Abstract
Acute ischemic stroke (AIS) is followed by a strong inflammatory response contributing to brain damage and making early diagnosis and treatment inevitable. Hence, obesity is a state of chronic inflammation with amplified oxidative stress; this study aimed to assess the role played by thrombomodulin (TM)/alarmin signaling pathway and copeptin in AIS initiation and severity in addition to the implication of abnormal body weight. The study was conducted on 50 participants; 30 were patients with AIS (15 overweight/obese and 15 normal weight), 10 were overweight/obese, and 10 were normal weight. Plasma TM, copeptin, high mobility group box1 (HMGB1), and lipocalin 2 (LCN2) levels were immunoassayed. Toll-like receptor 4 (TLR4) mRNA expression was evaluated by real-time PCR, National Institutes of Health Stroke Scale (NIHSS), carotid intima media thickness; atherogenic index and glycemic status were also assessed. TM, copeptin, HMGB1, and LCN2 levels were significantly increased in overweight/obese AIS patients and in AIS patients with NIHSS score ≥ 7 when compared to other groups (p value=, ˂ 0.001*). Receiver operating characteristic (ROC) curve elaborated HMGB-1 and LCN2 as the best biomarker for diagnosis and prediction of AIS severity, respectively. Regression analysis avails LCN2 and TM as best biomarker for AIS severity predication. In conclusion, these results highlighted detrimental role of alarmin signaling with increased adaptive response to block this pathway through TM in addition to increased copeptin level as an acute damage marker and their tight relation to WC not to BMI in AIS which clarify the implication of central adiposity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is considered one of the major leading causes of death worldwide, including age, smoking, diabetes, hypertension, and obesity as the most top risk factors. Stroke can be divided into ischemic and hemorrhagic stroke. Ischemic stroke (IS) accounts for 80% of all strokes and results from the obstruction of the brain blood flow with subsequent cell death and irreversible brain damage. Meanwhile, the pathogenic mechanisms of IS remain largely elusive; the interactions between environmental factors and genetic backgrounds may have important role [1, 2].
After stroke, not all brain cells die at the same time but the activated cellular signaling shares in cross-talk between damaged and un-damaged cells triggering an inflammatory cascade with increased neuronal injury and death [3]. Innate immune cells have pattern recognition receptors such as toll-like receptor 4 (TLR4) which recognize invading pathogens or damaged tissues. TLR4 recognizes pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) as alarmin, high mobility group box 1 protein (HMGB1), followed by activation of nuclear factor kappa B (NF-κB), key transcription factor for inflammation and oxidative stress, results in the release of chemokines and cytokines and induction of an adaptive immune-inflammation response [2, 4].
HMGB1 is a non-histone nuclear-binding protein that normally maintains DNA structure, repair, and transcription. Under stress and inflammatory conditions as that associated with obesity and post stroke, HMGB1 undergoes nucleo-cytoplasmic shuttling to modulate inflammatory response [5]. It was demonstrated that the alarmin/HMGB1 is involved in obesity pathogenesis by its immune-inflammatory signaling and has an important role in the outcome of IS patients [2, 6]. Lipocalin2 (LCN2), a 25 kDa small secretory glycoprotein, is a member of the small molecule transporting protein with an important role in iron, prostaglandins, and retinol transport in addition to its role in cell growth, migration/invasion, differentiation, death, and survival. In the central nervous system, LCN2 expression and secretion by glial cells are induced by inflammatory stimuli [7]. Thrombomodulin (TM), a membrane glycoprotein, is mainly expressed by endothelial and inflammatory cells. TM binds thrombin to inhibit coagulation and fibrinolysis; exhibits an anti-inflammatory effect through binding to the HMGB-1, a pro-inflammatory mediator; stabilizes barrier function; and increases blood flow under pathological conditions [8]. Copeptin is the carboxy-terminal part of the arginine vasopressin (AVP) precursor, produced in an equimolar ratio in hypothalamus and processed during axonal transport [9]. It was found to be increased in critically ill patients with various clinical conditions such as sepsis, trauma, and acute vascular events [10].
As the pathogenic mechanisms of IS and initial risk assessment remain largely elusive in addition, IS severity and prognosis is critical for improving the care and distribution of healthcare resources to improve outcome especially in obese subjects as they are subjected to many health hazards hoping for developing approaches targeted towards decreasing the risk, initial prognosis, and control of IS progression. So, the present study was accomplished to shed light on the role played by TM/alarmin signaling pathway in addition to copeptin (acute vascular damage marker) in acute ischemic stroke (AIS) development and initial severity with the specific consideration to the role played by central adiposity.
Patients and methods
Chemicals
All chemicals and solvents used unless otherwise described were purchased from Sigma-Aldrich (St. Louis, MO, USA) and were of high analytical grade.
Study subjects and inclusion and exclusion criteria
All subjects or their close relatives (in case of aphasia) have given their informed written consent, and the study was institutionally approved by the Research Ethical Committee of Faculty of Medicine, Tanta University, Egypt. This cross-sectional comparative study included 50 purposively selected subjects who were attending to the neurology department of Tanta University Hospital during the period from June 2016 to January 2017. From the hospital records, it is evident that during the study period, a number of 200 subjects attended the hospital. Out of these 200, only 50 met the inclusion criteria to have in our study analysis. Albeit small in sample size, we wanted to have a fare idea of the obesity on the outcome variable (AIS), and we had to divide the subjects into four groups. Group I (n = 10) represents control normal weight group. Group II (n = 10): age- and sex-matched subjects who were recruited as control overweight/obese group. Group III (n = 15): age- and sex-matched overweight/obese AIS patient admitted to the hospital within 24 h from the onset of AIS symptom (left temporal infarction 20%; right temporal infarction 20%; bilateral temporal infarction 33.3%; basal ganglionic infarction 13.3%; right cerebellar infarction 13.3%). Group IV (n = 15): age- and sex-matched normal-weight AIS patients admitted to the hospital within 24 h from the onset of AIS symptoms (left temporal infarction 33.3%; right temporal infarction 33.3%; bilateral temporal infarction 13.3%; basal ganglionic infarction 13.3%; right cerebellar infarction 6.67%). Diagnosis of AIS was made according to the standards established by the World Health Organization (WHO), 1989 [11]. Smokers, alcohol intake, peripheral vascular disease, history of cancer, liver failure, renal insufficiency (creatinine > 1.5 mg/dL), severe cardiac dysfunction, history of recent cerebral infarction or hemorrhage (less than 3 months before the recruitment), syndrome of inappropriate anti-diuretic hormone secretion, severe sepsis, heart failure/arrhythmias, history of recent surgery or trauma during the past 2 months, or autoimmune diseases with or without immunosuppressive therapy were excluded. None of our enrolled patients were on regular anticoagulants and/or anti-platelet agents. All controls were free from cerebro-or-cardiovascular diseases, liver/kidney or autoimmune diseases, immunological disorder, and hormone supplementation. All individuals of the study were subjected to history taking, thorough clinical examination, anthropometric measurements, and carotid ultrasound. Meanwhile, patients were also subjected to compute tomography (CT) scan and echocardiograph (ECG), in addition to the assessment of initial neurological impairment and short-term outcome.
Anthropometric measurements
Anthropometric measurements recorded were height, weight, and waist circumference (WC). Measurements of WC were carried out to assess abdominal obesity and WC > 88 cm in women and > 102 cm in men diagnose abdominal (central) obesity [12]. Body mass index (BMI) was determined as weight in kilograms divided by the square of the height in meters (kg/m2). BMI was categorized according to the World Health Organization classification (WHO): normal weight, BMI < 25; overweight, 25–29; obesity, ≥ 30) [13].
Assessment of early neurological impairment
Acute neurological impairment and short-term outcome were evaluated by using National Institutes of Health Stroke Scale (NIHSS) on admission, and patients were divided into two groups according to NIHSS score ≥ 7 or < 7 [14].
Doppler ultrasound examination
Carotid ultrasound was performed using B-mode ultrasound (Siemens G60S, Munich, Germany) equipped with a linear transducer, and carotid intima media thickness (CIMT) was determined in millimeters as a surrogate measure of atherosclerosis [15].
Blood sampling
Five milliliter venous blood samples were obtained after an overnight fast (within 24 h of admission) from each participant. Blood was collected on sterile EDTA-treated tubes for plasma and on sterile plain tube for serum separation and centrifuged at 3000 r/min for 15 min. The recovered serum, plasma aliquots, and EDTA-treated blood were stored at − 80 °C for further analysis.
Routine laboratory measurements
Serum lipid profile including triacylglycerol (TAG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured by commercial kits (Spin react Company, Spain). Fasting blood glucose (FBS) was detected by oxidase method using commercial kit (Biodiagnostic., Egypt). Atherogenic Index (AI) was calculated using the formula LDL-C/HDL-C ratio as previously described [16].
Assessment of endothelial stress and immune-inflammatory status by enzyme-linked immunosorbent assays
Plasma copeptin and HMGB1 levels were assessed by commercial enzyme-linked immunosorbent assay (ELISA) kits supplied by USCN Business Co., Ltd., Wuhan, China and Sunred Bio, Shanghai, China, respectively. TM and LCN2 levels were assessed by commercial ELISA kit supplied by (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. Color development was monitored by absorbance at 450 nm with a microplate reader (Stat Fax 2100, NY, USA).
RNA extraction, cDNA synthesis, and real-time PCR for TLR4 mRNA expression
Total RNA was extracted from EDTA peripheral blood using Fast HQ RNA Extraction Kit (no. 17213, iNtRON Biotechnology Inc., Seongnam, Korea) according to the manufacturer’s instructions. Total RNA was treated with DNase I to eliminate genomic DNA contamination, followed by synthesis of the first-strand cDNA using Prime Script 1st Strand cDNA Synthesis Kit (TaKaRa Bio, Shiga, Japan) according to the manufacturer’s instructions. The resulting cDNA was amplified using Power SYBR® Green PCR Master Mix (Life Technologies, Carlsbad, CA, USA) and sequence-specific primers according to the manufacturer’s instructions. TLR4 mRNA transcripts were quantified, relative to the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was used as an internal control. Sequence-specific primers were designed by Primer3 software: (http://bioinfo.ut.ee/primer3/) as follows: TLR4 (NM_138557.2) forward (5′-GATTGCTCAGACCTGGCAGTT-3′), reverse (5′-TGTCCTCCCACTCCAGGTAAGT-3′); GAPDH (no. NM_001289746.1): forward primer (5′-GGTGGTCTCCTCTGACTTCAACA-3′) and reverse primer (5′-GTTGCTGTAGCCAAATTCGTTGT-3′). PCR cycling was set as follows: a single cycle of DNA polymerase activation for a 10-min hold at 95 °C followed by 40 cycles of 95 °C for 15 s for denaturation, 60 °C for 1 min for annealing, and 60 °C for 1 min for extension. Amplification and data analysis were conducted on a Rotor-Gene Q 6plex and its specific software (Qiagen, Valencia, CA, USA). Relative gene expression was automatically calculated from the cycle threshold (Ct) method then normalized against the housekeeping gene GAPDH that was used as endogenous control in all experiments.
Statistical analysis
Data analysis was performed using Statistical Package for Social Sciences (SPSS), version 23.0 for Windows (SPSS, Chicago, IL), expressed as mean ±SD for continuous variables and as frequencies and percentages for categorical variables. Comparisons between more than two groups were carried out by one-way ANOVA, within two groups by Student’s t test or Man-Whitney test for parametric and non-parametric parameters respectively in addition to chi-square test. Correlations were analyzed using Spearman rank or Pearson’s correlation coefficients. Regression analysis was conducted to predict AIS severity from HMGPB1, LCN2, TM, TLR4, and copeptin level. Receiver operating characteristics (ROC) analysis was used to identify the optimal threshold values of the studied parameters. The area under the curve can range from 0.5 to 1, and diagnostic tests that approach 1 indicate a perfect discriminator. p value < 0.05 was considered significant.
Results
Basic demographic and clinical characteristics
Demographic variables and clinical characteristics of the studied groups are depicted in Table 1. There were no significant differences in age or sex between the studied groups. Meanwhile, BMI, WC, and FBG were statistically significantly different among all of the studied groups (p value ˂ 0.001*) with higher values for the overweight/obese group with AIS except for BMI where no significant difference was recorded between both overweight/obese subjects with or without AIS.
Endothelial stress marker, AI, and vascular measurements
This current study revealed statistically significant differences among all of the studied groups regarding lipid profile, CIMT, AI, and TM level (p value ˂ 0.001*). Overweight/obese AIS group showed statistically significantly increased TC, TG, LDL-C, CIMT, AI, and TM values when compared to control (normal weight and obese) groups and normal weight AIS groups (Tables 1 and 2; Fig. 1).
Alarmin and immune-inflammatory status
This current study revealed statistically significant differences among the studied groups regarding; copeptin, HMGB1, LCN2, and TLR4 mRNA expression levels (p value ˂ 0.001*) with higher values for AIS groups. There were statistically significant difference between overweight/obese AIS groups and normal-weight AIS group as regards copeptin, HMGPB1, and LCN2 levels with higher values for overweight/obese AIS groups. Meanwhile, there was no significant difference between both AIS groups as regards TLR4 mRNA expression levels (Table 2; Fig. 1).
Early neurological impairment
Early neurological impairment and short-term outcome were assessed by using NIHSS on admission (Table 1) and showed statistically significant differences between both AIS groups, with higher values for overweight/obese AIS group (p value ˂ 0.045*). In addition, AIS patients were divided into two subgroups according to NIHSS score ≥ 7 or < 7 (Table 3) with WC, AI, CIMT, TAG, LDL-c, HMGPB1, copeptin, LCN2, and TLR4 mRNA expression levels revealed statistically significant differences between both AIS groups with higher values for those with NIHSS score ≥ 7 (p values were 0.35, 0.014, 0.001, 0.012, 0.002, 0.04, 0.007, ˂ 0.001, and 0.018, respectively).
Correlation matrix of the studied biochemical and molecular parameters with obesity indices, CIMT, and NIHSS scores
All correlations are summarized in Table 4. In AIS cases, WC was correlated positively with NIHSS score, AI, CIMT, TM, copeptin, HMGB1, LCN2, and TLR4 mRNA levels revealing the role of central adiposity in modulation of the previously studied parameters. NIHSS score was significantly positively correlated with AI, CIMT, TM, copeptin, HMGPB1, LCN2, and TLR4 mRNA levels and no correlations were found between BMI and NIHSS score levels revealing the role of the previously studied parameters in modulation of initial neurological impairment and short-term outcome. BMI exhibited significantly positive correlation only with AI, CIMT, TM, and copeptin levels.
ROC curve analysis
Receiving operating characteristics curve study for copeptin, HMGPB1, LCN2, and TLR4 mRNA levels in AIS cases versus control (normal weight and obese non-stroke) groups is presented in Table 5. Area under the curve (AUC) for TM was 0.969 with optimal cutoff value of > 2427 pg/mL. Using this cutoff value, TM showed a sensitivity of 93% and a specificity of 83% for early diagnosis of AIS. AUC for copeptin was 0.96, with optimal cutoff value of > 2434.5 pg/mL. Using this cutoff value, copeptin showed a sensitivity of 90% and a specificity of 87% for early diagnosis of AIS. AUC for HMGB1 was 1, with optimal cutoff value of > 49.5 ng/mL. Using this cutoff value, HMGB1 showed a sensitivity of 100% and a specificity of 90% for early diagnosis of AIS. AUC for TLR4 mRNA expression level was 0.926, with optimal cutoff value of > 0.81. Using this cutoff value, TLR4 showed a sensitivity of 90% and a specificity of 70% for early diagnosis of AIS. AUC for LCN2 was 1, with optimal cutoff value of > 2981.5 pg/mL. Using this cutoff value, LCN2 showed a sensitivity of 93% and a specificity of 100% for early diagnosis of AIS.
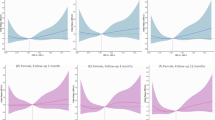
Receiving operating characteristics for copeptin, HMGB1, TLR4, and LCN2 level in AIS group with NIHSS ≥ 7 versus AIS group with NIHSS ˂ 7 were depicted in Table 6. AUC for TM was 0.681 with optimal cutoff value of > 2996.5 pg/mL. Using this cutoff value, TM showed a sensitivity of 75% and a specificity of 50% for early diagnosis of AIS severity. AUC for copeptin was 0.796, with optimal cutoff value of > 3125 pg/mL. Using this cutoff value, copeptin showed a sensitivity of 92% and a specificity of 62% for early diagnosis of AIS severity. AUC for HMGB1 was 0.692 with optimal cutoff value of > 97.5 ng/mL. Using this cutoff value, HMGB1 showed a sensitivity of 83% and a specificity of 56% for early diagnosis of IS severity. AUC for LCN2 was 0.903 with optimal cutoff value of > 4525 pg/mL. Using this cutoff value, LCN2 showed a sensitivity of 92% and a specificity of 78% for early diagnosis of AIS severity. Furthermore, regression model was performed to predict the severity of AIS in obese and non-obese patients with AIS. While looking at the severity of the AIS scores, it was found that only LCN2 could predict the severity outcome of AIS significantly adjusted for the other four factors (suppl. Fig. 1A; suppl. Table 1). Meanwhile, for non-obese cases with AIS, both LCN2 and TM significantly predicted the severity of AIS adjusted for the other three factors (suppl. Fig. 1B; suppl. Table 1).
Discussion
Stroke particularly ischemic one is the second leading cause of death and disability worldwide. It elicits apoptotic and inflammatory signaling pathways with further brain damage [17]. Obesity has been largely associated with increased risk of cardiovascular disease; however, its relationship to cerebrovascular diseases remained to be fully clarified. Visceral fat cell accumulation and its altered metabolic milieu are the key players in the development of atherogenic and inflammatory sequel of obesity [18, 19]. Clinical features and severity scores are insufficient to predict initial stroke outcome; therefore, searching for a newly, expedient measured biochemical and molecular biomarkers could enable early diagnosis and accurate assessment of severity and outcome in AIS with respect to central adiposity which ultimately would help for early therapeutic interventions and patient management [20].
So, the present study went further to assess the role played by TM/alarmin signaling and copeptin levels, in AIS pathogenesis and initial severity (NIHSS score) in relation to WC, a measure of central obesity. The present study revealed that WC but not BMI showed higher values in overweight/obese AIS patients than their allied normal-weight AIS group, obese controls, and normal-weight controls reporting the importance of central adiposity not BMI in cerebrovascular complications of obesity. In addition, WC in AIS group with NIHSS score ≥ 7 was significantly higher than those with score ˂ 7 with no difference between both groups regarding BMI. Further, WC was correlated positively with NIHSS indicating the importance of WC as risk factors for both AIS initiation in overweight/obesity and in early neurological severity in AIS. Yatsuya et al. (2010) reported a positive linear relationship between obesity indices and AIS incidence [21]. Also, increased visceral adipose tissue (VAT) has been observed in patients with embolic stroke [22].
In the present study, overweight/obese AIS patients experienced statistically significant altered lipid profile, glycemic status, and atherosclerosis indices when compared to other groups. Intriguingly, AIS subjects with NIHSS score ≥ 7 showed statistically significantly increased LDL-C, TAG, AI, and CIMT than those with lower scores recalling the importance of disturbed lipid profile in AIS development and short outcome. Moreover, a positive correlation was found between NIHSS severity score and the abovementioned parameters. This is consistent with Zeljkovic et al. (2010) who reported increased LDL-c as an independent predictor for AIS onset and mortality [23]. Similarly, hypertension, hyperlipidemia, and diabetes are the most prevalent risk factors for AIS with positive correlation to increased CIMT and atherogenic indices [24, 25].
All AIS patients showed increased HMGB1 levels, mostly due to excitatory inflammatory process and ischemia-induced neuronal death [26], and upregulated TLR4 gene expressions. However, overweight/obese AIS group had statistically significantly higher HMGB1 levels when compared to normal-weight AIS group, and TLR4 gene expressions showed insignificant difference between both AIS groups indicating that the inflammatory condition that associated with obesity is aggravated by that occurs post ischemic which may have an important role in pathogenesis and initial severity of the AIS in obese than normal-weight individuals. In coherence with that explanation, the results of the present study revealed positive correlation between HMGB1, TLR4 gene expression and CIMT, AI, NIHSS severity score, and WC, indicating their importance in early neurological assessment and highlighting the relation of immune-inflammatory markers to WC but not to BMI. TLR4 gene expression is upregulated in stroke patients which might account for the probable inflammatory injury before or after stroke [27]. Furthermore, AIS group with NIHSS score ≥ 7 showed statistically significantly increased HMGB1 andTLR4 gene expression levels when compared to those with lower scores.
Downregulation of TLR-4 mRNA expression in hypothalamic arcuate nucleus improved glucose homeostasis, insulin sensitivity, and abnormal gluconeogenesis in obese rats [28]. HMGB1 is produced mainly by macrophages in atherosclerotic lesions in response to inflammatory cytokines and may contribute to atherosclerosis progression and chronic inflammation [29]. Yao et al. reported increased HMGB1 levels in ischemic stroke, with positive relation to severity that could be helpful in evaluation of AIS severity and prognosis [30]. HMGB1 has been reported to be increased in adipose tissue from obese individuals. Depending on its redox state, extracellular cytokine-inducing isoform of HMGB1 promotes pro-inflammatory IL-6 secretion from human mature primary adipocytes via the activation of TLR4 and the downstream NF-κB and P38 MAPK signaling pathways [4]. Consequently, blocking HMGB1 inflammatory pathway may be a therapeutic target to overcome obesity-associated drawbacks. LCN2 is gliocalin primarily released by glial cells such as astrocytes after brain injury and promotes neuroinflammation [31]. Results presented herein demonstrated significant higher LCN2 levels in overweight/obese AIS particularly those with higher NIHSS scores. Also, LCN2 correlated well with stroke severity, WC, and indices of vascular damages pointing to its role in early diagnosis and neurological assessment. The increased LCN2 level during AIS may be due to the release of DAMPs as HMGP1 from dying necrotic neurons which stimulate inflammatory reactions via binding to TLR4 with resultant increased LCN2 level [32]. In line with our results, LCN2 expression has been shown to be increased in response to pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6 via signal transducer and activator of transcription 1 (STAT1) and NF-κB respectively, pathways known to be active and have important role in obesity-associated chronic inflammation with a sequel of obesity-associated health hazards [33].
Concomitantly, a critical role of LCN2 in brain injury and neuronal cell death has been described in a rodent model of transient cerebral ischemia through promoting neurotoxic glial activation, neuroinflammation, and blood-brain barrier disruption [34]. Furthermore, LCN2-induced inflammasome activation in heart tissue via HMGB1/TLR4-dependent signaling was attenuated by TLR4 chemical inhibition [35].
Thrombomodulin (TM) is an integral membrane protein with anticoagulant, anti-inflammatory, and anti-apoptotic properties synthesized by endothelial cells; in addition, it can improve endothelial barrier integrity. It is associated with atherosclerosis as an indicator of endothelial cell activation, stress, and injury [36, 37]. Here, we noticed increased TM levels in all overweight/obese AIS patients with no significant difference between patients with higher NIHSS scores and others. Meanwhile, TM correlated positively with WC, severity, and vascular damage indices. The noted increased TM level may be due its release from injured endothelial cells during AIS to prevent ongoing thrombosis and inflammation. Consistent with current data, Meyer et al. revealed increased CIMT, impaired endothelial function, and elevated TM levels in obese children relevant to obesity [38]. Pilarska et al. reported that elevated TM levels in AIS, in relation to severity or parenchymal brain injury duration, are important in diagnosis, management, relapse, and prevention of AIS [39].
Activation of the hypothalamo-pituitary-adrenal axis is one of the first measurable physiological responses to cerebral ischemia. Arginine vasopressin (AVP), a vital hormone with numerous effects, is one of the main hormones of the hypothalamic-pituitary-adrenal axis. The main stimulus for AVP secretion is hyper-osmolarity and endogenous stress. AVP levels increase apparently during the process of some acute and chronic diseases, but its clinical value is restricted because of its short half-life. Copeptin is released in an equimolar ratio to vasopressin, and it is a more stable easy measurable marker that mirrors AVP level [40, 41].
In the present study, increased copeptin level was detected in all overweight/obese AIS patients as compared to their allied control groups and normal-weight AIS groups. However, AIS patients with higher NIHSS score showed significantly increased copeptin levels compared to patients with lower scores. Also, copeptin correlated with anthropometric measures, severity, and vascular damage indices. This might be attributed to the role played by vasopressin in brain edema and ischemic neuronal injury, as previously reported [42]. Relevantly, increased inflammation and endogenous stress in obesity and after ischemic attack stimulate AVP release with increased copeptin levels have been linked to obesity, metabolic syndrome, and insulin resistance [43]. Prior reports have detected the association of copeptin level with both initial stroke severity and relapse with an unfavorable outcome [44, 45].
Collectively and based on the present study observed ROC curve analysis, HMGB1 followed by LCN2 showed the best specificity and sensitivity as diagnostic markers in AIS. Meanwhile, LCN2 followed by copeptin was the best to predict initial AIS severity assessment that may help in decision making for early prediction and therapeutic interventions. The limitations of the present study was small population size because only acute onset overweight or obese hospitalized patients are included in the study in addition to the strict inclusion and exclusion criteria that were planned to depend on in this study aiming to decrease the effect of different factors on the initiation and initial impairments of AIS as possible; therefore, future large community-based studies are still needed.
Conclusions
Based on our model in Fig. 2, obesity-induced signaling, redox, and metabolic derangements may play an important role in AIS development and initial severity. The present study declare the role played by TM and alarmin signaling through TLR4/LCN2 and copeptin in AIS development and their correlation to central adiposity and initial disease severity, illustrating new mechanistic insights on the detrimental role of HMGB1/TLR4/LCN2 and the protective role of TM against this pathway in addition to the role of copeptin as an acute vascular damage marker. This result may raise the possibility for further research to develop small-molecule inhibitors or neutralizing antibodies to disrupt HMGB1/TLR4/LCN2 signaling pathway as new therapeutics for stroke prevention especially centrally obese who suffer from many risk factors for stroke in addition to initial interventions against brain injury.
Model of immunoinflammatory signaling pre and post stroke in obesity. Central adiposity with increased waist circumference leads to increased visceral adipocytes activity and chronic inflammation due to cytokine production and increased oxidative stress and DNA damage and release of HMGB1 that activates TLR4 mRNA expression and binds to it sequentially with increased synthesis and release of LCN2 and disruption of blood-brain barrier and neuronal damage. Furthermore, increased FFA with impaired glycemic status leads to endothelial dysfunction and atherogenesis that leads increased copeptin and vascular damage as well as increased release of TM that antagonize HMGB1 release and TLR4 binding. Post ischemic neuronal death leads to neurotoxic glial activation and increased HMGB1/TLR4/LCN2 signaling as well as TM. Obesity particularly waist circumference heightened the risk of stroke and worsens the prognosis. AIS acute ischemic stroke, TM thrombomodulin, HMGB1 high mobility group box-1, LCN2 lipocalin2, TLR4 toll-like receptor 4, OS oxidative stress
References
Bonaventura A, Liberale L, Vecchié A et al (2016) Update on inflammatory biomarkers and treatments in ischemic stroke. Int J Mol Sci 17(12). https://doi.org/10.3390/ijms17121967
Gu L, Huang J, Liang B, Chen Q, Xie J, Yang J, Yan Y, Tang Q (2018) TLR4 polymorphisms affect stroke risk and inflammatory response in Chinese ischemic stroke patients. Neurol Sci 39:127–133. https://doi.org/10.1007/s10072-017-3151-y
Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ (2016) Inflammatory disequilibrium in stroke. Circ Res 119:142–158. https://doi.org/10.1161/CIRCRESAHA.116.308022
Zhu S, Li W, Ward MF, Sama A, Wang H (2010) High mobility group box 1 protein as a potential drug target for infection- and injury-elicited inflammation. Inflamm Allergy Drug Targets 9:60–72. https://doi.org/10.2174/187152810791292872
Gunasekaran MK, Virama-Latchoumy AL, Girard AC, Planesse C, Guérin-Dubourg A, Ottosson L, Andersson U, Césari M, Roche R, Hoareau L (2016) TLR4-dependant pro-inflammatory effects of HMGB1 on human adipocyte. Adipocyte 5:384–388. https://doi.org/10.1080/21623945.2016.1245818
Zhang J, Zhang L, Zhang S, Yu Q, Xiong F, Huang K, Wang CY, Yang P (2017) HMGB1, an innate alarmin, plays a critical role in chronic inflammation of adipose tissue in obesity. Mol Cell Endocrinol 454:103–111. https://doi.org/10.1016/jmce201706.012
Banjara M (2014) Lipocalin-2: a new regulator of non-pathogen-associated neuroinflammation. Int J Clin Exp Neurol 2:8–15. https://doi.org/10.12691/ijcen-2-1-3.
Wenzel J, Assmann JC, Schwaninger M (2014) Thrombomodulin—a new target for treating stroke at the crossroad of coagulation and inflammation. Curr Med Chem 21:2025–2034. https://doi.org/10.2174/0929867321666131228204839
Afsar B (2017) Pathophysiology of copeptin in kidney disease and hypertension. Clin Hypertens 23:13. https://doi.org/10.1186/s40885-017-0068-y
Wendt M, Ebinger M, Kunz A, Rozanski M, Waldschmidt C, Weber JE, Winter B, Koch PM, Nolte CH, Hertel S, Ziera T, Audebert HJ, STEMO Consortium (2015) Copeptin levels in patients with acute ischemic stroke and stroke mimics. Stroke 46:2426–2431. https://doi.org/10.1161/STROKEAHA.115.009877
Stroke--1989 (1989) Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke 20(10):1407–1431. https://doi.org/10.1161/01.STR.20.10.1407
Kuczmarski RJ, Flegal KM (2000) Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr 72:1074–1081
Lean ME, Han TS, Morrison CE (1995) Waist circumference as a measure for indicating need for weight management. BMJ 311:158–161
Hacke W, Albers G, Al-Rawi Y et al (2005) The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 36(1):66–73. https://doi.org/10.1161/01.STR.0000149938.08731.2c
Zineldeen DH, Keshk WA, Ghazy AH et al Sucrose non-fermenting AMPK related kinase/pentraxin 3 and DNA damage axis: a gate way to cardiovascular disease in systemic lupus erythematosus among Egyptian patients. Ann Clin Biochem 201653(Pt 2):240–251. https://doi.org/10.1177/0004563215578190
Abbott RD, Wilson PW, Kannel WB, Castelli WP (1988) High density lipoprotein cholesterol, total cholesterol screening, and myocardial infarction. The Framingham Study. Arteriosclerosis 8:207–211
Strong K, Mathers C, Bonita R (2007) Preventing stroke: saving lives around the world. Lancet Neurol 6(2):182–187. https://doi.org/10.1016/S1474-4422(07)70031-5
Karcher HS, Holzwarth R, Mueller HP, Ludolph AC, Huber R, Kassubek J, Pinkhardt EH (2013) Body fat distribution as a risk factor for cerebrovascular disease: an MRI-based body fat quantification study. Cerebrovasc Dis 35:341–348. https://doi.org/10.1159/000348703
Lim S, Meigs JB (2014) Links between ectopic fat and vascular disease in humans. Arterioscler Thromb Vasc Biol 34:1820–1826. https://doi.org/10.1161/ATVBAHA.114.303035
Katan M, Fluri F, Morgenthaler NG, Schuetz P, Zweifel C, Bingisser R, Müller K, Meckel S, Gass A, Kappos L, Steck AJ, Engelter ST, Müller B, Christ-Crain M (2009) Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol 66:799–808. https://doi.org/10.1002/ana.21783
Yatsuya H, Yamagishi K, North KE et al (2010) Associations of obesity measures with subtypes of ischemic stroke in the ARIC study. J Epidemiol 20(5):347–354. https://doi.org/10.2188/jea.je20090186
Muuronen AT, Taina M, Hedman M et al (2015) Increased visceral adipose tissue as a potential risk factor in patients with embolic stroke of undetermined source (ESUS). PLoS One 10(3):e0120598. https://doi.org/10.1371/journal.pone.0120598
Zeljkovic A, Vekic J, Spasojevic-Kalimanovska V, Jelic-Ivanovic Z, Bogavac-Stanojevic N, Gulan B, Spasic S (2010) LDL and HDL subclasses in acute ischemic stroke: prediction of risk and short-term mortality. Atherosclerosis 210(2):548–554. https://doi.org/10.1016/j.atherosclerosis.2009.11.040
Sujatha R, Kavitha S (2017) Atherogenic indices in stroke patients: a retrospective study. Iran J Neurol 16(2):78–82
Haq S, Mathur M, Singh J et al (2017) Colour Doppler evaluation of extracranial carotid artery in patients presenting with acute ischemic stroke and correlation with various risk factors. J Clin Diagn Res 11(3):TC01–TC05. https://doi.org/10.7860/JCDR/2017/25493.9541
Faraco G, Fossati S, Bianchi ME, Patrone M, Pedrazzi M, Sparatore B, Moroni F, Chiarugi A (2007) High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem 103:590–603. https://doi.org/10.1111/j.1471-4159.2007.04788.x
Wu D, Sheu JS, Liu HC, Yuan RY, Yu JM, Sheu JJ, Hung CH, Hu CJ (2012) Increase of toll-like receptor 4 but decrease of interleukin-8 mRNA expression among ischemic stroke patients under aspirin treatment. Clin Biochem 45:1316–1319. https://doi.org/10.1016/j.clinbiochem.2012.04.022
Zhao Y, Li G, Li Y et al (2017) Knockdown of Tlr4 in the arcuate nucleus improves obesity related metabolic disorders. Sci Rep 7:7441. https://doi.org/10.1038/s41598-017-07858-6
Kalinina N, Agrotis A, Antropova Y et al (2004) Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol 24:2320–2325. https://doi.org/10.1161/01.ATV.0000145573.36113.8a
Yao HC, Zhao AP, Han QF et al (2013) Correlation between serum high-mobility group box-1 levels and high-sensitivity C-reactive protein and troponin I in patients with coronary artery disease. Exp Ther Med 6:121–124. https://doi.org/10.3892/etm.2013.1095
Suk K (2016) Lipocalin-2 as a therapeutic target for brain injury: an astrocentric perspective. Prog Neurobiol 144:158–172. https://doi.org/10.1016/j.pneurobio.2016.08.001
Nam Y, Kim JH, Seo M, Kim JH, Jin M, Jeon S, Seo JW, Lee WH, Bing SJ, Jee Y, Lee WK, Park DH, Kook H, Suk K (2014) Lipocalin-2 protein deficiency ameliorates experimental autoimmune encephalomyelitis: the pathogenic role of lipocalin-2 in the central nervous system and peripheral lymphoid tissues. J Biol Chem 289:16773–16789. https://doi.org/10.1074/jbc.M113.542282
Zhao P, Stephens JM (2013) STAT1, NF-κB and ERKs play a role in the induction of lipocalin-2 expression in adipocytes. Mol Metab 2:161–170. https://doi.org/10.1016/j.molmet.2013.04.003
Jin M, Kim JH, Jang E, Lee YM, Han HS, Woo DK, Park DH, Kook H, Suk K (2014) Lipocalin-2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab 34:1306–1314. https://doi.org/10.1038/jcbfm.2014.83
Song E, Jahng JW, Chong LP, Sung HK, Han M, Luo C, Wu D, Boo S, Hinz B, Cooper MA, Robertson AA, Berger T, Mak TW, George I, Schulze PC, Wang Y, Xu A, Sweeney G (2017) Lipocalin-2 induces NLRP3 inflammasome activation via HMGB1 induced TLR4 signaling in heart tissue of mice under pressure overload challenge. Am J Transl Res 9:2723–2735
Gerdes VE, Kremer Hovinga JA, Ten Cate H et al (2004) Soluble thrombomodulin in patients with established atherosclerosis. J Thromb Haemost 2:200–201. https://doi.org/10.1111/j.1538-7836.2004.0562f.x
Dharmasaroja P, Dharmasaroja PA, Sobhon P (2012) Increased plasma soluble thrombomodulin levels in cardioembolic stroke. Clin Appl Thromb Hemost 18:289–293. https://doi.org/10.1177/1076029611432744
Meyer AA, Kundt G, Steiner M, Schuff-Werner P, Kienast W (2006) Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics 117:1560–1567. https://doi.org/10.1542/peds.2005-2140
Pilarska E, Lemka M, Bakowska A (2010) Thrombomodulin and antibeta2-glycoprotein I in stroke in children. Med Sci Monit 16:CR348–CR351
Jochberger S, Mayr VD, Luckner G, Wenzel V, Ulmer H, Schmid S, Knotzer H, Pajk W, Hasibeder W, Friesenecker B, Mayr AJ, Dünser MW (2006) Serum vasopressin concentrations in critically ill patients. Crit Care Med 34:293–299. https://doi.org/10.1097/01.ccm.0000198528.56397.4f
Zhang P, Wu X, Li G, Sun H, Shi J (2017) Prognostic role of copeptin with all-cause mortality after heart failure: a systematic review and meta-analysis. Ther Clin Risk Manag 13:49–58. https://doi.org/10.2147/TCRM.S124689
Vakili A, Kataoka H, Plesnila N (2005) Role of arginine vasopressin V1 and V2 receptors for brain damage after transient focal cerebral ischemia. J Cereb Blood Flow Metab 25:1012–1019. https://doi.org/10.1038/sj.jcbfm.9600097
Vintilă M, Gheorghiu ML, Caragheorgheopol A, Baculescu N, Lichiardopol C, Badiu C, Coculescu M, Grigorescu F, Poiană C (2016) Increased copeptin levels in metabolic syndrome from a Romanian population. J Med Life 9:353–357
Perovic E, Mrdjen A, Harapin M, Tesija Kuna A, Simundic AM (2017) Diagnostic and prognostic role of resistin and copeptin in acute ischemic stroke. Top Stroke Rehabil 24:1–5. https://doi.org/10.1080/10749357.2017.1367454
Tang WZ, Wang XB, Li HT, Dong M, Ji X (2017) Serum copeptin predicts severity and recurrent stroke in ischemic stroke patients. Neurotox Res 32:420–425. https://doi.org/10.1007/s12640-017-9754-5
Acknowledgements
We would like to thank Dr. Zaytoun H. (Radiology Department, Tanta University) for the radiological examinations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Ethical Committee of Tanta Faculty of Medicine approved this study (31649).
Electronic supplementary material
ESM 1
(DOCX 295 kb)
Rights and permissions
About this article
Cite this article
Keshk, W.A., Zineldeen, D.H., El-heneedy, Y.A. et al. Thrombomodulin, alarmin signaling, and copeptin: cross-talk between obesity and acute ischemic stroke initiation and severity in Egyptians. Neurol Sci 39, 1093–1104 (2018). https://doi.org/10.1007/s10072-018-3396-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3396-0