmiedebergs Arch Pharmacol 374(3):163–175
Franco-Cereceda A, Rudehill A, Lundberg JM (1987) Calcitonin gene-related peptide but not substance P mimics capsaicin-induced coronary vasodilation in the pig. Eur J Pharmacol 142(2):235–243
Bouchelet I, Case B, Olivier A, Hamel E (2000) No contractile effect for 5-HT1D and 5-HT1F receptor agonists in human and bovine cerebral arteries: similarity with human coronary artery. Br J Pharmacol 129(3):501–508
Sheykhzade M, Amandi N, Pla MV, Abdolalizadeh B, Sams A, Warfvinge K, Edvinsson L, Pickering DS (2017) Binding and functional pharmacological characteristics of gepant-type antagonists in rat brain and mesenteric arteries. Vascul Pharmacol 90:36–43
Maassenvandenbrink A, Chan KY (2008) Neurovascular pharmacology of migraine. Eur J Pharmacol 585(2–3):313–319
De Vries P, Villalón CM, Saxena PR (1999) Pharmacological aspects of experimental models in relation to acute antimigraine therapy. Eur J Pharmacol 375(1–3):61–74
Williamson DJ, Hargreaves RJ, Hill RG, Shepheard SL (1997) Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat—intravital microscope studies. Cephalalgia 17:525–531
Akerman S, Kaube H, Goadsby PJ (2004) Anandamide acts as a vasodilator of dural blood vessels in vivo by activating TRPV1 receptors. Br J Pharmacol 142:1354–1360
Akerman S, Kaube H, Goadsby PJ (2003) Vanilloid type 1 receptors (VR1) on trigeminal sensory nerve fibres play a minor role in neurogenic dural vasodilatation, and are involved in capsaicin-induced dural dilation. Br J Pharmacol 140:718–724
Haanes KA, Labastida-Ramírez A, Blixt FW, Rubio-Beltrán E, Dirven CM, Danser AH, Edvinsson L, MaassenVanDenBrink A (2019) Exploration of purinergic receptors aspotential anti-migraine targets using established pre-clinical migraine models. Cephalalgia 39(11):1421–1434
Petersen KA, Birk S, Doods H, Edvinsson L, Olesen J (2004) Inhibitory effect of BIBN4096BS on cephalic vasodilatation induced by CGRP or transcranial electrical stimulation in the rat. Br J Pharmacol 43(6):697–704
Connor HE, Stubbs CM, Feniuk W, Humphrey PP (1992) Effect of sumatriptan, a selective 5-HT1-like receptor agonist, on pial vessel diameter in anaesthetized cats. J Cereb Blood Flow Metab 12(3):514–519
Juhl L, Petersen KA, Larsen EH, Jansen-Olesen I, Olesen J (2006) The in vivo effect of adrenomedullin on rat dural and pial arteries. Eur J Pharmacol 538(1–3):101–107
Wang X, Fang Y, Liang J, Yan M, Hu R, Pan X (2014) 5-HT7 receptors are involved in neurogenic dural vasodilatation in an experimental model of migraine. J Mol Neurosci 54(2):164–170
Malhotra R (2016) Understanding migraine: potential role of neurogenic inflammation. Ann Indian Acad Neurol 19(2):175–182
Zakharov A, Vitale C, Kilinc E, Koroleva K, Fayuk D, Shelukhina I, Naumenko N, Skorinkin A, Khazipov R, Giniatullin R (2015) Hunting for origins of migraine pain: cluster analysis of spontaneous and capsaicin-induced firing in meningeal trigeminal nerve fibers. Front Cell Neurosci 9:287
Knyihar-Csillik E, Tajti J, Mohtasham S, Sari G, Vecsei L (1995) Electrical stimulation of the Gasserian ganglion induces structural alterations of calcitonin gene-related peptide-immunoreactive perivascular sensory nerve terminals in the rat cerebral dura mater: a possible model of migraine headache. Neurosci Lett 184(3):189–192
Limmroth V, Katsarava Z, Liedert B, Guehring H, Schmitz K, Diener HC, Michel MC (2001) An in vivo rat model to study calcitonin gene related peptide release following activation of the trigeminal vascular system. Pain 92(1–2):101–106
Markowitz S, Saito K, Moskowitz MA (1987) Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci 7:4129–4136
Buzzi MG, Moskowitz MA (1990) The antimigraine drug, sumatriptan (GR43175), selectively blocks neurogenic plasma extravasation from blood vessels in dura mater. Br J Pharmacol 99:202–206
Knyihar-Csillik E et al (1997) Effect of a serotonin agonist (sumatriptan) on the peptidergic innervation of the rat cerebral dura mater and on the expression of c-fos in the caudal trigeminal nucleus in an experimental migraine model. J Neurosci Res 48(5):449–464
Bohár Z, Fejes-Szabó A, Tar L, Varga H, Tajti J, Párdutz Á, Vécsei L (2013) Evaluation of c-Fos immunoreactivity in the rat brainstem nuclei relevant in migraine pathogenesis after electrical stimulation of the trigeminal ganglion. Neurol Sci 34(9):1597–1604
Buzzi MG, Carter WB, Shimizu T, Heath H III, Moskowitz MA (1991) Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology 30:1193–1200
Zhang Q, Han X, Wu H, Zhang M, Hu G, Dong Z, Yu S (2019) Dynamic changes in CGRP, PACAP, and PACAP receptors in the trigeminovascular system of a novel repetitive electrical stimulation rat model: relevant to migraine. Mol Pain 15:1744806918820452
Körtési T, Tuka B, Tajti J, Bagoly T, Fülöp F, Helyes Z, Vécsei L (2018) Kynurenic acid inhibits the electrical stimulation induced elevated pituitary adenylate cyclase-activating polypeptide expression in the TNC. Front Neurol 8:745
Humphrey PP, Feniuk W, Perren MJ, Connor HE, Oxford AW, Coates LH, Butina D (1988) GR43175, a selective agonist for the 5-HT1-like receptor in dog isolated saphenous vein. Br J Pharmacol 94:1123–1132
Zagami AS, Goadsby PJ, Edvinsson L (1990) Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides 16(2):69–75
Robert C, Bourgeais L, Arreto CD, Condes-Lara M, Noseda R, Jay T, Villanueva L (2013) Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J Neurosci 33(20):8827–8840
Holland PR, Akerman S, Andreou AP, Karsan N, Wemmie JA, Goadsby PJ (2012) Acid-sensing ion channel 1: a novel therapeutic target for migraine with aura. Ann Neurol 72(4):559–563
Kaube H, Keay KA, Hoskin KL, Bandler R, Goadsby PJ (1993) Expression of c-Fos-like immunoreactivity in the caudal medulla and upper cervical spinal cord following stimulation of the superior sagittal sinus in the cat. Brain Res 629(1):95–102
Kaube H, Hoskin KL, Goadsby PJ (1994) Acetylsalicylic acid inhibits cerebral cortical vasodilatation caused by superior sagittal sinus stimulation in the cat. Eur J Neurol 1(2):141–146
Goadsby PJ, Hoskin KL (1999) Differential effects of low dose CP122,288 and eletriptan on fos expression due to stimulation of the superior sagittal sinus in cat. Pain 82:15–22
Hoskin KL, Goadsby PJ (1998) Comparison of a more and less lipophilic serotonin (5HT1B/1D) agonist in a model of trigeminovascular nociception in cat. Exp Neurol 150:45–51
Kurosawa M, Messlinger K, Pawlak M, Schmidt RF (1995) Increase of meningeal blood flow after electrical stimulation of rat dura mater encephali: mediation by calcitonin gene-related peptide. Br J Pharmacol 114:1397–1402
Messlinger K, Hotta H, Pawlak M, Schmidt RF (1997) Effects of the 5-HT1 receptor agonists, sumatriptan and CP 93,129, on dural arterial flow in the rat. Eur J Pharmacol 332:173–181
Mitsikostas DD, Sanchez del Rio M, Waeber C, Moskowitz MA, Cutrer FM (1998) The NMDA receptor antagonist MK-801 reduces capsaicin-induced c-fos expression within rat trigeminal nucleus caudalis. Pain 76:239–248
Cutrer FM, Mitsikostas DD, Ayata G, Sanchez del Rio M (1999) Attenuation by butalbital of capsaicin-induced c-fos-like immunoreactivity in trigeminal nucleus caudalis. Headache 39:697–704
Mitsikostas DD, Sanchez del Rio M (2001) Receptor systems mediating c-fos expression within trigeminal nucleus caudalis in animal models of migraine. Brain Res Rev 2001(35):20–35
Burstein R (2001) Deconstructing migraine headache into peripheral and central sensitization. Pain 89:107–110
Laborc KF, Spekker E, Bohár Z, Szűcs M, Nagy-Grócz G, Fejes-Szabó A, Vécsei L, Párdutz Á (2020) Trigeminal activation patterns evoked by chemical stimulation of the dura mater in rats. J Headache Pain 21(1):101
Becerra L, Bishop J, Barmettler G, Kainz V, Burstein R, Borsook D (2017) Brain network alterations in the inflammatory soup animal model of migraine. Brain Res 1660:36–46
Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, de Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F (2009) Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol 2009(65):184–193
Burgos-Vega CC, Quigley LD, Trevisan Dos Santos G, Yan F, Asiedu M, Jacobs B, Motina M, Safdar N, Yousuf H, Avona A, Price TJ, Dussor G (2019) Non-invasive dural stimulation in mice: a novel preclinical model of migraine. Cephalalgia. 39(1):123–134
Boyer N, Signoret-Genest J, Artola A, Dallel R, Monconduit L (2017) Propranolol treatment prevents chronic central sensitization induced by repeated dural stimulation. Pain. 158(10):2025–2034
Zhang M, Liu Y, Zhao M, Tang W, Wang X, Dong Z, Yu S (2017) Depression and anxiety behaviour in a rat model of chronic migraine. J Headache Pain 18(1):27
Chen N, Su W, Cui SH, Guo J, Duan JC, Li HX, He L (2019) A novel large animal model of recurrent migraine established by repeated administration of inflammatory soup into the dura mater of the rhesus monkey. Neural Regen Res 14(1):100–106
Ashina M, Hansen JM, Olesen J (2013) Pearls and pitfalls in human pharmacological models of migraine: 30 years’ experience. Cephalalgia 2013 33(8):540–553
Demartini C, Greco R, Zanaboni AM, Sances G, De Icco R, Borsook D, Tassorelli C (2019) Nitroglycerin as a comparative experimental model of migraine pain: from animal to human and back. Prog Neurobiol 177:15–32
Greco R, Mangione AS, Sandrini G, Maccarrone M, Nappi G, Tassorelli C (2011) Effects of anandamide in migraine: data from an animal model. J Headache Pain 12(2):177–183
Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A (2014) Characterization of a novel model of chronic migraine. Pain 155(2):269–274
Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, Ptácek LJ, Ahn AH (2010) Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia 30(2):170–178
Greco R, Bandiera T, Mangione AS, Demartini C, Siani F, Nappi G, Sandrini G, Guijarro A, Armirotti A, Piomelli D, Tassorelli C (2015) Effects of peripheral FAAH blockade on NTG-induced hyperalgesia--evaluation of URB937 in an animal model of migraine. Cephalalgia. 35(12):1065–1076
De Icco R, Fiamingo G, Greco R, Bottiroli S, Demartini C, Zanaboni AM, Allena M, Guaschino E, Martinelli D, Putortì A, Grillo V, Sances G, Tassorelli C (2020) Neurophysiological and biomolecular effects of erenumab in chronic migraine: an open label study. Cephalalgia. 26:333102420942230
Greco R, Demartini C, Zanaboni AM, Tassorelli C (2018) Chronic and intermittent administration of systemic nitroglycerin in the rat induces an increase in the gene expression of CGRP in central areas: potential contribution to pain processing. J Headache Pain 19(1):51
Tassorelli C, Greco R, Cappelletti D, Sandrini G, Nappi G (2005) Comparative analysis of the neuronal activation and cardiovascular effects of nitroglycerin, sodium nitroprusside and L-arginine. Brain Res 1051(1–2):17–24
Koulchitsky S, Fischer MJ, Messlinger K (2009) Calcitonin gene-related peptide receptor inhibition reduces neuronal activity induced by prolonged increase in nitric oxide in the rat spinal trigeminal nucleus. Cephalalgia. 29(4):408–417
Rea BJ, Wattiez AS, Waite JS, Castonguay WC, Schmidt CM, Fairbanks AM, Robertson BR, Brown CJ, Mason BN, Moldovan-Loomis MC, Garcia-Martinez LF, Poolman P, Ledolter J, Kardon RH, Sowers LP, Russo AF (2018) Peripherally administered calcitonin gene-related peptide induces spontaneous pain in mice: implications for migraine. Pain. 159(11):2306–2317
De Logu F, Landini L, Janal MN et al (2019) Migraine-provoking substances evoke periorbital allodynia in mice. J Headache Pain 20(1):18
Mason BN, Kaiser EA, Kuburas A, Loomis MCM, Latham JA, Garcia-Martinez LF, Russo AF (2017) Induction of migraine-like photophobic behavior in mice by both peripheral and central CGRP mechanisms. J Neurosci 37(1):204–216
Cumberbatch MJ, Williamson DJ, Mason GS, Hill RG, Hargreaves RJ (1999) Dural vasodilation causes a sensitization of rat caudal trigeminal neurones in vivo that is blocked by a 5-HT1B/1D agonist. Br J Pharmacol 126:1478–1486
Levy D, Burstein R, Strassman AM (2005) Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann Neurol 58:698–705
Bhatt DK, Ramachandran R, Christensen SL, Gupta S, Jansen-Olesen I, Olesen J (2015) CGRP infusion in unanesthetized rats increases expression of c-Fos in the nucleus tractus solitarius and caudal ventrolateral medulla, but not in the trigeminal nucleus caudalis. Cephalalgia 35:220–233
Akerman S, Goadsby PJ (2015) Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: relevance to migraine. Sci Transl Med 7(308):308ra157
Pedersen SH, la Cour SH, Calloe K, Hauser F, Olesen J, Klaerke DA, Jansen-Olesen I (2019) PACAP-38 and PACAP (6-38) Degranulate rat meningeal mast cells via the orphan MrgB3-receptor. Front Cell Neurosci 13:114
Costa C, Tozzi A, Rainero I, Cupini LM, Calabresi P, Ayata C, Sarchielli P (2013) Cortical spreading depression as a target for anti-migraine agents. J Headache Pain 14(1):62
Tozzi A, de Iure A, Di Filippo M et al (2012) Critical role of calcitonin gene-related peptide receptors in cortical spreading depression. Proc Natl Acad Sci U S A 109(46):18985–18990
Jansen-Olesen I, Jorgensen L, Engel U, Edvinsson L (2013) In-depth characterization of CGRP receptors in human intracranial arteries. Eur J Pharmacol 481:206–217
Edvinsson L, Gulbenkian S, Barroso CP, Cunha e Sá M, Polak JM, Mortensen A, Jørgensen L, Jansen-Olesen I (1998) Innervation of the human middle meningeal artery:immunohistochemistry, ultrastructure, and role of endothelium for vasomotility. Peptides 19(7):1213–1225
Tfelt-Hansen P, De Vries P, Saxena PR (2000) Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 60(6):1259–1287
Edvinsson L, Alm R, Shaw D, Rutledge RZ, Koblan KS, Longmore J, Kane SA (2002) Effect of the CGRP receptor antagonist BIBN4096BS in human cerebral, coronary and omental arteries and in SK-N-MC cells. Eur J Pharmacol 434:49–55
Sicuteri F, Del Bene E, Poggioni M, Bonazzi A (1987) Unmasking latent dysnociception in healthy subjects. Headache 27(4):180–185
Sances G, Tassorelli C, Pucci E, Ghiotto N, Sandrini G, Nappi G (2004) Reliability of the nitroglycerin provocative test in the diagnosis of neurovascular headaches. Cephalalgia 24(2):110–119
Iversen HK, Olesen J, Tfelt-Hansen P (1989) Intravenous nitroglycerin as an experimental model of vascular headache. Basic characteristics. Pain 38(1):17–24
Ashina H, Schytz HW, Ashina M (2019) CGRP in Human Models of Migraine. Handb Exp Pharmacol 255:109–120
Ashina H, Guo S, Vollesen ALH, Ashina M (2017) PACAP38 in human models of primary headaches. J Headache Pain 18(1):110
Falkenberg K, Rønde Bjerg H, Yamani N, Olesen J (2020) Sumatriptan does not antagonize CGRP-induced symptoms in healthy volunteers. Headache 60(4):665–676
Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M (2008) Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia 28:226–236
Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M (2009) PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 132:16–25
Afridi S, Kaube H, Goadsby PJ (2005) Occipital activation in glyceryl trinitrate induced migraine with visual aura. J Neurol Neurosurg Psychiatry 76(8):1158–1160
Hansen JM, Thomsen LL, Olesen J, Ashina M (2010) Coexisting typical migraine in familial hemiplegic migraine. Neurology 74(7):594–600
Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning Pv, Larsson HB, Olesen J, Ashina M (2011) Evidence for a vascular factor in migraine. Ann Neurol 69(4):635–645
Christiansen I, Daugaard D, Lykke Thomsen L, Olesen J (2000) Glyceryl trinitrate induced headache in migraineurs - relation to attack frequency. Eur J Neurol 7(4):405–411
Afridi SK, Kaube H, Goadsby PJ (2004) Glyceryl trinitrate triggers premonitory symptoms in migraineurs. Pain 110(3):675–680
Maniyar FH, Sprenger T, Schankin C, Goadsby PJ (2014) The origin of nausea in migraine-a PET study. J Headache Pain 15(1):84
Guo S, Vollesen AL, Olesen J, Ashina M (2016) Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain 157(12):2773–2781
de Hoon JN, Willigers JM, Troost J, Struijker-Boudier HA, van Bortel LM (2003) Cranial and peripheral interictal vascular changes in migraine patients. Cephalalgia 23(2):96–104
Di Clemente L, Coppola G, Magis D, Gérardy PY, Fumal A, De Pasqua V, Di Piero V, Schoenen J (2009) Nitroglycerin sensitises in healthy subjects CNS structures involved in migraine pathophysiology: evidence from a study of nociceptive blink reflexes and visual evoked potentials. Pain 144(1–2):156–161
Perrotta A, Serrao M, Tassorelli C, Arce-Leal N, Guaschino E, Sances G, Rossi P, Bartolo M, Pierelli F, Sandrini G, Nappi G (2011) Oral nitric-oxide donor glyceryl-trinitrate induces sensitization in spinal cord pain processing in migraineurs: a double-blind, placebo-controlled, cross-over study. Eur J Pain 15(5):482–490
Asghar MS, Becerra L, Larsson HB, Borsook D, Ashina M (2016) Calcitonin gene- related peptide modulates heat nociception in the human brain - an fmri study in healthy volunteers. PLoS One 11(3):e0150334
Tvedskov JF, Thomsen LL, Iversen HK, Gibson A, Wiliams P, Olesen J (2004) The prophylactic effect of valproate on glyceryltrinitrate induced migraine. Cephalalgia 24(7):576–585
Fullerton T, Komorowski-Swiatek D, Forrest A, Gengo FM (1999) The pharmacodynamics of sumatriptan in nitroglycerin-induced headache. J Clin Pharmacol 39(1):17–29
Van der Schueren BJ, Blanchard R, Murphy MG, Palcza J, De Lepeleire I, Van Hecken A, Depré M, de Hoon JN (2011) The potent calcitonin gene-related peptide receptor antagonist, telcagepant, does not affect nitroglycerin-induced vasodilation in healthy men. Br J Clin Pharmacol 71(5):708–717
Petersen KA, Birk S, Lassen LH, Kruuse C, Jonassen O, Lesko L, Olesen J (2005) The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia 25(2):139–147
Greco R, Demartini C, Zanaboni AM, Tumelero E, Reggiani A, Misto A, Piomelli D, Tassorelli C (2020) FAAH inhibition as a preventive treatment for migraine: A pre-clinical study. Neurobiol Dis 134:104624
Mogil JS, Miermeister F, Seifert F, Strasburg K, Zimmermann K, Reinold H, Austin JS, Bernardini N, Chesler EJ, Hofmann HA, Hordo C, Messlinger K, Nemmani KV, Rankin AL, Ritchie J, Siegling A, Smith SB, Sotocinal S, Vater A, Lehto SG, Klussmann S, Quirion R, Michaelis M, Devor M, Reeh PW (2005) Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci USA 102(36):12938–12943
Chu DQ, Choy M, Foster P, Cao T, Brain SD (2000) A comparative study of the ability of calcitonin generelated peptide and adrenomedullin (13–52) to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol 130(7):1589–1596
Marquez de Prado B, Hammond DL, Russo AF (2009) Genetic enhancement of calcitonin gene-related Peptide-induced central sensitization to mechanical stimuli in mice. J Pain 10(9):992–1000
Huang Y, Brodda-Jansen G, Lundeberg T, Yu LC (2004) Anti-nociceptive effects of calcitonin gene-related peptide in nucleus raphe magnus of rats: an effect attenuated by naloxone. Brain Res 873(1):54–59
Yao G, Huang Q, Wang M, Yang CL, Liu CF, Yu TM (2017) Behavioral study of a rat model of migraine induced by CGRP. Neurosci Lett 651:134–139
Christensen SL, Petersen S, Kristensen DM, Olesen J, Munro G (2019) Targeting CGRP via receptor antagonism and antibody neutralisation in two distinct rodent models of migraine-like pain. Cephalalgia 39(14):1827–1837
Greco R, Mangione AS, Siani F, Blandini F, Vairetti M, Nappi G, Sandrini G, Buzzi MG, Tassorelli C (2014) Effects of CGRP receptor antagonism in nitroglycerin-induced hyperalgesia. Cephalalgia 34(8):594–604
Sundrum T, Walker CS (2018) Pituitary adenylate cyclase-activating polypeptide receptors in the trigeminovascular system: implications for migraine. Br J Pharmacol 175(21):4109–4120
Moldovan Loomis C, Dutzar B, Ojala EW, Hendrix L, Karasek C, Scalley-Kim M, Mulligan J, Fan P, Billgren J, Rubin V, Boshaw H, Kwon G, Marzolf S, Stewart E, Jurchen D, Pederson SM, Perrino McCulloch L, Baker B, Cady RK, Latham JA, Allison D, Garcia-Martinez LF (2019) Pharmacologic Characterization of ALD1910, a Potent Humanized Monoclonal Antibody against the Pituitary Adenylate Cyclase- Activating Peptide. J Pharmacol Exp Ther 369(1):26–36
Demartini C, Tassorelli C, Zanaboni AM, Tonsi G, Francesconi O, Nativi C, Greco R (2017) The role of the transient receptor potential ankyrin type-1 (TRPA1) channel in migraine pain: evaluation in an animal model. J Headache Pain 18(1):94
Mirrasekhian E, Nilsson JLÅ, Shionoya K, Blomgren A, Zygmunt PM, Engblom D, Högestätt ED, Blomqvist A (2018) The antipyretic effect of paracetamol occurs independent of transient receptor potential ankyrin 1-mediated hypothermia and is associated with prostaglandin inhibition in the brain. FASEB J 32(10):5751–5759
Vila-Pueyo M (2018) Targeted 5-HT1F Therapies for Migraine. Neurotherapeutics 15(2):291–303
Pertwee RG (2001) Cannabinoid receptors and pain. Prog Neurobiol 63(5):569–611
Navratilova E, Behravesh S, Oyarzo J, Dodick DW, Banerjee P, Porreca F (2020) Ubrogepant does not induce latent sensitization in a preclinical model of medication overuse headache. Cephalalgia 40(9):892–902
Saengjaroentham C, Strother LC, Dripps I, Sultan Jabir MR, Pradhan A, Goadsby PJ, Holland PR (2020) Differential medication overuse risk of novel anti-migraine therapeutics. Brain 143(9):2681–2688
Funding
This manuscript was partly supported by grants from the Italian Ministry of Health to IRCCS Mondino 432 Foundation, Pavia, Italy (GR-2016-02363848, and RC2017-2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CT received honoraria for the participation to advisory boards or for oral presentations from 424 Allergan, ElectroCore, Eli-Lilly, Novartis, and Teva. CT has no ownership interest and does not 425 own stocks of any pharmaceutical company. CT serves as Chief Section Editor of Frontiers in 426 Neurology—Section Headache Medicine and Facial Pain and on the editorial board of The Journal of Headache and Pain.
The other authors have no potential conflicts of interest to declare.
Ethical approval
This article does not contain any study with human subjects.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Greco, R., Demartini, C., De Icco, R. et al. Migraine neuroscience: from experimental models to target therapy. Neurol Sci 41 (Suppl 2), 351–361 (2020). https://doi.org/10.1007/s10072-020-04808-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04808-5