Abstract
Background
Migraine is a common neurovascular disease associated with vascular risks, especially in young adult females, but the mechanism underlying these associations remains unknown. This study evaluated the relationships between plasma endothelial dysfunction biomarkers and carotid intima–media thickness (IMT) in young adult females with migraine.
Methods
This case–control study included 148 female patients (age range: 18–50 years). Migraine was diagnosed according to the International Headache Society-IIIb criteria. Endothelial dysfunction biomarkers, such as von Willebrand factor (vWF), C-reactive protein (CRP), homocysteine, total nitrate/nitrite concentration, and thiobarbituric acid-reactive substances (TBARS), were evaluated in plasma. Carotid IMT was measured by a radiologist with sonography.
Results
The CRP, TBARS, vWF, and IMT levels were increased in the migraine compared with the control group (p < 0.001, p = 0.02, p < 0.001, and p < 0.001, respectively). After adjusting for confounders, multiple linear regression analysis revealed that systolic arterial blood pressure, CRP, vWF, TBARS, and right and left internal carotid artery (ICA) IMT were independently positively correlated with migraine (p < 0.01, p = 0.004, p = 0.023, p = 0.024, p = 0.032, and p = 0.048, respectively). Multiple logistic regression analysis revealed that right ICA IMT was independently associated with ergotamine and triptan and left ICA IMT was independently associated with ergotamine (p = 0.013, p = 0.026, and p = 0.017, respectively). In addition, significant correlations were found between LDL lipoprotein and carotid IMT in the migraine group (p < 0.05).
Conclusions
Carotid IMT enhancement and elevated TBARS, vWF, and CRP levels in migraine subjects during a migraine attack could be regarded as consequences of migraine attack pathophysiology. The independent associations between triptan and ergotamine consumption and enhanced carotid IMT suggest that repeated use of these vasoconstrictive antimigraine agents may have additional effects on carotid IMT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine (M) is a common primary headache disorder characterized by recurrent attacks of headache and various combinations of symptoms related to the gastrointestinal and autonomic nervous systems. Approximately one third of patients experience an aura involving neurological symptoms, mostly visual [1]. The prevalence of M in the general population ranges from 6 to 13%, and females are affected at higher rates because of the influence of hormones [2]. M is a neurovascular disorder involving cortical spreading depression and neurogenic inflammation, but the pathophysiology of this condition is not completely understood. Repeated M attacks cause inflammation of the cranial blood vessels, and various neuropeptides are implicated in the neuroinflammatory process [3, 4].
M, particularly M with aura, is associated with vascular diseases, and a two-fold increased risk of ischemic stroke in M has been reported; however, the mechanism underlying these associations is unknown [5]. It has been reported that certain features of the systemic and cranial vascular structures change during an M attack and between attacks [6, 7]. Inflammatory markers, oxidative stress, and thrombosis in the systemic circulation have been reported in M [8,9,10].
The inflammatory process in M and the associated vascular risk may disrupt the vascular endothelial function and structure of the vascular wall and may lead to atherosclerosis and vascular disease. Repeated sterile vascular inflammation results in endothelial injury of the cranial vessels [6, 7].
Recent reports have identified carotid intima–media thickness (IMT) as a valuable predictor of vascular risk that operates independently of traditional risk factors. Carotid IMT measurement is a noninvasive method that can be used as a structural marker of subclinical atherosclerosis and is correlated with concomitant endothelial dysfunction [9, 11, 12].
Endothelial dysfunction has been reported in M, and carotid IMT was recently reported to be increased in M [9, 10, 13, 14]. Only a few studies have investigated the relationships between various endothelial function biomarkers and carotid IMT in M, and these have obtained conflicting results [9, 15]. Therefore, this study evaluated the plasma biomarkers related to endothelial function and the carotid IMT, as a marker of structural vascular impairment, in young adult females with M and assessed the correlations of endothelial plasma biomarkers with carotid IMT.
Methods
Subjects
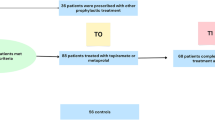
This prospective, cross-sectional case–control clinical study recruited subjects from the neurology and emergency outpatient clinics of Baskent Medical Faculty who had migraine between July 2015 and March 2017. Participants were recruited after the study was approved by the Institutional Review Board. The patients were diagnosed with M using the International Headache Society-IIIb (ICHD-IIIb) criteria [1]. Study eligibility criteria included (1) females who had M with and without aura, (2) aged between 18 and 50 years, (3) headache at the time of enrollment, (4) visual analog scale (VAS) score ≥ 7/10 during the headache, (5) M symptoms ≥ 1 year, (6) headache frequency ≥ 2 attacks/month, and (7) no known vascular risk factors, inflammatory disease, chronic illness, metabolic disease, or infections. Exclusion criteria included (1) history of M < 1 year; (2) > 15 days of headache per month; (3) use of antimigraine drugs > 10 days/month; (4) history of cerebrovascular or cardiovascular disease, arterial hypertension (blood pressure > 140/90 mmHg), diabetes mellitus, or hyperlipidemia (low-density lipoprotein cholesterol ≥ 160 mg/dL); (5) body mass index (BMI) < 18 kg/m2 or > 35 kg/m2; (6) smoke > 1 pack/day cigarettes; (7) current pregnancy, lactation, or hormonal contraceptive use; (8) alcohol or substance abuse; (9) drug use (e.g., antiplatelet agents, anticoagulants, statins, or hormonal drugs); and (10) possible “symptomatic migraine” in which magnetic resonance imaging showed arteriovenous malformations, ischemic infarcts, brain tumors, or other conditions that may be associated with M. Patients with high CRP (≥ 10 mg/L) levels were excluded from the study because such levels may represent nonspecific inflammation and lack positive predictive value [16]. The study included 74 patients aged between 18 and 50 years (mean age 35.1 ± 3.4) who were diagnosed with M.
Healthy control subjects aged between 18 and 50 years (mean age 31.9 ± 4.1) were recruited from the hospital and laboratory staff, patients’ relatives, and the general population. All control subjects met the following inclusion criteria: (1) female gender, (2) no headaches (e.g., M, tension-type headache, or cluster headache), (3) aged 18 to 50 years, and (4) absence of other neurological or systemic diseases. Exclusion criteria for the control subjects were as follows: (1) history of cerebrovascular or cardiovascular disease, arterial hypertension (blood pressure > 140/90 mmHg), diabetes mellitus, or hyperlipidemia (low-density lipoprotein cholesterol ≥ 160 mg/dL); (2) BMI < 18 kg/m2 or > 35 kg/m2; (3) smoke > 1 pack/day cigarettes; (4) current pregnancy, lactation, or hormonal contraceptive use; (5) alcohol or substance abuse; and (6) drug use (e.g., antiplatelet agents, anticoagulants, statins, or hormonal drugs).
Evaluation
The study included participants who were diagnosed with M according to the ICHD-IIIb criteria and healthy controls without headache. A detailed history of M was obtained, including disease duration (years), age at onset, average duration of current headache (hours), presence of aura, trigger factors, accompanying symptoms, frequency per month, and location and severity of pain. Severity of headache was evaluated with a VAS (range, 1 [minimum pain] to 10 [maximum pain]). M headache attack frequency was defined as the number of attacks per month. All patients and controls underwent complete physical and neurological examinations. Comorbidities (coronary artery disease, stroke, diabetes mellitus, or thyroid disease) and intercurrent illnesses, such as respiratory or urinary infections, were determined from the patient history, physical examination, and laboratory tests. Blood pressure, body weight, height, smoking habits, and educational level were recorded for all M subjects and controls. The patients may have previously used medications, such as acetaminophen, nonsteroidal anti-inflammatory drugs, triptans, or caffeine, for acute headache pain; however, patients who used analgesics on a daily basis were excluded from the study. Patients who were treated for M prophylaxis with drugs such as propranolol, topiramate, or valproic acid were also excluded.
Laboratory evaluation
Blood for TBARS, nitrate, total nitrite and nitrate, and biochemistry
First, 10 mL of venous blood was collected from an antecubital vein in each subject, centrifuged at 3000 rpm for 20 min to separate the serum, and stored at − 70 °C. The blood was drawn into collection tubes with sodium citrate for vWF activity, coagulation factor II, and homocysteine assays. The analysis was performed by personnel who were blind to the participants’ health and laboratory information.
Thiobarbituric acid-reactive substances
We used the reaction with thiobarbituric acid (TBA) at 90–100 °C to determine the malondialdehyde (MDA) level under the assumption that MDA and similar substances react with TBA and produce a pink pigment that has an absorption maximum at 532 nm (Rayto RT-6000 Elisa Micro Plate Reader, China). To ensure protein precipitation, the sample was mixed with cold 42 mM sulfuric acid, and the precipitate was then centrifuged to form a pellet. An aliquot of the supernatant was placed in an equal volume of 0.6% (w/v) TBA in a boiling water bath for 60 min. After cooling, the sample and blank absorbance are read at 532 nm and the results expressed as nmole per milliliter (Lipid peroxidation MDA assay Kit, Sigma AK085, St. Louis, USA).
Nitrite/nitrate
The sum of nitrites and nitrates, which are the final products of nitric oxide metabolism in vivo, was determined in plasma using the Griess method. Briefly, 100-μL aliquots of serum samples were mixed with an equal volume of Griess reagent. The absorbance at 540 nm was determined in an automated plate reader (Rayto RT-6000 Elisa Micro Plate Reader, China). Nitrite concentrations were calculated from a sodium nitrite standard curve and expressed in nmoles/L. Nitrate concentrations were determined with a spectrophotometric assay using oxidation by nitrate reductase (Nitrite/Nitrate assay Kit, colorimetric, Sigma 23479-1KT-F. St. Louis, USA).
Von Willebrand factor and coagulation factor II (Erlangen, Germany) were run on a Sysmex Cs2100 fully automatic coagulometer (Chuo-ku Kobe, Japan) (reference range for vWF 50–160% and for coagulation factor II 60–140%).
Homocysteine was determined on an I1000 Architect Abbott (Abbott Park, IL, USA) (reference range for homocysteine 4.44–13.56 μmol/L).
Low-density lipoprotein (LDL) was determined on a C8000 Architect Abbott (IL 60064, USA) biochemical autoanalyzer (reference range for LDL 60–130 mg/dL). LDL analyses were performed for only 27 participants.
C-reactive protein was determined on a C8000 Architect Abbott biochemical autoanalyzer (reference range for CRP 0–5 mg/L).
Measurement of carotid intima–media thickness
The carotid IMT was measured with a Siemens Acuson S 2000 sonography device (Siemens, Munich, Germany) using a 7.5–12 MHz transducer on B mode by a single, experienced radiologist who was blind to the patients’ clinical histories. Carotid imaging was performed while the patients were in a supine position with cervical angulation of 30 °C toward the contralateral side. Measurements were made at three levels: 1 cm from the most distal part of the right and left main common carotid arteries (CCA), at the carotid bifurcation, and 2 cm from the most proximal part of the internal carotid arteries (ICA). Carotid IMT was measured only at the posterior wall, longitudinally as the distance between the areas of vessel lumen and media/adventitia echogenicity using B mode. The carotid IMT that was used for the analysis was obtained by averaging three measurements performed in both carotid arteries. The Mannheim carotid IMT and Plaque Consensus describe the use of carotid IMT measurements [17]; our measurements are compatible with these methods, but we did not use automatic software for the measurements.
Statistical analysis
For discrete and continuous variables, descriptive statistics (mean, standard deviation, n, and percentile) are given. The homogeneity of the variances, which is a prerequisite for parametric tests, was checked using Levene’s test. The assumption of normality was tested with the Shapiro–Wilks test. To compare the differences between the two groups, the Student’s t test was used when the parametric test prerequisites were met, and the Mann–Whitney U test was used when such prerequisites were not met. To compare the differences between three or more groups, one-way analysis of variance was used when the parametric test prerequisites were met, and the Kruskal–Wallis test was used otherwise. The Bonferroni correction, which is a multiple comparison test, was used to evaluate the significance of results involving three or more groups. The Chi-square test was used to assess the relationships between two discrete variables. When the expected sources were less than 20%, values were determined using a Monte Carlo simulation method to include such sources in the analysis. Additionally, the variables age and BMI were determined as the covariance (to exclude the effect), and the groups were compared. The relationship between two continuous variables was assessed using the Pearson correlation coefficient or the Spearman correlation coefficient when the parametric test prerequisites were not met. In this study, binary logistic regression analysis was used to determine the model of the relationship between independent and dependent variables. Regression analysis was performed for the right and left ICA-dependent variables in the study. Relationships between the sets were examined by canonical correlation analysis.
The data were evaluated using SPSS 20 for Windows, ver. 20.0 (IBM Corp., Armonk, NY, USA). Values of p < 0.05 and < 0.01 were set as levels of significance. The power analysis of this study was 80.81%.
Results
Table 1 compares the characteristics of the M and control groups. After adjusting for age, differences between the M and control groups persisted for vWF, TBARS, and carotid IMT. After adjusting for age, differences between the M and control groups persisted for TBARS, vWF, and carotid IMT (Table 2). However, differences between the M and control groups persisted for carotid IMT after adjusting for age and BMI (Table 2).
The characteristics of the subjects with M with and without aura revealed significant differences in nitrate (1.5 ± 1.2, 1.02 ± 0.4, p = 0.30) and total nitrite/nitrate (2.3 ± 1.6, 1.6 ± 0.5, p = 0.20). After adjusting for confounders, multiple linear regression analysis revealed no significant differences between M with and without aura.
Univariate analysis revealed significant correlations between LDL lipoprotein and right common carotid artery (CCA; r = 0.451, p = 0.018) and right bulbus (r = 0.376, p = 0.028) IMT in the M group. There were significant correlations between VAS and vWF (r = 0.230, p = 0.049) and between M duration and right CCA (r = 0.281, p = 0.015), left CCA (r = 0.261, p = 0.025), right bulbus (r = 0.344, p = 0.003), and left bulbus (r = 0.282, p = 0.015) IMT. Additionally, VAS correlated with right ICA (r = 0.249, p = 0.032) and right bulbus (r = 0.232, p = 0.047) IMT.
After adjusting for confounders, multiple linear regression analysis revealed that systolic arterial blood pressure, CRP, vWF, TBARS, and right and left ICA IMT were independently positively correlated with membership in the M compared with the control group (Table 3). In addition, after adjusting for confounders, a multiple linear regression analysis revealed that M duration was independently positively correlated with right ICA IMT. After adjusting for confounders, a multiple linear regression analysis revealed that headache frequency was independently negatively correlated with age and positively correlated with systolic blood pressure, CRP, and right CCA IMT (Table 3).
In the multiple logistic regression analysis, right ICA IMT was independently associated with ergotamine and triptan, whereas left ICA IMT was independently associated with ergotamine (Table 3).
Discussion
The main goal of this study was to evaluate associations between endothelial dysfunction biomarkers and carotid IMT in M sufferers. We found that CRP, vWF, TBARS, and carotid IMT were significantly elevated in M during attacks (Tables 1 and 2), but we found no differences between those with M with and without aura. Systolic arterial blood pressure, CRP, vWF, TBARS, and right and left ICA IMT were independent factors associated with M (Table 3). M duration was independently and positively correlated with right ICA IMT, and headache frequency was independently and negatively correlated with age and positively correlated with systolic blood pressure, CRP, and right CCA IMT (Table 3). We found no association between endothelial dysfunction biomarkers and carotid IMT, whereas triptan and ergotamine consumption were independently associated with right and left ICA IMT (Table 3). It is through this mechanism of action that M attacks directly activate the endothelium, and the resultant inflammation, oxidative stress, and coagulation may lead to endothelial dysfunction and pathological vascular changes. These findings suggest that endothelial dysfunction and carotid IMT, which lead to subclinical atherosclerosis in M, are multifactorial and that vasoconstrictor drugs also contribute to this process.
Ergots and triptans have been consistently linked to increased risks of stroke and other ischemic events, at least when taken at recommended doses [18, 19]. Repeated exposure to vasoconstrictor drugs may cause vascular injury, and differences in medication could influence the observed vascular changes in patients with longstanding M. Experimental studies have demonstrated that triptan and ergotamine affect the vascular wall via an endothelial mechanism associated with vascular endothelial cell dysfunction [19, 20]. In this study, triptan and ergotamine use were independently associated with right and left ICA IMT (Table 3). To our knowledge, these findings have not been reported previously. Further studies need to confirm these results.
In this study, the levels of endothelial dysfunction biomarkers were high in M patients, which is consistent with previous studies [9, 10, 13]. However, previous studies reported no differences in the endothelial dysfunction biomarkers of the M and control groups [21, 22]. These study samples were small, included both sexes, and the endothelial biomarkers used differed from those in the present study [21, 22]. Consistent with our study, some previous studies reported that carotid IMT was high in M subjects [9, 14]. M headache frequency and M duration were independently correlated with carotid IMT (Table 3). Other studies found no differences in carotid IMT between M and control groups [15, 23]. The mean ages of the participants in the latter studies were older than that in our study, the participants had traditional cardiovascular risk factors, and the studies included both sexes [15, 23]. In our study, although the endothelial dysfunction biomarkers were high during an attack and the carotid IMT thickness was increased in the M group, we did not find any correlations between carotid IMT and endothelial dysfunction biomarkers. In contrast to our study, other studies reported correlations between carotid IMT and CRP, fibrinogen, and endothelin-1 [9, 14]. However, these studies had smaller samples, different study parameters (except CRP), and both sexes were included [9, 14].
Nitric oxide is an important vasodilator, but its synthesis is impaired during oxidative stress. A defect in the production or activity of nitric oxide leads to endothelial dysfunction. Nitric oxide is an unstable molecule that is rapidly degraded into nitrates and nitrites. Its half-life is a few seconds [24]. The nitrate and total nitrite/nitrate levels were significantly higher in those with M with aura compared with those with M without aura (1.5 ± 1.2, 1.02 ± 0.4, p = 0.30 and 2.3 ± 1.6, 1.6 ± 0.5, p = 0.20, respectively). However, after adjusting for confounders, multiple linear regression analysis revealed no significant differences between M with and without aura. As in our study, other studies found no differences in blood nitrate/nitrite concentrations between M and controls [24, 25]. However, the results were conflicting: one study found lower nitrate and nitrite in M [10, 26], whereas another study found that nitrate and nitrite were higher in M [27]. These studies had smaller sample sizes and included male and female participants [26, 27].
TBARS is a nonspecific biomarker of oxidative stress and oxidative damage. In our study, TBARS levels were independently significantly higher in M during the attack. Consistent with our study, previous studies reported that TBARS was elevated during and between M attacks [10, 24, 27].
Homocysteine is an active antioxidant form of glutathione, which has a major role in antioxidant defense, and is associated with an increased risk of premature vascular disease and thrombosis [28]. Conflicting results regarding the homocysteine level in M have been reported. Some studies reported no differences in plasma homocysteine levels in M [6, 10], which is consistent with our findings, whereas others reported high plasma homocysteine levels in the M group [29, 30]. In one of the latter studies, the mean patient age was higher than that in our study, and it included both sexes [29], whereas the mean patient age was older in the other study than in ours [30].
Von Willebrand factor plays a crucial role in maintaining normal hemostasis and contributes to thrombotic disorders following endothelial and platelet dysfunction. vWF, which is a large, pro-coagulopathy, multimeric glycoprotein that is produced and stored in and released from the vascular endothelium, is associated with vascular risk factors. vWF is an important plasma biomarker of endothelial dysfunction in vascular diseases and activates platelet glycoprotein receptors, causing platelet adhesion and aggregation [31]. vWF is increased during M headache attacks and between attacks [10, 32]. In this study, vWF was high during the period of headache pain, which revealed that the endothelium became activated in M and released vWF. These findings did not differ between those with M with and without aura. Contrasting our results, another study reported no differences in plasma vWF antigen in M compared with controls [13]. However, participants in that study had an older mean age and included both sexes and those with vascular risk factors [13].
Coagulation factor II, also known as plasma prothrombin, is a vitamin K-dependent pro-enzyme that functions in the blood coagulation cascade and is required in the formation of fibrin [33]. Consistent with a previous study, our results showed no differences in coagulation factor II between M and control groups [10]. Contrasting our study, coagulation factor II was associated with migraine, especially M with aura, in the previous study, which included older participants, both sexes, and those with vascular risk factors [10].
C-reactive protein, a marker of inflammation and vascular disease, was elevated during the M attack. Consistent with our study, other research has reported the association of CRP with migraine [8, 10, 13]. Contrasting our results, a previous study reported that the CRP levels of M patients and controls were similar; however, the previous study included those with vascular risk factors and concomitant diseases, and the mean age of participants in that research was 55 years [34].
M attacks can cause repeated endothelial damage. Endothelial dysfunction, which is more prevalent in M, may be the pathophysiological process underlying this widespread vasculopathy. Evidence suggests that the M attack is directly linked to endothelial dysfunction [19, 32]. Two mechanisms for endothelial pathology in M have been proposed. First, neurotransmitters, such as calcitonin gene-related peptide (CGRP) and neuropeptide Y, are released locally during activation of the trigeminovascular system. These molecules have multiple downstream effects via their receptors, including effects on vascular smooth muscle and angiogenesis [35]. Second, sterile neurogenic inflammation and the protein extravasation that occurs with trigeminovascular system activation may also contribute to endothelial dysfunction [4, 7]. Exposure to vascular risk factors initially leads to transient endothelial dysfunction and, with recurrent exposure, a state of persistent endothelial dysfunction and altered vascular wall milieu occurs, which promotes the structural changes of atherosclerosis [6].
Despite being within normal limits, the mean systolic blood pressure in this study was higher in the M group than in the control group, and systolic blood pressure was independently associated with M (Table 3). The relationship between blood pressure and headaches has always been controversial. In a population-based study, female M participants had higher diastolic blood pressures than control subjects [9, 36]. However, some studies found no differences in the systolic and diastolic blood pressure of M and control subjects [23]. The mean ages of participants in the other studies were older than that in our study, and participants with traditional cardiovascular risk factors and both sexes were included [23]. Dysregulation of the renin angiotensin pathway and endothelial dysfunction may be the biological basis of the association between M and high blood pressure [37, 38]. Furthermore, although LDL was within normal limits, we found a positive correlation between LDL-cholesterol and carotid IMT in M participants (p = 0.018 and p = 0.028). These results are consistent with previous studies [9, 12].
Additionally, the mean age of the M group was older than that of the controls, which may have affected the carotid IMT thickness and vascular biomarkers (Table 1). Carotid IMT also increased linearly with age. BMI was also significantly associated with carotid IMT [39]. In our study, age and BMI, M-associated cofactors related to atherosclerosis, may have affected the carotid IMT and vascular biomarkers.
The strength of our study is that it is the first to assess the relationship between endothelial dysfunction markers and carotid IMT in young adult female M participants with restricted vascular risk factors. Males are reported to have a greater carotid IMT than females across all age groups [40]. The limitations of this study are that it was restricted to female subjects, although prospective data suggest that the risk of stroke with M is also increased in male patients [10]. The M participants included in this study may not be entirely representative of the general population of people with M because subjects with comorbid vascular risk factors were excluded (except smoking). Furthermore, some risk factors, such as smoking, were not strictly excluded. Although we excluded heavy smokers (> 1 pack/day), we did not exclude all cigarette smokers, even though smoking may increase carotid IMT [12]. Nevertheless, we observed no significant differences between the M and control groups in smoking habit (Table 1). By excluding individuals with diabetes and cardiovascular conditions (angina, myocardial infarction, transient ischemic attack, and stroke), we may have missed important associations between M and endothelial dysfunction [6, 13]. M associated co-factors, such as a higher BMI and older mean age, were higher in the M group, which may have affected the results. Blood was collected from the subjects close to the headache attack, as this may have affected the values of some of the markers investigated in this study. Furthermore, a nitrite dietary restriction was not implemented. Additionally, the scarcity of data on LDL may have led us to miss some associations between biomarkers and carotid IMT. The values of the markers investigated in this study may vary over time and be affected by several confounders. A single measurement may not reflect the usual status of the variable.
Conclusions
Carotid IMT enhancement and elevated TBARS, vWF, and CRP levels in M subjects during M attack, which are signs of subclinical atherosclerosis and endothelial dysfunction, could be regarded as the consequences of M attack pathophysiology. The independent associations of triptan and ergotamine use with carotid IMT enhancement suggest that repeated use of these vasoconstrictive antimigraine agents may have an additional influence on the carotid IMT. Early diagnosis and adequate M prophylaxis to reduce M attack frequently could prevent the progression of atherosclerosis in young adult M females.
Abbreviations
- BMI:
-
body mass index
- CCA:
-
common carotid artery
- CRP:
-
C-reactive protein
- ICA:
-
internal carotid artery
- ICHD:
-
International Headache Society
- IMT:
-
intima–media thickness
- LDL:
-
low-density lipoprotein
- NSAID:
-
nonsteroidal anti-inflammatory drug
- TBARS:
-
thiobarbituric acid-reactive substances
- VAS:
-
visual analog score
- vWF:
-
von Willebrand factor
References
Headache Classification Committee of the International Headache Society (2013) The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33:629–808. https://doi.org/10.1177/0333102413485658
Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M (2001) Prevalence and burden of migraine in the United States: data from the American migraine study II. Headache 41:646–657
Waeber C, Moskowitz MA (2005) Migraine as an inflammatory disorder. Neurology 64(10 suppl 2):S9–S15
Moskowitz MA (2007) Pathophysiology of headache—past and present. Headache 47:558–567
Spector JT, Kahn SR, Jones MR, Jayakumar M, Dalal D, Nazarian S (2010) Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med 123:612–624
Kurth T, Schürks M, Logroscino G, Gaziano JM, Buring JE (2008) Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. BMJ 337:1–9. https://doi.org/10.1136/bmj.a636
Rajan R, Kuhrana D, Lal V (2014) Interictal cerebral and systemic endothelial dysfunction in patients with migraine: a case–control study. J Neurol Neurosurg Psychiatry 1–5. https://doi.org/10.1136/jnnp-2014-309571
Avci AY, Lakadamyali H, Arikan S, Benli US, Kilinc M (2015) High sensitivity C-reactive protein and cerebral white matter hyperintensities on magnetic resonance imaging in migraine patients. J Headache Pain 16:9. https://doi.org/10.1186/1129-2377-16-9
Hamed SA, Hamed EA, Eldin AME, Mahmoud NM (2010) Vascular risk factors, endothelial function, and carotid thickness in patients with migraine: relationship to atherosclerosis. J Stroke Cerebrovasc Dis 19:92–103. https://doi.org/10.1016/j.jstrokecerebrovasdis.2009.04.007
Tietjen GE, Herial NA, White L, Utley C, Kosmyna JM, Khuder SA (2009) Migraine and biomarkers of endothelial activation in young women. Stroke 40:2977–2982. https://doi.org/10.1161/STROKEAHA.109.547901
Yang EY, Nimbi V (2011) Ultrasound imaging of carotid intima–media thickness: an office-based tool to assist physicians in cardiovascular risk assessment. Curr Atheroscler Rep 13:431–436. https://doi.org/10.1007/s11883-011-0200-5
Naqvi TZ, Lee MS (2014) Carotid intima–media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging 7(10):1025–1038. https://doi.org/10.1016/j.jcmg.2013.11.014
Tietjen GE, Khubchandani J, Herial N, Palm-Meinders IH, Koppen H, Terwindt GM, Buchem MA, Launer LJ, Ferrari MD, Kruit MC (2017) Migraine and vascular disease biomarkers: a population-based case–control study. Cephalalgia 1–8. https://doi.org/10.1177/0333102417698936
Gonzales-Quintanilla V, Toriello M, Palacio E, Gonzalez-Gay MA, Castillo J, Montes S, Martinez-Nieto R, Fernandez J, Rojo A, Gutierrez S, Pons E, Otreino A (2015) Systemic and cerebral endothelial dysfunction in chronic migraine. A case–control study with an active comparator. Cephalalgia 36:552–560. https://doi.org/10.1177/0333102415607857
Stam AH, Weller CM, Janssens ACJW, Aulchenko YS, Oostra BA, Frants RR, Maagdenberg AMJM, Ferrari MD, Dujin CM, Terwindt GM (2012) Migraine is not associated with enhanced atherosclerosis. Cephalalgia 33:228–235. https://doi.org/10.1177/0333102412466966
Bassuk SS, Rifai N, Ridker PM (2004) High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol 29:439–493
Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS (2012) Mannheim carotid intima–media thickness and plaque consensus (2004-2006-2011). Cerebrovasc Dis 34:290–296. https://doi.org/10.1159/000343145
Albieri V, Olsen TS, Andersen KK (2016) Risk of stroke in migraineurs using triptans. Association with age, sex, stroke severity and subtype. EBioMedicine 6:199–205. https://doi.org/10.1016/j.ebiom.2016.02.039
Valentin JP, Bonnafous R, John GW (1996) Influence of the endothelium and nitric oxide on the contractile responses evoked by 5-HT1D receptor agonist in the rabbit isolated saphenous vein. Br J Pharmacol 119:35–42
Tfelt-Hansen P, Nilsson E, Edvinsson L (2007) Contractile responses to ergotamine and dihydroergotamine in the perfused middle cerebral artery of rat. J Headache Pain 8:83–89. https://doi.org/10.1007/s10194-007-0368-9
Vanmolkot FH, Hoon JN (2010) Endothelial function in migraine: a cross-sectional study. BMC Neurol 10:119. https://doi.org/10.1186/1471-2377-10-119
Fischer M, Gaul C, Shanib H, Holle D, Loacker L, Griesmacher A, Lackner P, Broessner G (2014) Markers of endothelial function in migraine patients: results from a bi-center prospective study. Cephalalgia 35:877–885. https://doi.org/10.1177/0333102414564890
Tzourio C, Gagniere B, El Amrani E, Alperovitch A, Bousser MG (2003) Relationship between migraine, blood pressure and carotid thickness. A population-based study in the elderly. Cephalalgia 23:914–920. https://doi.org/10.1046/j.1468-2982.2003.00613.x
Neri M, Frustaci A, Millic M, Valdiglesias V, Fini M, Bonassi S, Barbanti P (2015) A meta-analysis of biomarkers related to oxidative stress and nitric oxide pathway in migraine. Cephalalgia 35:931–937. https://doi.org/10.1177/0333102414564888
Silva FA, Rueda-Clausen CF, Silva SY, Zarruk JG, Guzman JC, Morillo CA, Vesga B, Pradilla G, Flórez M, López-Jaramillo P (2006) Endothelial function in patients with migraine during the interictal period. Headache 47:45–51. https://doi.org/10.1111/j.1526-4610.2006.00532.x
Heshmat-Ghahdarijani K, Javanmard SH, Sonbolestan SA, Saadatnia M, Sonbolestan SA (2015) Endothelial function in patients with migraine without aura during the interictal period. Int J Prev Med 6(2). https://doi.org/10.4103/2008-7802-151432
Ciancarelli I, Tozzi-Ciancarelli MG, Massimo CD, Marini C, Carolei A (2003) Urinary nitric oxide metabolites and lipid peroxidation by-products in migraine. Cephalalgia 23:39–42. https://doi.org/10.1046/j.1468-2982.2003.00447.x
Lippi G, Mattiuzzi C, Meschi T, Cervellin G, Borghi L (2014) Homocysteine and migraine. A narrative review. Clin Chim Acta 433:5–11. https://doi.org/10.1016/j.cca.2014.02.028
Moschiano F, D’Amico D, Usai S, Grazzi L, Stefano MD, Ciusani E, Erba N, Bussone G (2008) Homocysteine plasma levels in patients with migraine with aura. Neurol Sci 29:173–175. https://doi.org/10.1007/s10072-008-0917-2
Ipcioglu OM, Ozcan O, Gultepe M, Tekeli H, Senol MG (2008) Functional vitamin B12 deficiency represented by elevated urine methylmalonic acid levels in patients with migraine. Turk J Med Sci 38:409–414
Xiang Y, Hwa J (2016) Regulation of vWF expression, and secretion in health and disease. Curr Opin Hematol 23:288–293. https://doi.org/10.1097/MOH.0000000000000230
Cesar JM, Garcia-Avello A, Vecino AM, Sastre JL, Alvarez-Cermeno JC (1995) Increased levels of plasma von Willebrand factor in migraine crisis. Acta Neurol Scand 91:412–413
Furie B, Furie BC (1990) Molecular basis of vitamin K-dependent γ-carboxylation. Blood 75:1753–1762
Gudmundsson LS, Aspelund T, Scher AI, Thorgeirsson G, Johannsson M, Launer LJ, Gudnason V (2009) C-reactive protein in migraine sufferers similar to that of non-migraineurs: the Reykjavik study. Cephalalgia 29:1301–1310. https://doi.org/10.1111/j.1468-2982.2009.01865.x
Goadsby PJ, Edvinsson L, Ekman R (1990) Vasoactive peptide release in the extracerebral circulation of humans during headache. Ann Neurol 28:183–187
Gudmundsson LS, Thorgeirsson G, Sigfusson N, Sigvaldason H, Johannsson M (2005) Migraine patients have lower systolic but higher diastolic blood pressure compared with controls in a population-based study of 21537 subjects. The Reykjavik study. Cephalalgia 26:436–444. https://doi.org/10.1111/j.1468-2982.2005.01057.x
Agostoni E, Aliprandi A (2008) Migraine and hypertension. Neurol Sci 29:S37–S39. https://doi.org/10.1007/s10072-008-0883-8
Finocchi C, Sassos D (2017) Headache and arterial hypertension. Neurol Sci 38(suppl1):s67–s72. https://doi.org/10.1007/s10072-017-2893-x
Folsom AR, Eckfeldt JH, Weitzman S, Ma J, Chambless LE, Barnes RW, Cram KB, Hutchinson RG (1994) Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis risk in communities (ARIC) study investigators. Stroke 25:66–73
Howard G, Sharrett AR, Heiss G, Evans GW, Chambless LE, Riley WA, Burke GL (1993) Carotid artery intimal–medial thickness distribution in general populations as evaluated by B-mode ultrasound. ARIC investigators. Stroke 24:1297–1304
Funding
This study was supported by the Baskent University Research Fund.
Author information
Authors and Affiliations
Contributions
A.Y.A. and M.H.A. conceived and designed the study; E.T. measured the carotid IMT; S.A. acquired and analyzed the laboratory data; M.A.T analyzed the statistics; A.Y.A. acquired and interpreted the data and wrote the paper; A.Y.A. and U.C. critically revised the manuscript for important intellectual content.
Corresponding author
Ethics declarations
The study was approved by the Baskent University Institutional Review Board and Ethics Committee (project no: KA15/133) and conducted in accordance with the Declaration of Helsinki. All M and control subjects gave informed consent for participation in the study and for carotid artery Doppler ultrasound and laboratory tests.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by Baskent University Institutional Review Board and Ethics Committee (project no: KA15/133) and conducted in accordance with the Declaration of Helsinki of 1964.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yilmaz Avci, A., Akkucuk, M.H., Torun, E. et al. Migraine and subclinical atherosclerosis: endothelial dysfunction biomarkers and carotid intima-media thickness: a case-control study. Neurol Sci 40, 703–711 (2019). https://doi.org/10.1007/s10072-019-3710-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-019-3710-5