Abstract
Spontaneous intracranial hypotension (SIH) is a rare neurological condition caused by low cerebrospinal fluid (CSF) volume, most commonly due to a CSF leak. The most common presenting symptom is an orthostatic headache, but some patients may present with atypical neurological manifestations such as cranial nerve palsies, an altered mental status, and movement disorders, which complicate the clinical diagnosis. Therefore, the diagnosis is based on the combination of clinical signs and symptoms, neuroimaging, and/or a low cerebrospinal fluid pressure. In this review, we describe the wide variety of neurological manifestations and complications seen in patients with SIH as well as the most common features described on imaging studies, including both subjective and objective measurements, in order to lead the clinician to a correct diagnosis. The prompt and correct management of patients with SIH will help prevent the development of life-threatening complications, such as subdural hematomas, cerebral venous thrombosis, and coma, and avoid unnecessary invasive procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous intracranial hypotension (SIH), first identified in 1938 by German neurologist George Schaltenbrand, is a rare neurological condition usually caused by a spontaneous cerebrospinal fluid leak. If this alteration goes unnoticed, several clinical manifestations develop, primarily a postural headache [1]. The lack of a clear etiologic factor and its variety of presentations and imaging findings make it a frequently misdiagnosed neurological disorder. In this review article, we describe the main aspects of SIH, and focus on the essential clinical and imaging studies necessary for an accurate diagnosis. We also discuss key pathophysiological aspects and the initial treatment of this pathology.
Definition
Spontaneous intracranial hypotension is an uncommon, benign, and generally self-limiting condition caused by low cerebrospinal fluid (CSF) volume and pressure usually caused by a CSF leak [2]. This process results in a downward traction of the brain that may cause headaches, subdural fluid collections, and possible brain herniation, among other complications [3].
The International Classification of Headache Disorders (ICHD-3) assorts SIH under “secondary headaches attributed to non-vascular intracranial disorder,” as a headache attributed to spontaneous intracranial hypotension. It is described as an orthostatic headache caused by low CSF pressure of spontaneous origin, usually accompanied by neck stiffness and subjective hearing symptoms that remit after normalization of CSF pressure [4].
Incidence
SIH is a rare entity with a reported incidence of 5:100,000 patients per year. Women are commonly more affected than men (twice as likely) and the peak incidence is usually around 40 years old [5]. A report of 24 cases by Lyoubi et al. established a rate of 37% for patients with a serious form of SIH (including subdural hematoma, disturbance of consciousness, and cerebral venous thrombosis) [6].
Etiology
The exact cause of SIH often remains unnoticed; nerve root sleeve diverticulum, osteophyte spurs, and CSF to venous fistula are known as its main causes. Nerve root sleeve diverticulum is considered the most common presentation of a CSF leak in such pathology, by tearing of the dura and exposure of the arachnoid [1, 7,8,9]. This leak, thought to be the initial step of the pathophysiology, is not always well located even though it is known that the most common site of leakage is the thoracic spine [1, 10,11,12]. Another important aspect to consider is the size of the defect: the greater the defect the greater the leak, with secondary changes in clinical manifestations and imaging findings [7]. Most of the cases are considered to be caused by spine pathology [13].
Several authors have proposed a classification for CSF leaks. Schievnick et al. described one based on the etiology of the CSF leak; type 1: a dural tear—most commonly due to an osteophyte spur (with a prevalence of 26.6%), type 2: a meningeal diverticulum (42.3%), type 3: CSF-venous fistula (CVF) (2.5%), and type 4: indeterminate (28.7%) [14]. However, a newer classification scheme was provided by Farb et al., based on the morphology and distance of the CSF leak from the midline (Table 1) [10, 14]. This new classification is useful, as it provides information for a more specific management, depending on the etiology of each leak.
A relationship has been found between SIH, slender patients, and connective tissue disorders such as weakness of dura mater, isolated joint hypermobility, spontaneous retinal detachment, Marfan syndrome, Ehlers-Danlos syndrome type II, and autosomal dominant polycystic kidney disease [5, 15]. There is also a theory that suggests an imbalance of the pressure gradient between epidural space veins and CSF as a cause of increased CSF resorption [6].
Pathophysiology
Intracranial pressure is a perfectly balanced system of fluids and tissue: CSF, blood, and brain. Each of these components physiologically contributes to balance this pressure in a way that if any of them decreases or increases its volume, it would cause either compensation by the other two elements or a sudden damage to brain tissue, known as the Monro-Kellie doctrine. In SIH, there is a loss of CSF and subsequent hypovolemia; this leads to a downward displacement of the brain, pulls on pain sensitive structures, or dilates vascular structures and ultimately causes the characteristic headache of SIH. Standing up further decreases intracranial pressure; therefore, the pain is relieved by lying down [3, 16]. A common potential complication of SIH is a subdural hematoma, caused by the increased blood volume compensation mechanism directed towards cerebral veins due to their resistance and distention properties. Finally, patients can also develop a mild vasogenic edema due to impaired venous drainage [17].
Clinical manifestations
SIH integrates a myriad of signs and symptoms that can vary depending on the severity and time of establishment of the hypotension. The most common clinical manifestation is orthostatic headache due to further decrease of intracranial pressure while standing; other commonly found symptoms include nausea, vomiting, and posterior neck stiffness (occurring in about 50% of patients) [15, 16]. Even though these are the most common presenting symptoms, patients can have rare clinical findings that can mislead the diagnosis. The following manifestations have been reported in patients with SIH:
-
1.
Headache
It usually starts 15 min after standing up and has a bilateral occipital/suboccipital region presentation, although frontal and temporal types have also been described. Several case series report a frequency of 87.5–100% [6, 18]. Pain intensity varies from mild to severe and usually progresses rapidly over a few hours. It is worse during the Valsalva maneuver and head shaking. The orthostatic nature of the headache might become less obvious over time and some other patients may have chronic daily headaches with associated anxiety and/or depression. In rare cases, headache may not be postural but continuous [19]. Less typical presentations include non-positional, thunderclap, exertional, cough-related, and paradoxical (lying down) headaches [5]; a study by Ferrante et al. reported thunderclap headache in 15% of cases and observed seven patients (over 400 cases) who had as a single-symptom headache induced by the Valsalva maneuver [20].
SIH should also be considered as an alternative diagnosis for “reversible cerebral vasoconstriction syndrome,” a rare severe acute headache, often thunderclap in nature, mimicking that of a ruptured aneurysm. The major complications are localized cortical subarachnoid hemorrhages (20–25%) and ischemic or hemorrhagic strokes (5–10%). This syndrome typically resolves in 1 to 3 months and consists of a headache (that may or may not be accompanied by other neurological symptoms) and constriction of cerebral arteries [21, 22].
-
2.
Neck pain/back pain
These are considered some of the most common manifestations apart from headaches (71% in a case series by Idrissi et al.) [6]. They can present at the same time as orthostatic headaches, or occur days to weeks before the classic manifestations. A correlation between the site of pain and CSF leak level is not to be expected, and low back pain has also been described [16].
-
3.
Altered mental status
Somnolence, lethargy, or coma appear in advanced stages of SIH; they are usually fluctuating and progress over time, and they have been reported in 12–23% of patients [6, 23]. The displacement of cranial structures causes a brain herniation syndrome with a cephalic-caudal progression of signs and symptoms that include cranial nerve palsies, changes in breathing patterns, and decorticate or decerebrate posturing and may lead to respiratory arrest and coma [5]. Coma is a rare but serious complication of SIH; Schievinck et al. describe 15 patients with SIH that developed a coma. Compared with patients with SIH without coma, the patients who had this complication were more often older males who required surgical closure of CSF leaks. Fortunately, this type of coma is usually reversible with the aforementioned procedure [24].
-
4.
Frontotemporal brain sagging syndrome
Behavioral variant frontotemporal dementia (bvFTD) is a neurodegenerative disease defined by insidious and progressive decline in behavior and executive function. Frontotemporal brain sagging syndrome (FBSS) has been described as cognitive and behavioral changes that mimic a behavioral variant frontotemporal dementia but with evidence also suggestive of brain sagging. It is characterized by structural and functional changes affecting the frontal and anterior temporal regions such as disinhibition, poor impulse, antisocial behavior, and stereotypical or ritualized behaviors [25]. However, these symptoms are associated to atypical findings such as daytime somnolence, headache, dysarthria, dysphagia, gait disturbance, ocular abnormalities, and movement disorders. Therefore, SIH should be suspected in patients with unexplained rapidly progressing frontotemporal dementia, especially when it is associated with other neurological symptoms and an absence of brain atrophy on the MRI [26,27,28].
-
5.
Weakness
Patients can present with generalized weakness, subjectively described by the patient as constant fatigue or malaise, and may be associated to tremor [5, 29, 30].
-
6.
Cranial nerve palsies
Patients may present with an alteration in any cranial nerve (4–42.5%), due to the traction of the brainstem as intracranial pressure decreases [31, 32]. These findings can be divided as follows:
-
a.
Ocular findings
Patients may have optic nerve alterations, such as visual blurring, transient obscurations, photophobia, and visual field defects (involving the upper nasal and upper temporal quadrants). Extraocular muscle nerves (III, IV, VI) may also be affected, resulting in diplopia [3, 5, 16, 33]. The most common ophthalmoplegia is abducens nerve paresis (83%) due to its long intracranial course, which makes it susceptible to traction. Unilateral palsies have been more commonly reported (60%), compared with bilateral abducens nerve palsies (24%). Isolated oculomotor nerve and trochlear nerve paresis are less common, and have been reported with a prevalence of 14 and 7%, respectively [34,35,36].
-
b.
Extraocular findings
Patients may present with signs and symptoms related to traction of any cranial nerve, these include facial paresthesia, facial palsy, dysgeusia, and hiccups [3, 5]. Cochleovestibular manifestations (unilateral hearing loss, dizziness, tinnitus, and vertigo) may be related to the traction of the VIII cranial nerve; however, it has also been attributed to an altered pressure in the perilymphatic/endolymphatic fluid of the inner ear [16].
-
7.
Seizures/nonconvulsive status epilepticus
Nonconvulsive status epilepticus may occur in patients with advanced stages of SIH, and should be considered in patients with electrographic epileptiform discharges that do not respond to typical antiepileptic drugs. Patients have also been described with isolated seizures, such as myoclonus [5, 29].
-
8.
Movement disorders
Parkinsonism, ataxia, postural tremor, and chorea have been described as atypical findings of SIH [5, 37]. Duvall et al. reported a frequency of 12.5% of patients with movement disorders in a case series of 44 patients [38].
-
9.
Galactorrhea
Elevated prolactin levels may be found in up to 24% of patients with SIH. It is not related to pituitary size, rather to the presence of brain sagging [2, 39] .
-
10.
Radiculopathy/myelopathy
Upper extremity radiculopathy (usually in the cervical nerve roots) has also been reported, with a prevalence of 6% of patients. This occurs at the site of the leak and is usually caused by mass effect from the extradural CSF collection [3, 40, 41]. Transient quadriplegia has been attributed to compression of the upper spinal cord by severe herniation of the hindbrain [42].
-
11.
Hemorrhagic/thrombotic complications
Cerebral venous thrombosis has been found in about 2% of patients. It should always be considered as a possible complication in patients with SIH, especially if the headache pattern changes from orthostatic to continuous. However, this pattern change is not present in every patient that develops cerebral venous thrombosis and its absence should not exclude the diagnosis [43]. Hemorrhagic complications have also been reported, such as cerebellar hemorrhage and subdural hematomas. Takahashi et al. state an incidence of 16–57% of subdural hematoma among patients with SIH, with an average onset age of 44.9 years (older than SIH alone) [44]. It is important to consider the clinical manifestations that these complications cause, as they are similar to those primarily caused by SIH [5, 42, 45].
Imaging findings
Imaging techniques are crucial in order to assess anatomical changes in the central nervous system due to SIH; findings can be divided into two main groups: cranial/spinal findings and CSF leak–related findings. The latter is considered to be the determinant of this condition, as well as the cause of the main cranial findings to appear. Overall, brain and spinal MRI findings have a sensitivity and specificity of 83% and 94%, respectively [46].
Cranial findings
MRI is considered the imaging study of choice to evaluate SIH’s cranial findings. The most common alterations observed in this condition are as follows: pachymeningeal enhancement, subdural fluid collections (subdural hygromas), brain sagging, downward displacement of tonsils, distension of venous structures, and enlargement of the pituitary gland [47].
-
1.
Meningeal findings
Pachymeningeal enhancement (Fig. 1) is defined as a diffuse hyperintensity involving supratentorial and infratentorial compartments when contrasted with gadolinium, not involving the leptomeninges; it is reported in approximately 83% of patients [5]. It is important to consider that postoperative pachymeningeal irritation and idiopathic hypertrophic pachymeningitis can cause a similar enhancement, and therefore should be taken into account while studying a patient with possible SIH [40]. The presence of signs and symptoms suggestive of a different disorder may prompt an evaluation for infectious (meningitis), granulomatous (i.e., sarcoidosis, granulomatosis with polyangiitis), and neoplastic (metastatic disease) pathologies, as these disorders also present with meningeal enhancement [48].
-
2.
Downward displacement of intracranial structures
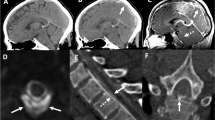
Common indicators of brain displacement are a downward drooping splenium of the corpus callosum, described as the droopy penis sign (Fig. 2), as well as a downward tonsillar displacement (> 5 mm in adults) [40, 49]. Brainstem sagging, seen in 61% of patients, is one of the most specific MRI signs and also the one with the most variable interobserver agreement. It is detected with ventricular effacement, effacement of the suprasellar and prepontine cisterns (Figs. 2 and 3), a third ventricle at or below the level of the sella turcica, and the bowing of the optic chiasm over it, red nuclei below the tentorium level, an infundibulum with horizontal configuration, and a flattening of the ventral pons [40, 47, 49].
-
3.
Engorgement of venous structures and pituitary gland
-
4.
Prominent venous sinuses are seen in 61% of patients (Fig. 1) [47]; engorgement is usually seen at the midportion of the dominant transverse sinus [5, 30]. Enlargement of the pituitary gland is seen as a supportive feature of venous engorgement [40].Subdural collections
T1-weighted sagittal head MRI showing a the downward dropping splenium of the corpus callosum (droopy penis sign), and b enlargement of the pituitary gland (white arrow) and effacement of the suprasellar (arrowhead) and prepontine cisterns (black arrow), as well as a decreased mamillopontine distance (line)
These are caused by transudation of fluid when vascular volume does not match CSF loss and can be seen overlying the cerebral convexities and the posterior fossa; it presents in up to 72% of patients [40].
-
5.
Orbital findings
SIH causes decreased optic nerve sheath CSF and a straightened optic nerve angle (measuring close to 180°) [50].
Spinal MRI findings
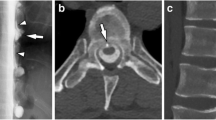
Spinal findings important to consider are as follows: dural enhancement, epidural fluid collection, distention of epidural veins, and abnormal visualization of root sleeves [5]. Most patients present with a spinal longitudinal extradural CSF collection; this is an important CSF leak finding, and is seen in patients with ventral dural tears. Patients who do not have spinal longitudinal extradural CSF collections usually have a more lateral leakage site, which can be caused by a CSF-venous fistula or a distal nerve root sleeve tear (type 3 or 4, as described in Table 1) [10, 14].
CSF leak–related findings
As variable as the clinical manifestations are, 20–30% of patients do not present with cranial findings [14], hence the importance of CSF leak study in order to achieve clinical correlation and a definitive diagnosis. The imaging modalities that have been reported to accurately detect leaks are as follows: CT myelography, dynamic CT myelography, digital subtraction myelography, and gadolinium-enhanced magnetic resonance myelography. CT myelography is the most commonly used modality and considered the study of choice for SIH; contrast material is injected into the thecal sac and CT images are taken from cervical to lumbar spine in order to detect leakage. A similar method is used with a different type of contrast and imaging: gadolinium-enhanced magnetic resonance myelography; in this procedure, gadolinium is injected into the thecal sac and MR images are obtained; however, it has not been approved by the Food and Drug Administration. To detect fast CSF leaks, the contrast material can be injected with the patient already located at the CT scanner, with CT images obtained right after the material enters the thecal sac, a method called dynamic CT myelography [5]. Another important method with diagnostic value is the radioisotope cisternography, where a radioisotope, typically 111-Indium-DPTA, is injected into the spinal subarachnoid space and followed at certain intervals (24–48 h) through spinal images, in order to detect abnormal paraspinal activity. Two-thirds of the radioisotope is cleared by the kidneys after 24 h of the procedure, and bladder activity can be seen in 70% of patients by 9 h; early detection of activity at the bladder (1–2 h) is usually seen in patients with CSF leaks [51]. The most common sites of CSF leak that are usually identified are the thoracic and lumbar spine [1]. A CT myelography is important to aid the diagnosis of SIH; however, it is positive in only 55% of patients [47].
Diagnosis
The diagnosis of SIH is made with the combination of clinical signs and symptoms, imaging techniques, and the measurement of CSF pressure. As previously described, there is a wide variety of presenting signs and symptoms for patients with SIH; therefore, it is of crucial importance to assess the imaging signs of SIH on the MRI. The measurement of CSF pressure also aids the diagnosis; however, there is weak correlation between individual MRI signs and CSF pressure. It should also be noted that CSF pressure measurement has a low sensitivity for detecting SIH, since patient-specific variables can influence CSF pressure in patients [47].
The International Classification of Headache Disorders (ICHD-3) establishes the diagnostic criteria for headache attributed to low CSF pressure. These include a headache that is usually, not invariably, orthostatic that must be accompanied by either CSF pressure < 60 mmH2O or imaging evidence. Furthermore, the headache must develop in temporal relation to CSF leakage or low pressure, or lead to its discovery (Table 2) [4] and the lack of an orthostatic component should not be used to rule out SIH in a case with findings concerning for this condition [3]. The diagnostic criteria are described in Table 1; it is important to emphasize that (1) SIH cannot be diagnosed in a patient who has had a dural puncture within the prior month, (2) a dural puncture to measure CSF pressure is not necessary in patients with positive MRI signs of leakage such as dural enhancement with contrast, and (3) it is not clear that all patients have an active CSF leak, despite a compelling history or brain imaging signs compatible with CSF leakage [4].
Certain efforts have been made in order to provide objective criteria for MRI evaluation. Shah et al. proposed a quantitative MRI assessment using standard value for the pontomesencephalic angle and mamillopontine distance in order to improve accuracy for brainstem sagging detection. They concluded that there was a significant difference between values of the intracranial hypotension group versus the control group. A mamillopontine distance of 5.5 mm or less and a pontomesencephalic angle of 50° or less were found sensitive and specific for intracranial hypotension [40]. An increased sag ratio, defined as the relation between the maximal anteroposterior diameter of the midbrain and the maximal bipendicular diameter, is often used with diagnostic value [44].
As no validated system has been established for evaluation and integration of these findings, Dobrocky et al. investigated 56 patients with confirmed SIH and CSF leaks in a case-control study. Compared with controls, and based on prevalence, interobserver agreement, and significance, they developed a SIH probability score using 6 imaging findings (Table 3): three weighted as mayor (pachymeningeal enhancement, engorgement of venous sinus, and effacement of the suprasellar cistern of 4 mm or less) and three as minor (subdural fluid collection, effacement of prepontine cistern of 5 mm or less, mamillopontine distance of 6.5 mm or less) (Fig. 4). On a scale of 9 points, where major criteria confers 2 points per finding and minor criteria, 1 point per finding, the patients are classified into 3 main groups based on the probability of finding a spinal CSF leak: high (≥ 5 points), intermediate (3–4 points), low (≤ 2 points). They recommend the score system to be used for further decision on invasive procedures: treatment should be promptly considered for patients with ≥ 5 points, and further work-up for scores ≤ 4 points [52].
In an attempt to improve the diagnostic accuracy of the different methods to detect intracranial hypotension, Schievink et al. proposed the following criteria: (a) orthostatic headache; (b) at least one of the following: low opening CSF pressure (≤ 60 mm H2O), improvement after epidural blood patching (EBP), demonstration of an active CSF leak, MRI changes suggestive of intracranial hypotension; (c) no recent history of dural puncture; (d) it is not attributable to another disorder [53]. These diagnostic criteria are somehow similar to the ones proposed by the ICHD-3: there must be a correlation between clinical presentation and a diagnostic method. Due to the usual variety of signs, symptoms, and imaging findings, it is recommended to consider all the reported manifestations and available imaging and diagnostic methods when suspecting intracranial hypotension. Either Schievink et al.’s or ICHD-3’s criteria, as well as the Dobrocky scoring system need to be further implemented in bigger prospective studies in order to analyze and establish their true relevance.
Treatment
The management of SIH involves four main approaches:
-
Conservative treatment
-
Epidural blood patch
-
Epidural fibrin glue injection
-
Surgical intervention
The most commonly used first approach is conservative treatment; it involves a combination of bed rest, hydration (oral or intravenous fluids), and oral caffeine [5]. Intravenous, intramuscular, and oral presentations of caffeine have been described and variable responses have been reported with different dosages; most of the recommended doses include 500 mg IV or 300 mg PO [54, 55]. The recumbent position helps reduce pressure at the leakage site, while caffeine causes venous vasoconstriction, decreasing CSF resorption [6]. Several other conservative treatments have been used: intravenous factor XIII, oral fludrocortisone, and theophylline [6, 56]. A study by Kong et al. found that 37.5% and 62.5% of patients with SIH treated conservatively had no recurrence of symptoms after 6 and 24 months, respectively [57].
An epidural blood patch (EBP) represents the first line of treatment after the failure of a conservative approach. EBP is defined as the use of homologous blood (10–30 ml) to control the CSF leakage by injecting it into the epidural space [6]. The correct management and control of the leak is achieved when the injected blood generates compression of the dural membrane (acute effect) and fibrosis at the dural tear (delayed effect) [56]. The EBP procedure itself can be divided into blind (lumbar) or guided by fluoroscopy or CT once the leakage site has been identified [5]. Usually, the blind approach is used first; if the CSF leakage site is identified or the signs and symptoms do not cease, the guided approach is implemented [6]. Retrospective studies have shown the efficacy of targeted over blinded EBP, although randomized trials still have to be developed in order to evaluate this relation. It is estimated that the efficacy of EBP goes from 30 to 57%, with a cumulative effect when using several EBPs and increased volume of autologous blood [1].
Another treatment alternative is the Epidural Fibrin Patch, which is CT-guided and requires CSF leakage site to be located. In this procedure, 1 ml of fibrin glue is injected over the leak [5]. After two or three EBPs and failure of the previous alternatives, surgical procedure is recommended if a clear site is identified (ligation of meningeal diverticula, suturing dural tears, packing epidural space) [5]. The detailed treatment and management of these patients go beyond the scope of this article; however, several algorithms have been proposed for the homogenization of clinical decision-making [2].
Conclusion
Spontaneous intracranial hypotension is a neurologic condition that represents the physiologic basis of the brain tissue–blood–CSF interrelation. It is important to consider the wide variety of clinical manifestations of SIH and always suspect of this entity in patients with atypical, unexplained, or rapidly progressing neurological symptoms. A complete neurological examination and imaging features are essential tools for the diagnosis of SIH, as well as the location of the main defect that originates this condition. It is crucial for future studies to prove the relationship of certain imaging findings with SIH and describe their sensitivity and specificity in randomized prospective studies in order to orient and systematize the recommendations for the management of this condition.
Change history
28 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10072-021-05323-x
References
Smith KA. (2016); Spontaneous intracranial hypotension: targeted or blind blood patch. J Clin Neurosci [Internet]. 25:10–2. Available from: https://doi.org/10.1016/j.jocn.2015.07.009
Michali-Stolarska M, Bladowska J, Stolarski M, Sąsiadek MJ (2018) Diagnostic imaging and clinical features of intracranial hypotension – review of literature. Polish J Radiol 83:e11–e18
Davidson B, Nassiri F, Mansouri A, Badhiwala JH, Witiw CD, Shamji MF, et al. (2017); Spontaneous intracranial hypotension: a review and introduction of an algorithm for management. World Neurosurg [Internet]. 101:343–9. Available from: https://doi.org/10.1016/j.wneu.2017.01.123
Vincent M, Wang S (2018) Headache classification committee of the International Headache Society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia 38(1):1–211
Urbach H (2014) Intracranial hypotension: clinical presentation, imaging findings, and imaging-guided therapy. Curr Opin Neurol 27(4):414–424
Idrissi AL, Lacour JC, Klein O, Schmitt E, Ducrocq X, Richard S (2015) Spontaneous intracranial hypotension: characteristics of the serious form in a series of 24 patients. World Neurosurg 84(6):1613–1620
Amrhein TJ, Kranz PG. (2019); Spontaneous intracranial hypotension: imaging in diagnosis and treatment. Radiol Clin North Am [Internet]. 57(2):439–51. Available from: https://doi.org/10.1016/j.rcl.2018.10.004
Cohen-Gadol AA, Mokri B, Piepgras DG, Meyer FB, Atkinson JLD (2006) Surgical anatomy of dural defects in spontaneous spinal cerebrospinal fluid leaks. Neurosurgery. 58(SUPPL. 2):238–245
Schievink WI, Reimer R, Folger WN (1994) Surgical treatment of spontaneous intracranial hypotension associated with a spinal arachnoid diverticulum. Case report. J Neurosurg 80(4):736–739
Farb RI, Nicholson PJ, Peng PW, Massicotte EM, Lay C, Krings T, terBrugge K (2019) Spontaneous intracranial hypotension: a systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. Am J Neuroradiol 40(4):745–753
Kranz PG, Luetmer PH, Diehn FE, Amrhein TJ, Tanpitukpongse TP, Gray L (2016) Myelographic techniques for the detection of spinal CSF leaks in spontaneous intracranial hypotension. Am J Roentgenol 206(1):8–19
Kranz PG, Stinnett SS, Huang KT, Gray L (2013) Spinal meningeal diverticula in spontaneous intracranial hypotension: analysis of prevalence and myelographic appearance. Am J Neuroradiol 34(6):1284–1289
Schievink WI, Schwartz MS, Maya MM, Moser FG, Rozen TD (2012) Lack of causal association between spontaneous intracranial hypotension and cranial cerebrospinal fluid leaks: clinical article. J Neurosurg 116(4):749–754
Schievink WI, Maya MM, Jean-Pierre S, Nuño M, Prasad RS, Moser FG (2016) A classification system of spontaneous spinal CSF leaks. Neurology. 87(7):673–679
Schievink WI. (2006); Spontaneous spinal cerebrospinal fluid and ongoing investigations in this area. Jama. 295(19)
Mokri B (2015) Spontaneous intracranial hypotension. Contin Lifelong Learn Neurol 21(4):1086–1108
Hadizadeh DR, Kovács A, Tschampa H, Kristof R, Schramm J, Urbach H (2010) Postsurgical intracranial hypotension: diagnostic and prognostic imaging findings. Am J Neuroradiol 31(1):100–105
Kim JH, Roh H, Yoon WK, Kwon TH, Chong K, Hwang SY, Kim JH (2019) Clinical features of patients with spontaneous intracranial hypotension complicated with bilateral subdural fluid collections. Headache. 59(5):775–786
Ferrante E, Trimboli M, Rubino F. (2019); Spontaneous intracranial hypotension: review and expert opinion. Acta Neurol Belg [Internet]. (0123456789). Available from: https://doi.org/10.1007/s13760-019-01166-8
Ferrante E, Savino A (2005) Thunderclap headache caused by spontaneous intracranial hypotension. Neurol Sci 26(SUPPL. 2):155–157
Marshall N, Maclaurin WA, Koulouris G (2007) MRA captures vasospasm in fatal migrainous infarction. Headache. 47(2):280–283
Ducros A, Bousser M-G (2009) Reversible cerebral vasoconstriction syndrome. Pract Neurol 9:256–257
Takai K, Niimura M, Hongo H, Umekawa M, Teranishi A, Kayahara T, et al. (2019); Disturbed consciousness and coma: diagnosis and Management of Intracranial Hypotension Caused by a spinal cerebrospinal fluid leak. World Neurosurg [Internet]. 121:e700–11. Available from: https://doi.org/10.1016/j.wneu.2018.09.193
Schievink WI, Jean-Pierre S, Maya MM, Moser FG, Nuño M (2018) Coma: a serious complication of spontaneous intracranial hypotension. Neurology. 90(19):E1638–E1645
Hodges JR. (2001); Frontotemporal dementia (Pick’s disease): clinical features and assessment. Neurology. 56(11 SUPPL. 4)
Sayao AL, Heran MKS, Chapman K, Redekop G, Foti D (2009) Intracranial hypotension causing reversible frontotemporal dementia and coma. Can J Neurol Sci 36(2):252–256
Wicklund MR, Mokri B, Drubach DA, Boeve BF, Parisi JE, Josephs KA (2011) Frontotemporal brain sagging syndrome: an SIH-like presentation mimicking FTD. Neurology. 76(16):1377–1382
Ozyigit A, Michaelides C, Natsiopoulos K (2018) Spontaneous intracranial hypotension presenting with frontotemporal dementia: a case report. Front Neurol 9(August):1–5
Hedna VS, Kumar A, Miller B, Bidari S, Salardini A, Waters MF, Hella M, Valenstein E, Eisenschenk S (2014) Intracranial hypotension masquerading as nonconvulsive status epilepticus: report of 3 cases. J Neurosurg 120(3):624–627
Kim SC, Ryoo I, Sun HY, Park SW (2019) MRI findings of spontaneous intracranial hypotension: usefulness of straight sinus distention. Am J Roentgenol 212(5):1129–1135
He FF, Li L, Liu MJ, Di Zhong T, Zhang QW, Fang XM (2018) Targeted epidural blood patch treatment for refractory spontaneous intracranial hypotension in China. J Neurol Surgery, Part B Skull Base 79(3):217–223
Christoforidis GA, Mehta BA, Landi JL, Czarnecki EJ, Piaskowski RA (1998) Spontaneous intracranial hypotension: report of four cases and review of the literature. Neuroradiology. 40(10):636–643
Horton JC, Fishman RA (1994) Neurovisual findings in the syndrome of spontaneous intracranial hypotension from dural cerebrospinal fluid leak. Ophthalmology [Internet] 101(2):244–251. Available from:. https://doi.org/10.1016/S0161-6420(94)31340-6
Porta-Etessam J, Di Capua D, Jorquera M, Cuadrado ML, Marcos A (2011) Orthostatic headache and bilateral abducens palsy secondary to spontaneous intracranial hypotension. J Headache Pain 12(1):109–111
Khemka S, Mearza AA (2006) Isolated sixth nerve palsy secondary to spontaneous intracranial hypotension. Eur J Neurol 13(11):1264–1265
Zada G, Solomon TC, Giannotta SL (2007) A review of ocular manifestations in intracranial hypotension. Neurosurg Focus 23(5):1–5
Pakiam AS, Christine L, Lang AE (1999) Intracranial hypotension with parkinsonism, ataxia, and bulbar weakness. Arch Neurol 56(7):869–872
Duvall JR, Robertson CE, Cutsforth-Gregory JK, Carr CM, Atkinson JLD, Garza I (2019) Headache due to spontaneous spinal cerebrospinal fluid leak secondary to cerebrospinal fluid-venous fistula: case series. Cephalalgia. 39(14):1847–1854
Schievink WI, Nuño M, Rozen TD, Maya MM, Mamelak AN, Carmichael J, Bonert VS (2015) Hyperprolactinemia due to spontaneous intracranial hypotension. J Neurosurg 122(5):1020–1025
Shah LM, McLean LA, Heilbrun ME, Salzman KL (2013) Intracranial hypotension: improved MRI detection with diagnostic intracranial angles. Am J Roentgenol 200(2):400–407
Schievink WI, Chu RM, Maya MM, Johnson JP, Cohen HCM (2013) Spinal manifestations of spontaneous intracranial hypotension: clinical article. J Neurosurg Spine 18(1):96–101
Schievink WI, Maya MM (2006) Quadriplegia and cerebellar hemorrhage in spontaneous intracranial hypotension. Neurology. 66(11):1777–1778
Schievink WI, Maya MM (2008) Cerebral venous thrombosis in spontaneous intracranial hypotension. Headache. 48(10):1511–1519
Takahashi K, Mima T, Akiba Y (2016) Chronic subdural hematoma associated with spontaneous intracranial hypotension: therapeutic strategies and outcomes of 55 cases. Neurol Med Chir (Tokyo) 56(2):69–76
De Noronha RJ, Sharrack B, Hadjivassiliou M, Romanowski CAJ (2003) Subdural haematoma: a potentially serious consequence of spontaneous intracranial hypotension. J Neurol Neurosurg Psychiatry 74(6):752–755
Watanabe A, Horikoshi T, Uchida M, Koizumi H, Yagishita T, Kinouchi H (2009) Diagnostic value of spinal MR imaging in spontaneous intracranial hypotension syndrome. Am J Neuroradiol 30(1):147–151
Kranz PG, Tanpitukpongse TP, Choudhury KR, Amrhein TJ, Gray L (2016) Imaging signs in spontaneous intracranial hypotension: prevalence and relationship to CSF pressure. Am J Neuroradiol 37(7):1374–1378
Kranz PG, Gray L, Amrhein TJ (2018) Spontaneous intracranial hypotension: 10 myths and misperceptions. Headache. 58(7):948–959
Kumar MK (2017) Spontaneous intracranial hypotension - MRI features. J Tumor Med Prev 1(3):100–106
Holbrook JF, Hudgins PA, Bruce BB, Saindane AM. (2017); Novel orbital findings of intracranial hypotension. Clin Imaging [Internet]. 41:125–31. Available from: https://doi.org/10.1016/j.clinimag.2016.10.019
Mokri B (2014) Radioisotope cisternography in spontaneous CSF leaks: interpretations and misinterpretations. Headache. 54(8):1358–1368
Dobrocky T, Grunder L, Breiding PS, Branca M, Limacher A, Mosimann PJ, Mordasini P, Zibold F, Haeni L, Jesse CM, Fung C, Raabe A, Ulrich CT, Gralla J, Beck J, Piechowiak EI (2019) Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol 76(5):580–587
Schievink WI, Dodick DW, Mokri B, Silberstein S, Bousser MG, Goadsby PJ (2011) Diagnostic criteria for headache due to spontaneous intracranial hypotension: a perspective. Headache. 51(9):1442–1444
Ragab A, Facharzt KN. (2014); Caffeine, is it effective for prevention of postdural puncture headache in young adult patients? Egypt J Anaesth [Internet] 30(2):181–186. Available from: https://doi.org/10.1016/j.egja.2013.11.005
Goadsby PJ, Boes C (2002) New daily persistent headache. J Neurol Neurosurg Psychiatry 72(II):ii6–ii9
Wu W, Hseu SS, Fuh JL, Lirng JF, Wang YF, Chen WT et al (2017) Factors predicting response to the first epidural blood patch in spontaneous intracranial hypotension. Brain. 140(2):344–352
Kong DS, Park K, Nam DH, Lee JI, Kim JS, Eoh W et al (2005) Clinical features and long-term results of spontaneous intracranial hypotension. Neurosurgery 57(1):91–95
Author information
Authors and Affiliations
Contributions
Victor Garcia-Navarro, MD FAANS, had the idea of the article. Carlos Perez-Vega, Pilar Robles-Lomelin, MD, and Isabel Robles-Lomelin performed the literature review and writing of the article. Victor Garcia-Navarro critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Perez-Vega, C., Robles-Lomelin, P., Robles-Lomelin, I. et al. Spontaneous intracranial hypotension: key features for a frequently misdiagnosed disorder. Neurol Sci 41, 2433–2441 (2020). https://doi.org/10.1007/s10072-020-04368-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04368-8