Abstract
Objectives
To study the effects of a standard acute medication withdrawal program on short-term cortical plasticity mechanisms in patients with medication overuse headache (MOH).
Methods
Thirteen patients with MOH and 16 healthy volunteers underwent repetitive transcranial magnetic stimulation (rTMS) over the left motor cortex; in patients with MOH, recordings were performed before and after a 3-week medication withdrawal program. Ten trains of 10 stimuli each (120% resting motor threshold) were delivered at 1 Hz or 5 Hz in two separate sessions in a randomised order. Motor evoked potential (MEP) amplitudes were measured from the right first dorsal interosseous muscle and the slope of the linear regression line from the first to the tenth stimuli was calculated for each participant.
Results
All subjects exhibited MEP amplitude inhibition in response to 1 Hz rTMS. Alternatively, the 5-Hz trains of rTMS inhibited rather than potentiated MEP amplitudes in patients with MOH. The physiological potentiating effect of 5 Hz rTMS on MEP amplitudes was restored after drug withdrawal and in proportion with the percentage reduction in monthly headache days in patients with MOH.
Conclusions
The results suggest that acute medication withdrawal normalises brain responses in patients with MOH. Clinical improvements after medication withdrawal may reflect the reversal of neurophysiological dysfunction. Accordingly, medication withdrawal should be offered to patients with MOH as early as possible in order to prevent the development of more pronounced alterations in brain plasticity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The International Classification of Headache Disorders (ICHD 3) [1] defines medication overuse headache (MOH) as headaches occurring ≥ 15 days per month for a period of at least 3 months as the result of excessive intake of acute medications such as non-steroidal analgesic drugs (NSAIDs) and triptans. Several electrophysiological studies have investigated the pathophysiology of MOH and demonstrated that patients with MOH exhibit characteristic neurophysiological abnormalities. For example, patients with MOH show response sensitisation of the somatosensory cortex in response to different repetitive sensorial stimulations, demonstrated by an initial increase in the amplitude of evoked potentials [2]. Patients with MOH also exhibit impaired amplitude habituation, defined as the absence of a decrease in amplitude in response to repeated stimulation [2,3,4]. Since habituation is a basic form of learning [5], these findings suggested that patients with MOH experience alterations in neural plasticity and learning processes.
We recently assessed neural plasticity in the motor cortex of chronic migraineurs with and without medication overuse using low- and high-frequency repetitive transcranial magnetic stimulation (rTMS). We found that, depending on the duration of overuse headache, patients did not show short-term potentiation of motor evoked potentials in response to facilitatory trains of rTMS [6]. In contrast, chronic migraineurs without medication overuse showed normal responses to inhibitory/facilitatory trains of rTMS. These observations led us to hypothesise that medication overuse induces a dysfunctional state of brain plasticity. On this premise, we further speculated that medication-induced alterations in short-term plasticity would normalise after the discontinuation of medication overuse.
The aim of this study was to examine responses of patients with MOH to both low- and high-frequency rTMS over the motor cortex before and after drug withdrawal in comparison to normal subjects in order to understand the characteristics of short-term plasticity dysfunction in MOH.
Material and methods
Subjects
We recruited 16 patients with de novo MOH (according to the International Classification of Headache Disorders III [1]) from our headache clinic. Of these, 3 patients were excluded because they did not meet the primary inclusion criteria (see below). We previously published the results of rTMS studies performed on the initial 8 patients [6] and have combined these data with data from 5 additional patients in order to verify the observed effect of acute medication withdrawal. Participants were included if they were between 18 and 65 years of age, had at least a 1-year clinical history of migraine and had never completed a detoxification program before their first screening visit. The inclusion criteria were restricted to patients with MOH as a result of NSAID use only (IHCD-III code 8.2.3.2) based on a previous study demonstrating that these patients exhibit more pronounced sensorimotor abnormalities than patients overusing acute migraine medications such as triptans [2, 7]. Participants were excluded from the study if they had been taking regular medications in the previous 3 months (e.g. antibiotics, corticosteroids, antidepressants, benzodiazepines or prophylactic migraine medication; contraceptive pills were allowed) or if they had a history of other neurological disorders, systemic hypertension, diabetes or other metabolic or autoimmune disease, or any other type of primary or secondary headache. All participants received a comprehensive description of the study and provided written informed consent prior to participation. The study was approved by the local ethics review board and was conducted in accordance with the Declaration of Helsinki.
After application of the inclusion/exclusion criteria, the final dataset comprised 13 patients. All patients had a clear history of episodic migraine without aura (ICHD-III code 1.1) prior to the development of MOH. With the exception of 2 patients who indicated the presence of mild headache (mean visual analogue scale score, 4/10), all patients underwent MEP recordings in a pain-free state. Recordings were performed at least 3 h after the last dose of medication; because patients with MOH self-administer acute medication in a compulsive manner, we were unable to prevent patients from taking medication on the day of recording. For comparison, we also recruited 16 healthy volunteers (HVs) with comparable distributions of age and sex and no personal or familial history (first- or second-degree relatives) of migraine or other health conditions. Neurophysiological data for these HVs were previously published elsewhere [6]. To avoid hormonal interference, female participants completed the experimental protocol between menstrual periods. All participants were right-handed.
Patients with MOH underwent a 3-week standard acute medication withdrawal program which consisted in the advice to abruptly withdraw the overused medication without any prophylactic medication [8]. Patients’ compliance with medication withdrawal was checked through headache diary. Eight patients out of 13 were able to completely avoid drug intake, while one tablet was taken by three patients, two tablets by one patient and four tablets by another patient for the entire period of 3-week acute medication withdrawal program. After the withdrawal period, patients were re-evaluated using the same experimental TMS protocol. We ensured that the post-withdrawal recording session occurred at least 3 days before and after a migraine attack, as verified by telephone or email interview.
TMS procedures
During the TMS procedure, patients were seated in a comfortable armchair and instructed to relax with their eyes closed. TMS was delivered through a high-frequency biphasic magnetic stimulator (MagstimRapid, The Magstim Company Ltd., Whitland, South West Wales, UK) connected to a figure-of-eight coil with a maximal output of 1.2 T. We first determined the optimal orientation and position of the coil (i.e. “hot spot”) over the left motor area for stimulating the first right dorsal interosseous (FDI) muscle. Thereafter, the resting motor threshold (RMT) was identified using single TMS pulses. The RMT was defined as the minimal intensity required to elicit an electromyographic (EMG) response of at least 50 μV with 50% probability in a fully relaxed muscle. Complete relaxation of the FDI muscle was verified by the absence of EMG signals as determined by visual (on a monitor) and acoustic feedback. Because all participants were right-handed and because patients did not always experience the headaches on the same side, rTMS trains were delivered exclusively over the left motor cortex. EMG activity in the right FDI muscle was recorded with surface electrodes. Thereafter, 10 consecutive trains of 10 single pulses of TMS (stimulus intensity, 120% of the RMT; inter-train interval, 1 min) were delivered at a frequency of 1 or 5 Hz in two separate sessions (with an intersession interval of at least 1 week) in a randomised order. The resulting EMG signal was filtered (20 Hz–1 kHz) and stored on a personal computer. All recordings were collected during a 3-h period in the morning between 09:00 and 12:00 by two investigators (C.L. and C.C.). The 10 trains of 10 stimuli were averaged and analysed offline in a blind manner by a single investigator (F.C.). Peak-to-peak MEP amplitudes (μV) were measured for each of the 10 responses within the train of 10 stimuli.
Statistical analysis
All data were analysed in a blinded manner by a single investigator (G.C.) using Statistica version 8.0 (StatSoft Inc., Tulsa, USA) for Windows (Microsoft Corporation, Redmond, WA, USA).
We first checked the normality of the data distribution using the Kolmogorov-Smirnov test. A preliminary descriptive analysis revealed that some the peak-to-peak MEP amplitudes within individual rTMS trains had non-normal distributions. After log transformation (log10[x]), all data satisfied a normal distribution (the Kolmogorov-Smirnov test, p > 0.05).
In order to compare the baseline findings in patients with MOH (MOH-b) with those of HVs, we performed a repeated measures analysis of variance (rm-ANOVA) with “group” as the between-subject factor (HV, MOH-b) and “stimuli” as the within-subject factor (n = 10). Moreover, as previously described [6], we calculated the slope of the linear regression line for all 10 stimuli using normalised data in order to quickly evaluate MEP amplitude trends within trains of rTMS stimuli. Baseline slope values were compared using independent Student’s t tests. Relative changes (RC) in mean monthly headache days and in the slope of the linear regression were assessed using the following formula: RC = 100 − ([MOH-a × 100] ÷ MOH-b), where MOH-a represents findings obtained after medication withdrawal. Electrophysiological variables before and after the 3-week acute medication withdrawal program were compared using paired Student’s t tests. The threshold for statistical significance was p < 0.05.
Results
Basic clinical and neurophysiological parameters
Complete rTMS trains of MEPs were obtained for all study participants. Baseline neurophysiological parameters (RMT and the first MEP amplitude) were not significantly different between groups for either condition (1 and 5 Hz rTMS) or after a 3-week withdrawal from acute medication in patients with MOH (Table 1).
Effects of rTMS on baseline neurophysiological parameters
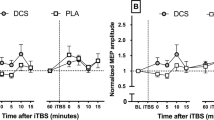
In a rm-ANOVA model using the rTMS 1-Hz MEP peak-to-peak amplitude as the dependent variable, there was a borderline significant main effect of stimuli (F9,243 = 1.867, p = 0.057), but not of group (F1,27 = 0.0255, p = 0.874) or the group × stimuli interaction effect (F9,243 = 0.340, p = 0.961) (Fig. 1, left panel). The slope of the linear regression of MEP amplitudes over all stimuli was not significantly different between groups (t = 0.490, p = 0.628) (Fig. 2, left panel).
Motor evoked potentials (MEPs) elicited by repetitive transcranial magnetic stimulation trains delivered at 1 Hz [left panel] and 5 Hz [right panel] at 120% resting motor threshold in healthy volunteers (HV) and patients with medication overuse headache (MOH) before (MOH-b) and after (MOH-a) completing a 3-week medication withdrawal program
Bar charts representing the motor evoked potential (MEP) amplitude slope of the linear regression line from the first to the tenth stimulus of 1 Hz [left panel] and 5 Hz [right panel] trains of transcranial magnetic stimulation in healthy volunteers (HV) and patients with medication overuse headache (MOH) before (MOH-b) and after (MOH-a) completing a 3-week medication withdrawal program
In a rm-ANOVA model using the rTMS 5-Hz MEP peak-to-peak amplitude as the dependent variable, there was a significant main effect of stimuli (F9,243 = 2.367, p = 0.014) and the group × stimuli interaction (F9,243 = 3.714, p = 0.0002) but not group (F1,27 = 1.029, p = 0.319) (Fig. 1, right panel). The slope of the linear regression of MEP amplitudes over all stimuli was significantly different between groups (t = 3.803, p = 0.0007) (Fig. 2, right panel).
Effects of drug withdrawal on neurophysiological and clinical parameters
There was no significant difference in the mean slope of the linear regression of MEP amplitudes over all stimuli obtained in response to 1 Hz rTMS before and after the 3-week drug withdrawal period in patients with MOH (t = − 0.810, p = 0.937) (Table 2). In contrast, there was a significant difference in the mean slope of the linear regression of MEP amplitudes recorded in response to 5 Hz rTMS between before and after drug withdrawal (t = − 2831, p = 0.015). Of note, the mean slope of MOH-a data was not significantly different from that for HVs (t = 0.854, p = 0.400).
The mean days with headache per month and the mean number of tablets taken per month were also significantly decreased 1 month after withdrawal compared to baseline in patients with MOH (t = 12.338, p < 0.001; t = 5.252, p < 0.001 respectively). Moreover, there was a significant negative correlation between the percentage reduction of days with headache at 1 month after withdrawal and the relative variation of the slope of the linear regression of MEP amplitudes recorded in response to 5 Hz rTMS (r = − 0.637, p = 0.019) (Fig. 3).
Discussion
The main finding of the present study was that a standard withdrawal program for patients overusing medication restored normal short-term synaptic potentiation in the primary motor cortex of patients with MOH. Several neurobiological factors can account for these results.
In healthy subjects, trains of rTMS alter MEP amplitudes during and immediately after stimulation depending on the frequency and intensity of stimulation. When applied over the motor cortex at suprathreshold intensity (120% RTM), high-frequency (5 Hz) rTMS increases MEP amplitudes [9], whereas low-frequency stimulation (1 Hz) diminishes MEP amplitudes. Therefore, rTMS produces plastic changes in motor cortex excitability that outlast the period of stimulation for a period of minutes to hours [9,10,11].
The results of this study confirm our previous finding of dysfunctional short-term synaptic potentiation in patients with MOH [6]; in this study, trains of high-frequency rTMS induced a paradoxical decrease in amplitude in patients with MOH prior to medication withdrawal. This neurophysiological dysfunction may reflect a general alteration in plasticity and learning processes in the MOH brain. Moreover, the absence of these abnormalities in another group of patients with chronic migraine patients without MOH suggests that these findings are specifically related to medication overuse. In our previous study, this conclusion was underscored by the observation that longer durations of medication overuse were associated with more pronounced dysfunction of short-term potentiation in the motor cortex [6], as previously demonstrated for the somatosensory cortex [2].
The present results expand on our previous findings by demonstrating that complete medication withdrawal restores normal short-term potentiation mechanisms within the motor cortex of patients with MOH. Moreover, withdrawal-related normalisation of rTMS responses corresponded to a change from chronic migraine to episodic migraine as indicated by a relative reduction in the number of monthly headache days.
Since the primary motor cortex is involved in several aspects of pain integration and modulation, likely influencing affective or sensory components of pain or by top-down activation of descending antinociceptive systems [12, 13], drug withdrawal may induce the normalisation of a complex network involving brain areas that participate in pain modulation and control such as M1. Consistent with this idea, previous studies have associated the discontinuation of medication overuse with the normalisation of several neurophysiological parameters and morphological features in brain areas of the salient network (also known as the “pain matrix”) [14].
Pain-related cortical potentials [4, 15] and spinal noxious flexion reflex responses [16] are sensitised in patients with MOH. These abnormal responses normalise after withdrawal treatment [4, 15, 16]. Perrotta and colleagues found that at the spinal level, the sensitisation process in MOH was related at least in part to insufficient descending inhibition from the brainstem, subserving the counterirritation phenomenon activated by heterotopic pain stimulation to suppress incoming nociceptive information [16]. The supraspinal antinociceptive structures include the periaqueductal grey, rostral ventromedial medulla, thalamus, nucleus raphe magnus and nucleus reticularis gigantocellularis [17]. Altered structural integrity and functional connectivity of descending pain modulatory areas such as the periaqueductal grey [18,19,20,21] and thalamic nuclei [22] has been repeatedly identified in patients with MOH. These structures are all interconnected with areas belonging to the salient network such as the sensorimotor cortex and orbitofrontal and anterior cingulate cortices [14].
A voxel-based morphometry study identified significant increases in grey matter volume in the midbrain (including periaqueductal grey matter) of patients with MOH and subsequent decreases in volume after the discontinuation of medication overuse. Of note, low grey matter volume in the orbitofrontal cortex before withdrawal was associated with a poor response to drug discontinuation in a previous study [18]. In another study, the orbitofrontal cortex was less connected both metabolically [23] and functionally to nociceptive input regions such as spinal trigeminal nucleus and cerebellum [24] in patients with MOH before drug withdrawal, whereas these connections were normalised after drug withdrawal [23, 24]. Taken together, these data support the hypothesis that medication overuse promotes maladaptive neurophysiological and morphological changes in the brain.
Conclusion
In conclusion, we demonstrate that the dysfunction of short-term plasticity mechanisms in patients with MOH is alleviated by the discontinuation of medication overuse. On this premise, clinical improvements associated with withdrawal treatment may be related, at least in a subgroup of patients, to the restoration of physiological brain plasticity. Our findings underscore the importance of initiating withdrawal treatment as early as possible in patients with MOH in order to facilitate normalisation of brain plasticity mechanisms. Future studies in a larger cohort of patients are necessary to determine the exact relationships between neurophysiological changes and clinical variables in patients with MOH and whether the normalisation of such brain processes allow patients to regain clinical efficacy from acute and prophylactic migraine medications.
References
No authors listed (2018) Headache Classification Committee of the International Headache Society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia 38:1–211. https://doi.org/10.1177/0333102417738202
Coppola G, Currà A, Di Lorenzo C et al (2010) Abnormal cortical responses to somatosensory stimulation in medication-overuse headache. BMC Neurol 10:126. https://doi.org/10.1186/1471-2377-10-126
Siniatchkin M, Gerber WD, Kropp P, Vein A (1998) Contingent negative variation in patients with chronic daily headache. Cephalalgia 18:565–569 discussion 531
Ferraro D, Vollono C, Miliucci R, Virdis D, de Armas L, Pazzaglia C, le Pera D, Tarantino S, Balestri M, di Trapani G, Valeriani M (2012) Habituation to pain in “medication overuse headache”: a CO2 laser-evoked potential study. Headache 52:792–807
Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu CF, Thompson RF (2009) Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem 92:135–138. https://doi.org/10.1016/j.nlm.2008.09.012
Cortese F, Pierelli F, Pauri F, di Lorenzo C, Lepre C, Malavolta G, Merluzzo C, Parisi V, Serrao M, Coppola G (2018) Short-term cortical synaptic depression/potentiation mechanisms in chronic migraine patients with or without medication overuse. Cephalalgia 033310241878474. https://doi.org/10.1177/0333102418784747
Currà A, Coppola G, Gorini M, Porretta E, Bracaglia M, di Lorenzo C, Schoenen J, Pierelli F (2011) Drug-induced changes in cortical inhibition in medication overuse headache. Cephalalgia 31:1282–1290. https://doi.org/10.1177/0333102411415877
Rossi P, Faroni JV, Tassorelli C, Nappi G (2013) Advice alone versus structured detoxification programmes for complicated medication overuse headache (MOH): a prospective, randomized, open-label trial. J Headache Pain 14:10. https://doi.org/10.1186/1129-2377-14-10
Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M (1994) Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117(Pt 4):847–858
Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá MD (1998) Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol 15:333–343
Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG (1997) Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48:1398–1403
García-Larrea L, Peyron R, Mertens P, Gregoire CM, Lavenne F, le Bars D, Convers P, Mauguière F, Sindou M, Laurent B (1999) Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain 83:259–273
Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LRG (2012) Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: possible pathways for antinociception. Pain 153:2359–2369. https://doi.org/10.1016/j.pain.2012.08.002
Uddin LQ (2017) Anatomy of the salience network. In: Uddin LQ (ed) Salience Network of the Human Brain. Academic Press, Cambridge, pp 5–10
Ayzenberg I, Obermann M, Nyhuis P, Gastpar M, Limmroth V, Diener HC, Kaube H, Katsarava Z (2006) Central sensitization of the trigeminal and somatic nociceptive systems in medication overuse headache mainly involves cerebral supraspinal structures. Cephalalgia 26:1106–1114. https://doi.org/10.1111/j.1468-2982.2006.01183.x
Perrotta A, Serrao M, Sandrini G, Burstein R, Sances G, Rossi P, Bartolo M, Pierelli F, Nappi G (2010) Sensitisation of spinal cord pain processing in medication overuse headache involves supraspinal pain control. Cephalalgia 30:272–284
Peyron R, García-Larrea L, Grégoire MC, Costes N, Convers P, Lavenne F, Mauguière F, Michel D, Laurent B (1999) Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain 122:1765–1780
Riederer F, Gantenbein AR, Marti M, Luechinger R, Kollias S, Sandor PS (2013) Decrease of gray matter volume in the midbrain is associated with treatment response in medication-overuse headache: possible influence of orbitofrontal cortex. J Neurosci 33:15343–15349
Chen Z, Chen X, Liu M, Liu S, Ma L, Yu S (2017) Disrupted functional connectivity of periaqueductal gray subregions in episodic migraine. J Headache Pain 18:36. https://doi.org/10.1186/s10194-017-0747-9
Chen Z, Chen X, Liu M, Liu S, Ma L, Yu S (2017) Texture features of periaqueductal gray in the patients with medication-overuse headache. J Headache Pain 18:14. https://doi.org/10.1186/s10194-017-0727-0
Michels L, Christidi F, Steiger VR, Sándor PS, Gantenbein AR, Landmann G, Schreglmann SR, Kollias S, Riederer F (2017) Pain modulation is affected differently in medication-overuse headache and chronic myofascial pain – a multimodal MRI study. Cephalalgia 37:764–779. https://doi.org/10.1177/0333102416652625
Chen Z, Jia Z, Chen X, Liu M, Liu S, Ma L, Yu S (2017) Volumetric abnormalities of thalamic subnuclei in medication-overuse headache. J Headache Pain 18:82. https://doi.org/10.1186/s10194-017-0791-5
Fumal A, Laureys S, Di Clemente L et al (2006) Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain 129:543–550. https://doi.org/10.1093/brain/awh691
Mehnert J, Hebestreit J, May A (2018) Cortical and subcortical alterations in medication overuse headache. Front Neurol 9:499. https://doi.org/10.3389/fneur.2018.00499
Funding
The contribution of the G.B. Bietti Foundation to this article was supported by the Italian Ministry of Health and Fondazione Roma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cortese, F., Pierelli, F., Pauri, F. et al. Withdrawal from acute medication normalises short-term cortical synaptic potentiation in medication overuse headache. Neurol Sci 40, 963–969 (2019). https://doi.org/10.1007/s10072-019-03735-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-019-03735-4