Abstract
Background
Multiple sclerosis (MS) is an autoimmune, neuroinflammatory, and neurodegenerative disease of the central nervous system. B cells have recently emerged as a promising target to significantly reduce inflammatory disease activity in MS, with successful trial studies using antiCD20 therapies. However, real-life data about safety and efficacy are limited.
Objectives
To analyze the clinical and radiological inflammatory activity, adherence to therapy, and safety of rituximab (RTX) in an MS patients’ sample, treated from 2015 to 2018 in our center
Patients and methods
Retrospective study on prospectively collected data about relapses, disability progression, and radiological activity (new T2 lesions and Gd-enhancing lesions) were recorded and used to assess no evidence of disease activity (NEDA) at 12 months. RTX-related adverse events were recorded. RTX was administered intravenously at a dosage of 1000 mg twice 2 weeks apart, then every 6 months.
Results
Sixty-nine patients were included. Fifty-three (76.8%) had a relapsing-remitting, two a primary progressive course, and 14 a secondary progressive course. The mean follow-up period was 16 ± 9.7 months. Thirty-five (50.7%) patients had relapses in the year prior to RTX therapy, with a mean annualized relapse rate of 0.75, significantly reduced to 0.36 at 12 months (p < 0.001). Among the 36 patients included in the study who had an MRI available at 12 months, MRI activity was reduced from 88% (n = 32) to 8.3% (n = 3) at follow-up (p < 0.001). Twelve (17.4%) patients suspended RTX during the study.
Conclusions
Our real-life experience confirms that off-label therapy with RTX may represent a valid, cost-effective therapeutic option in MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is an autoimmune, neuroinflammatory, and neurodegenerative disease of the central nervous system, most often characterized at onset by a relapsing-remitting (RR) course. In the past few years, B cells have emerged as a promising target to significantly reduce inflammatory disease activity, with the first successful trials using rituximab (RTX) in RRMS [1,2,3]. The standard administration dosage for MS patients consists of an induction phase, with two 1000-mg infusions at a 15-day interval, and a maintenance regimen, with reinfusions every 6 months. More recently, a phase III clinical trial is evaluating two different dosing regimens, comparing—after 1 year with a 500-mg infusion in the induction phase and every 6 months—an extended-dose administration arm (with a 12-month reinfusion regimen) with a standard-interval administration regimen (ClinicalTrials.gov Identifier: NCT03979456). Even if an increasing body of evidence suggests the efficacy of RTX as a highly efficacious disease-modifying treatment (DMT) for MS, prospective real-life data are still needed [4,5,6,7,8,9]. The aim of the present study was to analyze efficacy and safety of RTX administration in an Italian real-life cohort of MS patients.
Methods
We included MS patients, diagnosed according to the 2010 McDonalds criteria [10], followed by the MS center of the University of Florence and treated with RTX as an “off-label” therapy from 2015 to 2018. RTX was administered intravenously at the dosage of 1000 mg twice with 8 weeks apart, in a single course. A maintenance regimen was then started, with reinfusions every 6 months using the same dosage of 1000 mg. Time on treatment was defined as time from first RTX infusion until data censure. When addressing reasons for discontinuation of the last treatment before RTX, poor efficacy was defined as clinical (one or more relapses) and/or radiological activity (new T2 lesions and or gadolinium-enhancing lesions in two consecutive MRIs) under treatment [11].
In this retrospective study on prospectively acquired clinical data, we evaluated both efficacy and safety outcomes. Efficacy outcomes were the reduction of annualized relapse rate (ARR) at 12 months; disability progression at 6 and 12 months (defined as a worsening of 1.5 Expanded Disability Status Scale, EDSS [12] points if the baseline EDSS was 0.0; 1 point if it was 1–5.5; 0.5 points if it was ≥ 5.5 [13]); MRI activity at 6 and 12 months (defined as new T2 lesions and/or gadolinium (Gd)-enhancing lesions). These data were combined to obtain the no evidence of disease activity (NEDA)-3 score for a patients’ subgroup. As for safety outcomes, we assessed the occurrence of adverse events (AEs) that were classified as infusion-related (IR), infectious, and non-infectious. The AEs were also classified accordingly to the Common Terminology Criteria for Adverse Events (CTCAE v 5.0, 2017 [14]) into mild (1); moderate (2); severe (3); and life-threatening (4).
Blood samples for safety and B cell monitoring were drawn immediately before RTX infusions. B cell levels were not used to guide treatment decisions. This study was approved by the local ethic committee.
Statistical analysis
Group comparisons were performed using the Student t test, Mann-Whitney U test, and chi-square test when appropriate, with Bonferroni’s correction for multiple comparisons. The McNemar test was used for dichotomic non-parametric variables. Comparison of efficacy and safety variables before and after treatment was performed using the paired t test, Wilcoxon signed-rank test, and McNemar test when appropriate. Possible predictors of NEDA at 12 months were assessed using a multivariable logistic regression analysis, including sex, age at onset, age at RTX therapy, disease duration, disease course, ARR in the year before therapy, and baseline EDSS as covariates.
Likewise, possible predictors of infusion-related, infectious, and non-infectious AEs were assessed using multivariable logistic regression analyses, including the following covariates: sex, age at onset, age at RTX therapy, disease duration, disease course, baseline EDSS, previous immunosuppressive therapy.
A p value < 0.05 was considered statistically significant for all the analyses. IBM SPSS version 25.0 was used for the statistical analysis.
Results
Clinical and demographical data
Sixty-nine patients were included in our study: 51 (73.9%) females, 53 RR, 12 secondary progressive (SP), and four primary progressive (PP) patients. All the analyses were conducted considering SP and PP patients as a whole “progressive” group. In comparison with RR patients, the progressive cases were older at RTX therapy initiation (49.5 ± 7.0 years old vs 40.9 ± 9.7 years old in the RR patients, p < 0.0001) and had a higher median baseline EDSS score (6; IQR, 5.5–7 vs 2.5; IQR, 1.5–5, p < 0.0001). Furthermore, a trend (p = 0.09) was found toward a difference in disease duration (7.1 ± 5.1 years in RR vs 10.3 ± 6.7 years in progressive patients). The patients were followed for a mean of 16.0 ± 9.7 months. Other demographic and clinical characteristics of the whole sample are depicted in Table 1.
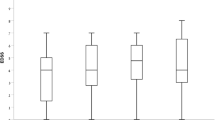
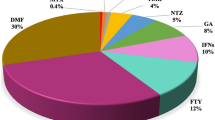
For 44 (68.8%) of the 64 previously treated patients, persistent disease activity during treatment with another DMT represented the most frequent reason for starting RTX. Thirteen patients, three receiving GA or IFN, two dimethyl-fumarate, five cyclophosphamide, and three fingolimod, switched to RTX due to AEs or reduced compliance. Seven patients switched from natalizumab to RTX due to safety reasons (high titer JC virus positivity). In the remaining five (7.2%) patients, RTX was administered as a first-line therapy due to a highly active disease course. The most frequent therapy used before RTX initiation was dimethyl-fumarate (23.2%), followed by fingolimod (18.8%). The proportion of previous treatments and their relative reason for discontinuation are depicted in more detail in Table 2.
Efficacy
Annualized relapse rate
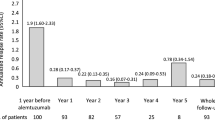
Seven patients were excluded from the efficacy analysis, since they were switching from natalizumab and in NEDA-3 status before starting RTX. Among the remaining 62 patients, 46 (74.2%) had a RR, and 16 (26.8%) had a progressive disease course. Sixty-two patients were included in the analysis. The mean ARR in the year before the first infusion was of 0.75, and it was significantly reduced at 12 months to 0.36 (p = 0.004). Considering RR and progressive patients separately, there was a trend (p = 0.07) toward a significant reduction in the ARR in both groups. Patients with a RR course had an ARR of 0.36 at 12 months, compared with 0.83 in the year before inclusion, while progressive patients had an ARR of 0.5 in the year before inclusion, reduced to 0.36 at 12 months. The time from last relapse to RTX initiation was 1.23 (SD, ± 1.5) years for the whole group, while the time from first RTX administration to first relapse was 0.74 years (SD, ± 0.54). Considering separately RR and progressive patients, the latency from last relapse to RTX was 0.67 (SD, ± 0.54) vs 3.2 (SD, ± 2.1) (p < 0.001), while from first RTX administration to first relapse 0.82 (SD, ± 0.6) in RR patients and 0.2 (SD, ± 0.2) (p = 0.442) for progressive patients.
MRI activity
Data on MRI activity were available in 43 subjects at 6 months and 36 at 12 months. At 6 months, there was a significant reduction in the proportion of patients showing MRI activity that decreased from 67.4 to 23.2% considering the whole group (p < 0.001). Focusing on different disease courses, there was a 55.4% reduction in MRI activity at 6 months for RR patients (p < 0.001), with a similar reduction in progressive patients (53.2%, p < 0,039). Among the 36 patients included in the study who had an MRI available at 12 months, MRI activity was reduced from 88% (n = 32) to 8.3% (n = 3) at follow-up (p < 0.001).
EDSS progression
Of the 55 patients who had an EDSS evaluation at 6 and 12 months, 13 (23.2%) showed an EDSS progression at 6 months, while only one patient showed an EDSS progression at 12 months, compared with baseline. Distinguishing RR and progressive patients, ten progressive and three RR patients showed an EDSS progression at 6 months, while no RR and one progressive patient had an EDSS worsening at 12 months.
NEDA
We evaluated the NEDA-3 [15] status at 12 months for 36 patients. Twenty-two of them (61.1%) were labeled as NEDA-3 at 12 months, eighteen RR and four progressive patients. In the multivariable model, no significant predictor was found (data not shown).
Efficacy data for RR and progressive patients are reported in more detail in Table 3.
Safety
The whole sample was included in the safety analysis. The frequency and severity of the AEs that occurred during the follow-up period are depicted in Table 4.
Infusion-related AEs were the most common and occurred in 30 cases (43.5%). They were most often represented by a mild pricking sensation in the throat and resolved by reducing the infusion speed or temporarily stopping the infusion, without further treatments. In only one case, there was a more serious reaction, characterized by a maculopapular rash located in the trunk and neck that prompted RTX interruption and required corticosteroid administration. The patients who experienced infusion-related AEs had a lower EDSS (3.0 ± 1.8 vs 4.4 ± 2.2, p = 0.008). At the multivariable analysis, a lower EDSS (OR 0.69 CI 95% 0.52–0.92; p = 0.012) and the absence of previous immunosuppressive treatments (OR 0.25, CI 95% 0.08–0.85; p = 0.026) were associated with a higher frequency of infusion-related AEs.
In nineteen cases (27.5%), non-infectious AEs recorded were most commonly leukopenia or neutropenia (26.3%). One patient developed a pancreatic carcinoma that prompted therapy interruption. However, this event was recorded after 4 months following the induction phase.
Infectious AEs were less common than non-infectious in our cohort, occurring in 16 (23.2%) cases under RTX therapy. The most common infections recorded were urinary tract infections, reported to affect 10 (62.5%) subjects. Four patients had respiratory tract infection, and two had gastrointestinal infection. One patient experienced Gram+ sepsis as a consequence of persistent neutropenia. At the multivariable analysis, a significant association between infections and a longer disease duration was noted (11.1 ± 6.0 years. vs 6.6 ± 4.9 years old in the infectious vs non-infectious group; p = 0.013).
Analyzing the correlation between non-infectious AEs and clinical and demographic characteristics, we observed that the four subjects with evidence of leukopenia had a significantly lower weight compared with the rest of the sample (56.8 ± 2.4 vs 66.1 ± 13.0 kg, p < 0.0001). No significant relationships were found between clinical characteristics and non-infectious AEs at the multivariable analysis.
Twelve (17.4%) patients suspended RTX during the study. The reasons for discontinuation are reported in Table 5.
The 27 patients who experienced CTCAE grade ≥ 2 infusion-related AEs to RTX administration were tested for anti-drug antibodies that were positive in one of them.
Discussion
From the first small case series published in 2004 that described a favorable experience with RTX in neuromyelitis optica spectrum disorder and aggressive MS patients [16], RTX has been suggested to be a highly effective DMT for MS patients [1, 3, 7, 17, 18]. This was confirmed in a recent review including 48 clinical studies involving at least 2151 RRMS and 1437 progressive MS [9].
As a consequence of the abovementioned promising real-word data about its usage in MS, and following the positive results of the phase III clinical trials of ocrelizumab and its subsequent approval, the off-label use of RTX has gained attention for the patients who do not respect the inclusion criteria for ocrelizumab therapy [19].
Our observational study that included both RR and progressive patients, with a mean follow-up time of 16 months, confirms the previously shown good efficacy and safety profile of RTX therapy for MS patients.
First, the ARR was significantly reduced at the 12-month follow-up visit (0.75 vs 0.36, p = 0.004) for the whole patient group. These results are in line with both the phase II clinical trial conducted by Hauser et al. [1] in 2008 and other studies that focused on real-life data obtained by national clinical registries [4] or in other cohorts, including other patients with acquired demyelinating disorders [6].
Regarding radiological disease activity, the significant 12-month reduction in MRI activity showed in our patients corroborates the efficacy of RTX therapy on MRI measures of ongoing disease activity, documented in other studies [1, 5, 6]. Regarding Gd+ lesions, a 2010 study focusing on highly active RRMS patients with recent relapses [20] showed a significant reduction of the number of these lesions. However, differently from the other studies, we considered Gd+ lesions and new T2 lesions as a measure of MRI activity, without distinguishing them.
Fifty-five patients had an EDSS evaluation at baseline and at 6 and 12 months. Thirteen of them showed an EDSS worsening that was not confirmed at 12 months for most of them. For progressive patients, even if in a reduced sample size, the medium EDSS worsened at 12 months. The data on RR patients are in line with some other real-life studies, where the EDSS ameliorated for RR patients at 12 months [4, 7]. However, other studies demonstrated a stability of the EDSS score at 12 months for RR patients, and one registry study even reported an EDSS worsening at 12 and 18 months for this patients’ subgroup [8]. As for progressive disease course, a phase II/III placebo-controlled RCT explored the efficacy of RTX in PPMS: a significant reduction in time to confirmed disability progression (CDP) in younger (< 51 years old) patients and in those with Gd-enhancing lesions at baseline was confirmed at 96 weeks, even if the time to CDP was not significantly different between the two arms [21]. Moreover, a real-life study including a progressive patient subgroup demonstrated that the EDSS score ameliorated for SP patients, while remained the same for PP patients [4]. Finally, a recent retrospective study assessed efficacy of RTX-treated patients in reducing EDSS score and CDP in SPMS patients at a mean follow-up of 3.5 years, compared with propensity-score-matched individuals who were never treated with RTX [22]. We were not able to replicate these findings, due to the limited sample size of our progressive cohort.
In interpreting the above results, due to the absence of a control group, a regression to the mean phenomenon has to be taken into account. Nevertheless, our efficacy data taken together with other data in the literature suggest an early action of RTX into reducing clinical and radiological activity, while its action may be more delayed regarding disability worsening, and it could be more evident later or during the treatment. However, the observational design and the relatively limited sample size and duration of the follow-up prevent us to take strong evidence supporting our hypotheses.
The NEDA-3 status was evaluated at 12 months from the start of RTX therapy: this condition was observed in 61.1% of patients, consistent with the result reported by D’Amico et al. [6] but slightly lower than the percentage reported in another recent observational study (74%) [23].
Concerning safety issues, in our patients, we observed a frequency of infusion-related adverse events similar to the data reported in other studies [24]. In most cases, these AEs were mild-to-moderate (CTCAE 1–2) and spontaneously resolved or only needed a temporary slowing in the infusion rate. In one case, a severe allergic reaction (CTCAE 4) occurred and led to therapy discontinuation.
Within our sample, infectious and not infectious AEs were almost equally balanced in their proportion (23.2% vs 27.5%, respectively), conversely from what has been reported in another study [4], where infectious AEs were more represented.
As discussed before, 27.5% of our sample experienced non-infectious AEs: in only 3 (4.3%) subjects, these were serious in nature (CTCAE 3 to 4) and led to discontinuation of therapy. In particular, in a single patient, the presence of an infiltrating pancreatic neoplasm was found after 4 months from the beginning of RTX therapy: however, in consideration of other previous immunosuppressive treatments and the short time of exposure to RTX, the direct relationship with RTX administration appears to be doubtful. A large cohort study reported a low frequency of all type of malignancies for RTX in MS, without significant differences with the general population, with an estimated incidence rate of 34.4 for 10,000 patient-years, compared with 31.0 in the general population [25].
Interestingly, in our study, some of the adverse events were hematologic (10.1%); in particular, our data confirm what had been reported in the literature for MS or neuromyelitis optica spectrum disorder patients for hypogammaglobulinemia during RTX therapy [26, 27].
In our sample, despite adequate premedication with antihistaminics and iv corticosteroids, more than 40% of patients experienced IR AEs. In the multivariable analysis, a higher incidence of IR AEs was associated with lower EDSS score and absence of previous immunosuppressive treatments. Literature data about IR AEs related to RTX administration suggest that—except for rare cases of hypersensitivity—these AEs are mainly linked to cytokine-release by immune cells (B and NK cells), resulting from the binding of the Fc portion of RTX to FcyRIIIA/CD16 expressed in these cells, and thus could be influenced by peripheral B cell level and CD16 expression [28]. It is possible to speculate that previous immunosuppressive treatment could influence B and NK cell activities, reducing the risk of IR AEs after RTX administration. However, larger studies are needed in the attempt to find a predictive model for these events and identify the patients who may need a more aggressive premedication before RTX infusion.
Analyzing the possible correlations between clinical and demographic parameters and AEs, we observed that the four subjects with evidence of leukopenia had a significantly lower weight compared with the rest of the sample. It is interesting to note that a Swedish study [5] showed no differences in efficacy between the two groups with different maintenance doses (500 mg vs 1000 mg every 6 months); moreover, this study revealed a reduction in AEs in the group that received a reduced maintenance dose. In light of these results and with the aim of customizing the therapeutic schemes of RTX therapy, future studies comparing different dosages of the drug in larger and prospective cohorts are necessary.
Conclusion
Despite few limitations, our study confirms that RTX can be considered an efficacious and reasonably safe therapy for MS, especially for patients with a RR disease course. Future multicentric and comparative trials are needed to evaluate the long-term efficacy and tolerability of this low-cost therapy compared with other monoclonal antibodies used for MS.
Data availability
The data that support the findings of this study are available from the corresponding author, MPA, upon reasonable request.
References
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH, HERMES Trial Group (2008) B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 358:676–688. https://doi.org/10.1056/NEJMoa0706383
Naismith RT, Piccio L, Lyons JA, Lauber J, Tutlam NT, Parks BJ, Trinkaus K, Song SK, Cross AH (2010) Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology. 74:1860–1867. https://doi.org/10.1212/WNL.0b013e3181e24373
Bar-Or A, Calabresi PAJ, Arnlod D et al (2008) Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 63:395–400. https://doi.org/10.1002/ana.21363
Alcalá C, Gascón F, Pérez-Miralles F, Gil-Perotín S, Navarré A, Boscá I, Coret F, Casanova B (2018) Efficacy and safety of rituximab in relapsing and progressive multiple sclerosis: a hospital-based study. J Neurol 265:1690–1697. https://doi.org/10.1007/s00415-018-8899-3
Salzer J, Svenningsson R, Alping P, Novakova L, Björck A, Fink K, Islam-Jakobsson P, Malmeström C, Axelsson M, Vågberg M, Sundström P, Lycke J, Piehl F, Svenningsson A (2016) Rituximab in multiple sclerosis. Neurology. 87:2074–2081. https://doi.org/10.1212/WNL.0000000000003331
D’Amico E, Zanghì A, Chisari CG et al (2019) Effectiveness and safety of rituximab in demyelinating diseases spectrum: an Italian experience. Mult Scler Relat Disord 27:324–326. https://doi.org/10.1016/j.msard.2018.09.041
Spelman T, Frisell T, Piehl F, Hillert J (2018) Comparative effectiveness of rituximab relative to IFN-β or glatiramer acetate in relapsing-remitting MS from the Swedish MS registry. Mult Scler J 24:1087–1095. https://doi.org/10.1177/1352458517713668
Scotti B, Disanto G, Sacco R, Guigli M’, Zecca C, Gobbi C (2018) Effectiveness and safety of rituximab in multiple sclerosis: an observational study from southern Switzerland. PLoS One 13:e0197415. https://doi.org/10.1371/journal.pone.0197415
Ineichen BV, Moridi T, Granberg T, Piehl F (2019) Rituximab treatment for multiple sclerosis. Mult Scler J 135245851985860:137–152. https://doi.org/10.1177/1352458519858604
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302. https://doi.org/10.1002/ana.22366
Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, Clanet M, Comi G, Derfuss T, Fazekas F, Hartung HP, Havrdova E, Hemmer B, Kappos L, Liblau R, Lubetzki C, Marcus E, Miller DH, Olsson T, Pilling S, Selmaj K, Siva A, Sorensen PS, Sormani MP, Thalheim C, Wiendl H, Zipp F (2018) ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 24:96–120. https://doi.org/10.1177/1352458517751049
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 33:1444–1452. https://doi.org/10.1212/WNL.33.11.1444
Kappos L, Butzkueven H, Wiendl H, Spelman T, Pellegrini F, Chen Y, Dong Q, Koendgen H, Belachew S, Trojano M, On Behalf of the Tysabri® Observational Program (TOP) Investigators (2018) Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult Scler J 24:963–973. https://doi.org/10.1177/1352458517709619
NIH/NCI (2017) Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP. In: Protocol
Giovannoni G, Tomic D, Bright JR, Havrdová E (2017) “No evident disease activity”: the use of combined assessments in the management of patients with multiple sclerosis. Mult Scler 23:1179–1187. https://doi.org/10.1177/1352458517703193
Cree B, Lamb S, Chin A, Bonovich D, Islar J, Waubant E, Genain C (2004) Tolerability and effects of rituximab (anti-CD20 antibody) in neuromyelitis optica (NMO) and rapidly worsening multiple sclerosis (MS). Neurology 62:06.90
Salzer J, Svenningsson R, Alping P, Novakova L, Björck A, Fink K, Islam-Jakobsson P, Malmeström C, Axelsson M, Vågberg M, Sundström P, Lycke J, Piehl F, Svenningsson A (2016) Rituximab in multiple sclerosis a retrospective observational study on safety and efficacy. Neurology 87:2074–2081
Granqvist M, Boremalm M, Poorghobad A, Svenningsson A, Salzer J, Frisell T, Piehl F (2018) Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol 75:320–327. https://doi.org/10.1001/jamaneurol.2017.4011
Berntsson SG, Kristoffersson A, Boström I, Feresiadou A, Burman J, Landtblom AM (2018) Rapidly increasing off-label use of rituximab in multiple sclerosis in Sweden—outlier or predecessor? Acta Neurol Scand 138:327–331. https://doi.org/10.1111/ane.12963
Piccio L, Naismith RT, Trinkaus K, Klein RS, Parks BJ, Lyons JA, Cross AH (2010) Changes in B- and T-lymphocyte and chemokine levels with rituximab treatment in multiple sclerosis. Arch Neurol 67:707–714. https://doi.org/10.1001/archneurol.2010.99
Hawker K, O’Connor P, Freedman MS et al (2009) Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 66:460–471. https://doi.org/10.1002/ana.21867
Naegelin Y, Naegelin P, Von Felten S et al (2019) Association of rituximab treatment with disability progression among patients with secondary progressive multiple sclerosis. JAMA Neurol 76:274–281. https://doi.org/10.1001/jamaneurol.2018.4239
Yamout BI, El-Ayoubi NK, Nicolas J et al (2018) Safety and efficacy of rituximab in multiple sclerosis: a retrospective observational study. J Immunol Res 2018:1–9. https://doi.org/10.1155/2018/9084759
D’Amico E, Zanghì A, Gastaldi M et al (2019) Placing CD20-targeted B cell depletion in multiple sclerosis therapeutic scenario: present and future perspectives. Autoimmun Rev 18:665–672. https://doi.org/10.1016/j.autrev.2019.05.003
Alping P, Askling J, Burman J, Fink K, Fogdell-Hahn A, Gunnarsson M, Hillert J, Langer-Gould A, Lycke J, Nilsson P, Salzer J, Svenningsson A, Vrethem M, Olsson T, Piehl F, Frisell T (2020) Cancer risk for fingolimod, natalizumab, and rituximab in multiple sclerosis patients. Ann Neurol 87:688–699. https://doi.org/10.1002/ana.25701
Valentino P, Marnetto F, Granieri L, Capobianco M, Bertolotto A (2017) Aquaporin-4 antibody titration in NMO patients treated with rituximab: a retrospective study. Neurol Neuroimmunol Neuroinflamm 4:e317. https://doi.org/10.1212/NXI.0000000000000317
Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Montalban X, Rammohan KW, Selmaj K, Traboulsee A, Wolinsky JS, Arnold DL, Klingelschmitt G, Masterman D, Fontoura P, Belachew S, Chin P, Mairon N, Garren H, Kappos L, OPERA I and OPERA II Clinical Investigators (2017) Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 376:221–234. https://doi.org/10.1056/NEJMoa1601277
Paul F, Cartron G (2019) Infusion-related reactions to rituximab: frequency, mechanisms and predictors. Expert Rev Clin Immunol 15:383–389
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
MP Amato received research grants and honoraria as a speaker and member of advisory boards from Bayer, Biogen, Merck, Novartis, Sanofi Genzyme, Teva, Almirall, and Roche. E Portaccio served as scientific advisory board for Biogen Idec and Merck Serono; received honoraria for speaking and funding for traveling from Biogen, Genzyme, Novartis, Merck, and Teva; and received research support from Merck Serono. L. Pastò and L. Razzolini received editorial grant from Teva, Almirall, Genzyme, and Merck. All other authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the institutional review board of the University of Florence, Italy, and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Informed consent was obtained from all patients.
Consent for publication
The authors declare that neither the article nor portions of it have been previously published elsewhere, that the manuscript is not under consideration for publication in another journal, and will not be submitted elsewhere until the Neurol Sci editorial process is completed. All authors consent for the publication of the manuscript in Neurol Sci, should the article be accepted by the Editor-in-Chief upon completion of the refereeing process.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bellinvia, A., Prestipino, E., Portaccio, E. et al. Experience with rituximab therapy in a real-life sample of multiple sclerosis patients. Neurol Sci 41, 2939–2945 (2020). https://doi.org/10.1007/s10072-020-04434-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04434-1