Abstract
To investigate the role of the Montreal Cognitive Assessment (MoCA) (Beijing version) and its memory tasks on detecting different mild cognitive impairment (MCI) subtypes including amnestic MCI (aMCI) and nonamnestic MCI (naMCI) in memory clinics. A total of 121 patients with MCI and 53 healthy controls were included. Fifty-six aMCI-multiple domains (amMCI), 32 aMCI-single domain (asMCI), and 33 naMCI patients were diagnosed according to extensive cognitive tests. All participants were administered by the Mini Mental State Examination (MMSE) and the MoCA. Patients with amMCI performed worse than patients with asMCI, naMCI, and healthy controls on the MMSE and the MoCA (p < 0.001). The area under the curve (AUC) value for the MoCA when comparing the amMCI and control groups was 0.884 (p < 0.001), which was superior to that of the MMSE. The AUC value decreased to 0.687 when applied to the naMCI and control groups (p = 0.007), which was still higher than that of the Rey Auditory Verbal Learning Test (RAVLT) or the Rey-Osterrieth complex figure (ROCF). Delayed free recall or category prompted recall in the MoCA had roles in differentiating asMCI and controls groups with AUC value of 0.717 (p = 0.002) and 0.691 (p = 0.005), respectively. The MoCA is a good screening tool for detecting different types of MCI and is suitable for patients in outpatient clinics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia has become a significant health problem within the light of the ever-increasing older population in China [1]. Mild cognitive impairment (MCI) is an intermediate state between dementia and normal cognitive aging, and could provide important information about the population at risk for developing dementia [2]. Identification of MCI is thought to be crucial to early intervention. The expanded concept of MCI distinguishes four clinical subtypes: amnestic MCI-single domain (asMCI), amnestic MCI-multiple domains (amMCI), nonamnestic MCI-single domain (nasMCI), and nonamnestic MCI-multiple domains (namMCI), which differ in etiology and outcome. Amnestic MCI (aMCI) is said to have a high likelihood of progressing to Alzheimer’s disease (AD), especially amMCI. Nonamnestic MCI (naMCI) is assumed to have a higher likelihood of progressing to a non-AD dementia [2,3,4,5].
The prevalence of MCI from the Mayo Clinic Study of Aging is estimated at approximately 15% of the nondemented population with a 2:1 ratio of aMCI to naMCI [3]. The expanded Mayo Clinic criteria for MCI have been used in most studies and require trained specialists and a battery of time-consuming, complicated cognitive tests. The application of these tests is suitable for memory clinics but too complex for general outpatient clinics. A brief screening tool would therefore be a more practical approach for clinicians. The Montreal Cognitive Assessment (MoCA) was specifically developed as a screening tool for MCI and has been shown to have high sensitivity and specificity in various countries, including China [6]. The purpose of the present study was to investigate the value of the MoCA in detecting and differentiating different MCI subtypes, including aMCI and naMCI, in memory clinics and the relationship between the MoCA and other special memory tests.
Methods
Subjects
One hundred seventy-four subjects were recruited from our memory clinics between 2013 and 2015. All participants underwent routine assessments, including standardized history taking, physical and neurological examinations, necessary laboratory tests, and a CT or MRI scan. One hundred twenty-one of these patients (63 men and 58 women, mean age 73.67 ± 7.72 years, age range 51 to 90 years) were diagnosed with MCI and fulfilled the following criteria: (1) cognitive complaint, preferably corroborated by an informant; (2) objective cognitive impairment, quantified as a performance score greater than 1.5 SD below the appropriate mean on one cognitive test of any domain; (3) normal general cognitive functioning (scores of the Mini Mental State Examination (MMSE) [7] equal to or above 26); (4) essentially intact activities of daily living (ADL); and (5) no dementia [8]. Subjects with a score less than 1.5 SD from the mean on a learning measure or delayed recall on the Rey Auditory Verbal Learning Test (RAVLT) [9] or the Rey-Osterrieth complex figure (ROCF) [10] were classified as having aMCI. Subjects with a score less than 1.5 SD from the mean on at least one test of attention, executive function, language facilities, and visuospatial capacity, but no memory impairment, were classified as having naMCI. Subjects with aMCI were subclassified as asMCI if they only had impairment in memory and as amMCI if they also had impairments in non-memory domains. Fifty-six amMCI, 32 asMCI, and 33 naMCI patients were included in the study. The Clinical Dementia Rating (CDR) Scale [11] was 0.5 in all MCI patients.
Fifty-three subjects were included as healthy controls including 18 men and 35 women with a mean age of 70.19 ± 9.72 years. The inclusion criteria for controls were as follows: (1) normal cognitive functions verified by informants, (2) scores of the MMSE equal to or above 26, (3) intact ADL, (4) a CDR of 0, and (5) scores of all cognitive tests higher than 1.5 SD from the mean. Exclusion criteria for the controls were severe medical illness, neurological disorder, psychiatric disease, hearing or eyesight loss, and obvious abnormalities on a cranial CT or MRI such as stroke and obvious white matter changes. Participants who were prescribed psychiatric drugs were also excluded.
The objectives of the research were explained to participants and their families and written informed consent was obtained. The research was approved by the Ethics Committee of the China-Japan Friendship Hospital.
Cognitive assessments
The cognitive assessments were administered according to a standard procedure by technicians and scored by a neuropsychologist. All of them knew nothing on the diagnosis of the participants. The time required for test administration was approximately 90 min.
The test battery included global cognitive screening, attention/processing speed, executive function, memory aptitude, language facilities, and visuospatial abilities. The MMSE and the MoCA (Beijing version) were used for global cognitive screening [6, 7]. The scores relative to the memory tasks in the MMSE include immediate recall (maximum = 3), delayed recall (maximum = 3), and total recall (maximum = 6). The scores regarding memory tasks in the MoCA were categorized as delayed free recall (maximum = 5), category prompted recall (maximum = 5), and multiple choice recognition (maximum = 5).
Other cognitive tests assessed each cognitive domain to diagnose MCI. The Digit Span Forward of the Wechsler Adult Intelligence Test-Revised Chinese (WAIS-RC) version [12], the Trail Making Test A [9], the Stroop Color Word Test (modified version) Part A [9], and the Digit Symbol Subtest of WAIS-RC [12] were used to assess processing speed/attention. Executive function was assessed using the Chinese Version of Trail Making Test B [13] and the Stroop Color Word Test (modified version) Part C [9]. The RAVLT included the sum of trials 1 to 5 and delayed recall to detect verbal memory [9]. Delayed recall (30 min) of the ROCF [10] was also used to measure visual memory. The Semantic Category Verbal Fluency Test (animal) [9] and the Boston Naming Test as modified by Cheung et al. [14] assessed language ability. Visuospatial skills were verified by the ROCF [10], the Block Design of WAIS-RC [12], and the Clock Drawing Test (CDT) [15], including the command and copy conditions, which were scored by the Rouleau system. The raw scores were achieved in all cognitive tests.
Statistical analysis
Statistical analyses were performed using SPSS, version 17.0 (SPSS Inc., USA). Data were expressed as the mean ± SD unless otherwise specified. A one-way ANOVA was applied for quantitative variables among the control, the amMCI, the asMCI, and the naMCI groups, including the demographic data, the results of the MMSE or the MoCA, and the scores from memory evaluations in either test. Post hoc analyses were Bonferroni corrected for multiple comparisons. The Chi-square test was used to compare the differences between the qualitative variables among the four groups, such as the sex ratio. Receiver operating characteristic (ROC) analysis was calculated to compare the diagnostic performance of the control group with the different MCI groups. The sensitivity and specificity of every memory task or the total scores from the MMSE or the MoCA were also assessed. The Pearson correlation coefficient (r) was used to evaluate the correlations between the MMSE, the MoCA, and the memory tasks from both and the scores from the memory tests. All statistical tests were two-tailed, and p < 0.05 was used to determine statistical significance.
Results
The demographic and clinical data of the patients with amMCI, asMCI, and naMCI and the healthy controls are summarized in Table 1. The healthy controls were younger than the patients with amMCI (p = 0.012). The educational years were similar among the four groups. There were more males in the asMCI group than in the other three groups (p = 0.020).
Patients with amMCI performed worse than patients with asMCI, naMCI, and the healthy controls on the MMSE, and total and delayed recall in the MMSE (p < 0.001), but there were no differences among the last three groups. Patients with amMCI (20.90 ± 3.29) had lower scores on the MoCA than both the asMCI (24.76 ± 2.17) and naMCI patients (23.76 ± 3.40), as well as the healthy controls (25.79 ± 2.25) (p < 0.001), while the scores from naMCI patients were lower than those of the healthy controls (p = 0.016). amMCI patients performed worse than naMCI patients and healthy controls on delayed free recall (p < 0.001), while the asMCI group had lower scores than the healthy controls (p = 0.005). The scores on category prompted recall of amMCI patients were lower than those of asMCI patients (p = 0.006), naMCI patients (p < 0.001), and healthy controls (p < 0.001); the scores from asMCI patients were lower than those of the healthy controls (p = 0.015). Regarding multiple choices recognition, patients with amMCI performed worse in comparison to patients with asMCI (p = 0.009), patients with naMCI (p = 0.017), and healthy controls (p < 0.001). Patients with naMCI performed worse than the healthy controls in this task (p = 0.026).
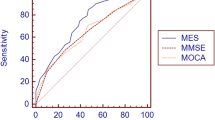
The area under the curve (AUC) values for the total scores on the MMSE and the MoCA when comparing the amMCI group and the control group were 0.819 (CI = 0.734–0.904) and 0.884 (CI = 0.814–0.955) (p < 0.001), respectively. The AUC values for the scores on delayed free recall and category prompted recall on the MoCA were 0.816 (CI = 0.724–0.909) and 0.845 (CI = 0.761–0.925) (p < 0.001), respectively. In the comparison of the asMCI and control groups, the AUC values for the scores on delayed free recall and category prompted recall on the MoCA were 0.717 (CI = 0.601–0.833) (p = 0.002) and 0.691 (CI = 0.569–0.814) (p = 0.005), respectively, whereas the AUC values for the MMSE decreased to 0.645 (p = 0.035). The MoCA had an inferior diagnostic role (p > 0.05). In the comparison of the naMCI and control group, only total scores from the MoCA and multiple choices recognition had a diagnostic role, and the AUC values were 0.687 (CI = 0.554–0.819) (p = 0.007) and 0.680 (CI = 0.549–0.811) (p = 0.009), respectively. The sum of trials 1 to 5, delayed recall of the RAVLT, and delayed recall of the ROCF had better diagnostic roles when comparing the amMCI or asMCI group and the control group. The AUC values of those decreased when comparing the naMCI group and the control group (Table 2).
If ≤ 27, ≤ 24, ≤ 2, and ≤ 3 were selected as cutoff values for the MMSE, the MoCA, delayed free recall, and category prompted recall on the MoCA when comparing amMCI patients with healthy controls, the sensitivity was 74.0, 88.0, 83.3, and 85.4%, respectively, and the specificity was 77.0, 66.7, 66.0, and 66.0%. If ≤ 28, ≤ 2, and ≤ 3 were selected as cutoff values for the MMSE, delayed free recall, and category prompted recall on the MoCA, the sensitivity was 44.8, 55.2, and 51.7%, respectively, and the specificity was still 77.0, 66.0, and 66.0%, respectively, when comparing asMCI patients with healthy controls. When ≤ 25 and ≤ 4 were selected as cutoff values for the MoCA and multiple choices recognition on the MoCA, the sensitivity was 65.5 and 44.8%, respectively, and the specificity was 56.3 and 89.6%, respectively, in the comparison of naMCI patients with healthy controls.
After controlling for age and education, the scores of the MoCA showed a positive relationship with the sum of trials 1 to 5 of the RAVLT (r = 0.407, p = 0.007) and delayed recall of the ROCF (r = 0.407, p = 0.007). Delayed free recall, category prompted recall, and multiple choices recognition had positive correlations with the sum of trials 1 to 5 (r = 0.580, 0.497, 0.404; p < 0.001, 0.001, 0.007, respectively), delayed recall of the RAVLT (r = 0.496, 0.500, 0.374; p = 0.001, 0.001, 0.013, respectively), and delayed recall of the ROCF (r = 0.512, 0.612, 0.508; p < 0.001, p < 0.001, p = 0.001, respectively) in the amMCI group. In the asMCI group, positive correlations were found between delayed recall of the RAVLT and the scores from the MoCA, delayed free recall, category prompted recall, and multiple choices recognition (r = 0.539, 0.495, 0.449, 0.478; p = 0.004, 0.009, 0.019, 0.012, respectively). In patients with naMCI, delayed recall of the RAVLT was positively correlated with the scores from the MoCA (r = 0.464, p = 0.015) and multiple choices recognition (r = 0.551, p = 0.003).
Discussion
The present study investigated the role of the MoCA (Beijing version) and memory tasks on detecting different MCI subtypes, including aMCI and naMCI, in our memory clinics. Our findings suggest that the MoCA is a useful screening tool for amMCI patients because the AUC value was 0.884. The test was still effective for patients with naMCI, who were included despite an AUC value of 0.687. The memory tasks, especially delayed free recall on the MoCA, were better methods for amMCI and asMCI patients; however, the AUC values decreased from 0.816 to 0.717. The scores of the RAVLT were clearly correlated with the total scores and memory tasks of the MoCA in different MCI subgroups. Although the correlation coefficients were not high, they still reflected the relationship between the MoCA and episodic memory.
The MoCA is growing in popularity as a brief cognitive screening measure that is freely available with multiple editions in various languages. The original MoCA reported a sensitivity of 100% and specificity of 87% in detecting mild Alzheimer’s disease (AD) using a cutoff score of 26 [6]. It reported a sensitivity of 90% in detecting MCI. The cutoff scores varied from 13/14 to 25/26 in the following studies [16,17,18,19], which is mainly attributed to the effects of age, education, and culture. The difference between amMCI and asMCI could be another important factor for the diagnostic role of the MoCA, although most studies have focused on patients with aMCI. The total scores of the MoCA could effectively discriminate amMCI patients from healthy controls when 24/25 was selected as the cutoff score in our study. The MoCA was ineffective in differentiating between asMCI patients and healthy controls.
There are five Chinese versions of the MoCA, but the Beijing version is the most widely used version in mainland China [16]. A study investigated the use of the MoCA (Beijing version) in 8411 community residents of five regions on mainland China and found high sensitivity (83.8%) and specificity (82.5%) in the MoCA for the detection of MCI when the AUC value was 0.899 [16]. Another community-based study of the MoCA reported modest sensitivity (68.7%) and specificity (63.9%) (AUC, 0.71) in Beijing when the optimal cutoff score was 22 [20]. Among older people in rural areas, the MoCA showed modest accuracy (AUC, 0.72) in detecting aMCI, especially in people with limited educational backgrounds [21]. Another hospital-based study of the MoCA found that the AUC value was 0.930 with 92% sensitivity and 85% specificity for screening aMCI [22]. The former two studies defined MCI according to the CDR, and the latter studies defined aMCI on the AVLT or the memory portion of the Alzheimer’s Disease Assessment Scale-cognitive (ADAS-cog) subscale, potentially resulting in different experimental outcomes. According to the Mayo Clinic Study of Aging, there are different incidence rates for cognitive (neuropsychological tests) and functional (CDR = 0.5) definitions of MCI, which were 95 and 55 per 1000 person-years, respectively [23]. The ratio between amMCI and asMCI also showed variable results except for the diagnostic criteria. Furthermore, the prevalence of asMCI (6.4 and 4.48%) was higher than that of amMCI (3.7 and 2.09%) in Beijing and Tianjin community-based studies [24, 25].
For the MMSE, some studies reported that the MoCA is superior to the MMSE in differentiating MCI patients from healthy controls (the AUC values were 0.899:0.842, 0.928:0.741) [16, 22], whereas other studies found that the diagnostic accuracy was similar between the MoCA and the MMSE (the AUC values were 0.71:0.70, 0.72:0.74) [20, 21]. The MoCA (0.884, 0.717) was superior to the MMSE (0.819, 0.645) in detecting amMCI or asMCI with better AUC value in the present study, although no significant difference was achieved, which may contribute to smaller samples. The MoCA was a good discriminator for naMCI, whereas the MMSE was not useful.
naMCI is another common MCI subtype with a prevalence of 5.6 or 7.0% according to the Beijing Ageing Brain Rejuvenation Initiative and the Shanghai Aging Study [24, 26]. The present study demonstrated that the MoCA had a diagnostic role in detecting naMCI because many non-memory tasks were included. The accuracy was lower than that of differentiating amMCI patients from controls but better than that of memory tests such as the RAVLT or the ROCF, although naMCI patients also experienced impairment of episodic memory [27].
The subtasks of the MoCA have important roles for cognitive screening, besides the total scores from the MoCA. A Brazilian study showed that word repetition, inverse digits, serial 7, phrases, verbal fluency, abstraction, and word recall could appropriately discriminate between MCI patients and controls [28]. Another study reported that serial subtraction, delayed recall, and orientation tasks were the best individual items for distinguishing between cognitively impaired subjects and control subjects [29]. These short versions of the MoCA had similar diagnostic accuracy as the standard version. Delayed recall was shown to be the most difficult item and the best index to differentiate MCI patients from the healthy controls in many studies [28,29,30]. Even the MoCA-Memory Index Score at the time of diagnosis could predict the risk of conversion to AD in a short follow-up period [31]. Delayed free recall or category prompted recall in the MoCA was a good discriminator for amMCI patients, although the accuracy was lower compared to the accuracy from using the total scores of the MoCA. Furthermore, these could differentiate asMCI patients from the healthy controls with modest accuracy. Multiple choices recognition had some roles on detecting naMCI patients, but with low sensitivity.
The present study has some limitations. First, the participants were selected from our memory clinics and not from the community, which may lead to bias. Second, the findings must be interpreted with caution because of the small size of our sample, especially for asMCI and naMCI. naMCI was not divided into nasMCI and namMCI for the same reason. Third, the selected healthy controls had cognitive preservation with a CDR of 0, which may not necessarily be representative of health.
In conclusion, the total score of the MoCA was a better discriminator for amMCI and had a modest accuracy in differentiating naMCI patients from healthy controls that was better than the MMSE. Delayed free recall or category prompted recall was effective in detecting amMCI and asMCI. All of these contributed to the relationship between the MoCA and episodic memory. Thus, the MoCA and its memory tasks are good tools for detecting MCI and are suitable for patients in outpatient clinics. Further studies should be performed in a larger community-based population to determine its usefulness.
References
Jia J, Wang F, Wei C, Zhou A, Jia X, Li F, Tang M, Chue L, Zhou Y, Zhou C, Cui Y, Wang Q, Wang W, Yin P, Hu N, Zuo X, Song H, Qin W, Wu L, Li D, Jia L, Song J, Han Y, Xing Y, Yang P, Li Y, Qiao Y, Tang Y, Lv J, Dong X (2014) The prevalence of dementia in urban and rural areas of China. Alzheimers Dement 10:1–9
Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B (2001) Current concepts in mild cognitive impairment. Arch Neurol 58:1985–1992
Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CR Jr (2009) Mild cognitive impairment: ten years later. Arch Neurol 66:1447–1455
Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L (2014) Mild cognitive impairment: a concept in evolution. J Intern Med 275:214–228
Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel-Heller SG (2006) Mild cognitive impairment: long-term course of four clinical subtypes. Neurology 67:2176–2185.4
Nasreddine Z, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699
Zhang M, Katzman R, Salmon D, Jin H, Cai GJ, Wang ZY, Qu GY, Grant I, Yu E, Levy P (1990) The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Ann Neurol 27:428–437
Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 25:183–194
Strauss E, Sherman EM, Spreen O (2006) A compendium of neuropsychological tests: administration, norms, and commentary, 3rd edn. Oxford University Press, New York
Osterrieth PA (1944) Le test de copie d’une figure complexe. Arch Psychol 30:206–356
Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43:2412–2414
Dai XY, Ryan JJ, Paolo AM, Harrington RG (1990) Factor analysis of the mainland Chinese version of the Wechsler Adult Intelligence Scale (WAIS-RC) in a brain-damaged sample. Int J Neurosci 55:107–111
Lu L, Bigler ED (2000) Performance on original and a Chinese version of trail making test part B: a normative bilingual sample. Appl Neuropsychol 7:243–246
Cheung RW, Cheung MC, Chan AS (2004) Confrontation naming in Chinese patients with left, right or bilateral brain damage. J Int Neuropsychol Soc 10:46–53
Rouleau I, Salmon DP, Butters N, Kennedy C, McGuire K (1992) Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain Cogn 18:70–87
Lu J, Li D, Li F, Zhou A, Wang F, Zuo X, Jia XF, Song H, Jia J (2011) Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol 24:184–190
Rossetti HC, Lacritz LH, Cullum CM, Weiner MF (2011) Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology 77:1272–1275
Ng TP, Feng L, Lim WS, Chong MS, Lee TS, Yap KB, Tsoi T, Liew TM, Gao Q, Collinson S, Kandiah N, Yap P (2015) Montreal Cognitive Assessment for screening mild cognitive impairment: variations in test performance and scores by education in Singapore. Dement Geriatr Cogn Disord 39:176–185
Kaya Y, Aki OE, Can UA, Derle E, Kibaroğlu S, Barak A (2014) Validation of Montreal Cognitive Assessment and discriminant power of Montreal cognitive assessment subtests in patients with mild cognitive impairment and Alzheimer dementia in Turkish population. J Geriatr Psychiatry Neurol 27:103–109
Yu J, Li J, Huang X (2012) The Beijing version of the Montreal cognitive assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry 12:156
Zhou S, Zhu J, Zhang N, Wang B, Li T, Lv X, Ng TP, Yu X, Wang H (2014) The influence of education on Chinese version of Montreal Cognitive Assessment in detecting amnesic mild cognitive impairment among older people in a Beijing rural community. ScientificWorldJournal 2014:689456
Hu JB, Zhou WH, Hu SH, Huang ML, Wei N, Qi HL, Huang JW, Xu Y (2013) Cross-cultural difference and validation of the Chinese version of Montreal Cognitive Assessment in older adults residing in Eastern China: preliminary findings. Arch Gerontol Geriatr 56:38–43
Ganguli M, Fu B, Snitz BE, Hughes TF, Chang CC (2013) Mild cognitive impairment: incidence and vascular risk factors in a population-based cohort. Neurology 80:2112–2120
Li X, Ma C, Zhang J, Liang Y, Chen Y, Chen K, Wang J, Zhang Z, Wang Y, Beijing Ageing Brain Rejuvenation Initiative (2013) Prevalence of and potential risk factors for mild cognitive impairment in community-dwelling residents of Beijing. J Am Geriatr Soc 61:2111–2119
Ma F, Wu T, Zhao J, Ji L, Song A, Zhang M, Huang G (2016) Prevalence of mild cognitive impairment and its subtypes among Chinese older adults: role of vascular risk factors. Dement Geriatr Cogn Disord 41:261–272
Ding D, Zhao Q, Guo Q, Meng H, Wang B, Luo J, Mortimer JA, Borenstein AR, Hong Z (2015) Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the Shanghai Aging Study. Alzheimers Dement 11:300–309
Wang B, Guo Q, Zhao Q, Hong Z (2012) Memory deficits for non-amnestic mild cognitive impairment. J Neuropsychol 6:232–241
Cecato JF, Martinelli JE, Izbicki R, Yassuda MS, Aprahamian I (2016) A subtest analysis of the Montreal Cognitive Assessment (MoCA): which subtests can best discriminate between healthy controls, mild cognitive impairment and Alzheimer’s disease? Int Psychogeriatr 28:825–832
Horton DK, Hynan LS, Lacritz LH, Rossetti HC, Weiner MF, Cullum CM (2015) An abbreviated Montreal Cognitive Assessment (MoCA) for dementia screening. Clin Neuropsychol 29:413–425
Freitas S, Prieto G, Simões MR, Santana I (2015) Scaling cognitive domains of the Montreal Cognitive Assessment: an analysis using the partial credit model. Arch Clin Neuropsychol 30:435–447
Julayanont P, Brousseau M, Chertkow H, Phillips N, Nasreddine ZS (2014) Montreal Cognitive Assessment Memory Index Score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer’s disease. J Am Geriatr Soc 62:679–684
Acknowledgements
The authors thank all participating investigators.
Funding
The study was supported by the China-Japan Friendship Hospital (2014-4-QN-32).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The objectives of the research were explained to participants and their families and written informed consent was obtained. The research was approved by the Ethics Committee of the China-Japan Friendship Hospital.
Rights and permissions
About this article
Cite this article
Li, X., Jia, S., Zhou, Z. et al. The role of the Montreal Cognitive Assessment (MoCA) and its memory tasks for detecting mild cognitive impairment. Neurol Sci 39, 1029–1034 (2018). https://doi.org/10.1007/s10072-018-3319-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3319-0