Abstract
Despite the availability of nucleic acid amplification tests (NAATs), most of aseptic acute meningitides, encephalitides, and meningoencephalitides (AAMEMs) in adults remain of unknown etiology so far. To shed light on such topic, we aimed to evaluate potential predictors for AAMEMs of unknown origin. We collected retrospectively data from all consecutive cases of AAMEMs in adults discharged from an Italian referral hospital, from January 2004 to December 2016. Laboratory analysis included common immunometric methods and NAATs. Potential predictors for unknown etiology (age, seasonality, serum C-reactive protein value, antibiotic use before lumbar puncture, immunodeficiency conditions, clinical symptoms and signs) were evaluated by a logistic regression analysis model. A p value ≤ 0.05 was considered to indicate statistical significance. The study included 92 patients (median age 39 years; 54.3% males) affected by meningitis (n = 57), encephalitis (n = 25), and meningoencephalitis (n = 10). The identified agents that cause AAMEMs were herpesviruses (20.7%), enteroviruses (5.4%), tick-borne encephalitis virus (3.3%), influenza virus A (2.2%), West Nile virus (1.1%), and parvovirus B19 (1.1%), while 66.3% of cases were of unknown etiology. No predictor was found to be significantly associated with AAMEMs of unknown etiology. We suggest that potential infectious agents causing undiagnosed cases should be investigated among non-bacterial, non-opportunistic, and non-seasonal organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute aseptic meningitis, encephalitis, and meningoencephalitis (AAMEMs) are inflammations of the central nervous system (CNS) generally distinguished from one another by the presence of meningeal and/or cerebral involvement. Despite being separate nosological categories, AAMEMs may have overlapping symptoms, signs, cerebrospinal fluid (CSF) findings, and etiologies [1,2,3]. AAMEMs are uncommon diseases, but they are clinically challenging and potentially associated with cognitive or physical long-term sequelae. In the past 10 years, causes of non-infectious encephalitis are increasingly reported [4, 5] such as autoimmune conditions as anti-NMDA receptor encephalitis [6, 7]. Despite being a healthcare issue of global concern, the epidemiology of AAMEMs is poorly studied, and a lack of data regarding their frequency, etiology, and outcome can be observed. For instance, more than 100 etiological agents can cause encephalitis but clinical studies in adults are often limited to single etiological agents or local geographical reports [8]. In fact, etiological diagnosis of AAMEM is quite challenging so far. At present, since most etiological agents causing AAMEMs are considered to be viruses, nucleic acid amplification tests (NAATs) are worldwide recognized as the gold standard for the etiological diagnosis of AAMEMs [9].
However, despite their undeniable usefulness, the detection of a pathogen nucleic acid alone in the CSF is not by itself a straightforward proof of that agent causality [10]. Even integrating molecular findings with other laboratory data, the recognition of an etiological diagnosis in AAMEMs could be intellectually demanding and, in different settings worldwide, up to 85% of cases of encephalitis are of unknown etiology [8]. The understanding of AAMEM etiology is critical both for the clinical management of some of these diseases and public health interventions. Moreover, failure to obtain etiological diagnosis results in unnecessary use of antimicrobial agents with increased antibiotic resistance, a potential onset of side effects, and additional costs.
Consequently, we aimed to study the incidence of AAMEMs, the frequency of etiological agents causing non-bacterial AAMEMs in adults referring to our institution, and the presence of potential predictors for unknown etiological diagnosis.
Patients and methods
We collected retrospectively clinical and laboratory data from all consecutive adult patients discharged from January 2004 to December 2016 from the university hospital of Trieste (Italy) with a diagnosis of aseptic acute “meningitis,” “encephalitis,” or “meningoencephalitis” (ICD-9-CM diagnosis codes 047.0, 047.1, 047.8, 047.9, 0.49.1, 052.0, 053.0, 053.3, 054.3, 054.72, 062.9, 063.2, 064, 066.41, 072.1, 072.2, 139, 322.0, 322.9, 323.01, 323.71, 323.81, 323.9), retrospectively evaluating every clinical diagnosis. “Aseptic” was defined by negative CSF Gram staining and sterile CSF standard culture for common bacteria and fungi. We defined “acute” every clinical condition of abrupt onset (≤ 72 h). We defined “meningitis” a clinical condition characterized by clinical signs of meningeal irritation associated with a “pathological” CSF analysis (defined by CSF proteins ≥ 50 mg/dL and/or CSF leukocytes ≥ 5 cells/μL). “Encephalitis” was defined as clinical, radiological, or electroencephalographic evidence of neurological dysfunction associated with “pathological” CSF analysis. “Meningoencephalitis” was defined as a subset of encephalitis characterized by an overlap of findings of both encephalitis and aseptic meningitis.
Laboratory investigations included common serologic methods (enzyme immunoassay [EIA], immunofluorescence assay [IFA], chemiluminescence immunoassay [CLIA]) and NAATs in the CSF and other biological samples (blood and/or urine). In detail, cytomegalovirus (CMV), herpes simplex virus (HSV), Epstein-Barr virus (EBV), varicella zoster virus (VZV), West Nile virus (WNV), and enteroviruses were detected by real-time PCR using commercial kits (Elitech, formerly Nanogen, Trezzano sul Naviglio, Italy). Identification of tick-borne encephalitis virus (TBEV) was performed by an in-house real-time RT-PCR [11]. In order to verify the sensitivity of assays and reproducibility of results, we have participated in External Quality Assurance programs, namely NEQAS for CMV, HSV, EBV, VZV, and enteroviruses since 2005, and QCMD for WNV. No EQA test was available for TBEV and sensibility has been evaluated with a plasmid containing the genome of TBEV and resulted in 100 copies/mL. Being a retrospective study, the choice of diagnostic tests in every individual case of AAMEM was at the clinical discretion of the attending physician. Real-time PCR on CSF specific for the aforementioned herpesviruses were tested in all cases (data not shown). On the other hand, the collection of other diagnostic tests specific for neurotropic agents resulted to be guided by the epidemiological and clinical history of the individual patient.
The University Hospital of Trieste is a 840-bed tertiary referral hospital for the northeastern Italian provinces of Trieste and Gorizia (320,706 adult residents up to the 2011 ISTAT census) [12]. The Italian provinces of Trieste and Gorizia are highly urbanized and our hospital is the referral institution for every suspected CNS infection.
Every single etiological diagnosis was re-evaluated according to the 2010 UK Health Protection Agency Aetiology of Encephalitis Study Group etiological case definitions (UK HPA AoESG) [10], retrospectively classifying every diagnosis as “confirmed,” “probable,” or “possible.” We excluded cases affected by bacterial AAMEM with sterile CSF culture (i.e., borreliosis, syphilis), tuberculosis, subacute/chronic AAMEM (i.e., subacute sclerosing panencephalitis, HIV-associated neurocognitive disorders), and non-infectious AAMEM (in a broad sense: autoimmune or metabolic or drug toxicity-associated or sepsis-associated encephalopathies). In addition, we excluded cases with incomplete available data defined as lack of baseline neurological examination and/or CSF NAATs and/or immunometric assays. Following exclusion of apparent infectious etiologies of AAMEM, diagnostic tests for autoimmune etiologies (e.g., CSF or serum antibodies against N-methyl-d-aspartate receptor) were employed.
In order to identify potential predictors for unknown etiological diagnosis, we used a univariate logistic regression analysis model including odds ratio with 95% confidence interval using the software SPSS Statistics® V21.0 (IBM, USA). We selected as predictors the following dichotomic variables in order to explore “hidden” reservoirs or host characteristics: (1) patient age at the onset of the disease (more or less than 40 years—in order to assess potential aging correlations with unidentified etiologies); (2) seasonality at AAMEM onset (every single season versus every other seasons—in order to identify a potential seasonality related to an unidentified etiologic agents such as arboviruses or respiratory viruses); (3) baseline serum C-reactive protein (CRP; more or less than 50 mg/L—as potential common biomarker consistent with a potential bacterial etiology); (4) any antibiotic therapy within 72 h before the baseline lumbar puncture (to exclude the presence of culture-negative bacterial infections in our sample); (5) immunodeficiency conditions (i.e., oncologic, hematologic, autoimmune disorders, diabetes mellitus, a concurrent immunosuppressive therapy, or HIV infection—in order to assess the presence of unidentified opportunistic pathogens); (6) common signs and symptoms of CNS infection (headache, fever, confusion, nausea or vomiting, and stiff neck) in order to explore the usefulness of clinical markers to estimate a priori the potential for an unknown etiological diagnosis, leading to a more extensive baseline diagnostic workout. A p value ≤ 0.05 was considered to indicate statistical significance.
At admission to our hospital, every patient or their next of kin included in our study signed an informed consent form to allow the collection of clinical data for scientific purposes. Since demographic data remained anonymous, no approval of the Ethics Committee was requested.
Results
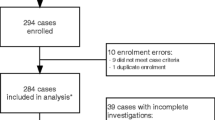
During the study period, 181 adult patients had a diagnosis of aseptic acute “meningitis,” “encephalitis,” or “meningoencephalitis,” fulfilling the above definitions. The total annual incidence rate of AAMEMs was 5.6 per 100,000 adults. We excluded 76 cases for incomplete data, 2 cases of acute disseminated encephalomyelitis, 2 cases of Miller Fisher syndrome, 1 case of neurolupus, 3 cases of neurolisteriosis, 2 cases of neuroborreliosis, 1 case of meningismus in a patient with a staphylococcal sepsis, 1 case of neurosyphilis, and 1 case of neurobartonellosis. Therefore, we included the remaining 92 patients in the following analysis. The annual distribution of AAMEMs cases was substantially stable, except for numerical increases in 2009 and 2013 without apparent epidemic events (Fig. 1).
Characteristics of the 92 patients are summarized in Table 1. The median age was 39 years (range 18–87) with a male-to-female ratio of 1:0.8. Most of the AAMEM cases (67.4%) occurred during summer and autumn. In 13 cases, the first lumbar puncture was preceded in the previous 72 h by antibiotic therapy. Twelve patients had underlying immunodeficiency conditions. No patient had HIV infection. The most common diagnosis of AAMEM was meningitis (57 cases), followed by encephalitis and meningoencephalitis (25 and 10 cases, respectively).
The most commonly reported symptoms at hospital admission were headache, fever, and nausea or vomiting (72.8, 67.4, and 35.9%, respectively) while stiff neck, mental confusion, and positive Lasègue sign were the most commonly recorded signs (40.2, 23.9, and 14.1%, respectively), as summarized in Table 2. Overall, serum markers of inflammation (CRP, white blood cells) were mildly elevated. The analysis of CSF showed mainly clear CSF samples with a mean CSF-to-serum glucose ratio of 0.57 (SD ± 0.1; median 0.6; interquartile range [IQR] 0.2), slightly increased CSF proteins (mean 84.8 mg/dL; SD ± 39.4; median 77.1 mg/dL; IQR 58.25), and slight pleocytosis (mean 144.5 cells/μL; SD ± 169; median 86 cells/μL; IQR 157.5).
As summarized in Table 3, the AAMEMs were found to be of unknown origin in 66.3% of cases. The most commonly reported agents were herpes simplex viruses (5 cases of HSV-1, 5 cases of HSV-2, and 2 cases of unidentified HSV), enteroviruses and VZV followed by TBEV, influenza A virus, EBV, and WNV. A probable parvovirus B19 (PVB19) meningitis was reported in a 29-year-old immunocompetent female, whose symptoms at the onset were fever and headache. No skin rash was seen. The analysis of her CSF sample showed CSF-to-serum glucose ratio of 0.6, proteins 83.5 mg/dL, and 100 leukocytes/μL (92% lymphomonocytes). No other pathogen was found, and only the presence of serum IgM and IgG specific to PVB19 was recorded. The patient was discharged with full clinical recovery after 20 days. Again, agents were defined as “confirmed,” “probable,” and “possible” accordingly to the 2010 UK HPA AoESG etiological case definition list [10]. For instance, the two cases caused by influenza A were defined as “possible” because the viral RNAs were amplified from nasopharyngeal swabs but influenza virus RNA was not tested on CSF.
No potential predictors resulted to be significantly associated with AAMEM with unknown etiological diagnosis by the logistic regression analysis (Table 4).
Two patients (2.2%) of the 92 cases included in the analysis died during hospitalization: 2 females 80- and 64-year-old, the latter having various comorbidities. Both the deceased patients did not have an identified etiological agent responsible for their CNS infection.
Discussion
In this 12-year-period study, the annual incidence rate of AAMEMs is similar to that reported in other clinical studies [6]. Regarding the 92 cases included in our analysis, most of them were of unknown etiology and the annual proportion of such cases remained approximately stable over time. We expected that the proportion of unknown etiological diagnosis should have decreased in more recent years, with the advancement of laboratory techniques. This has not happened since molecular diagnostic methods in the diagnosis of AAMEM have been introduced early in the laboratory of our hospital and only minor modifications in commercial kit supply occurred from 2004 to 2016.
We identified in our series herpesviruses as the main “confirmed” pathogens causing AAMEMs, followed by enteroviruses and VZV. Enterovirus encephalitis is considered notably rare [13] and, in fact, we did not find encephalitis or meningoencephalitis due by such viruses. In our series, the frequency of AAMEMs with unknown etiological diagnosis and those due by documented etiological agents is similar to what was reported by other studies regarding aseptic meningitis and encephalitis [9, 13,14,15,16]. On the other hand, Koskiniemi et al. showed the leading role of VZV in both encephalitis and meningitis (29% of identified etiology). However, the authors ascribed the high frequency of VZV findings to the improvement in diagnostic methods [17]. In our study, VZV accounted for only 16.1% of AAMEMs with identified etiology including 2 cases of meningitis, 1 case of encephalitis, and 2 cases of meningoencephalitis.
Numerous areas of northeastern Italy are endemic for TBEV and WNV infections. In detail, the annual incidence rate of TBEV encephalitis in our area was 0.1 for 100,000 population from 2000 to 2013 [18]. Moreover, most of our patients with AAMEMs belonged to geographical areas sharing borders with Slovenia, which is considered an endemic country for TBEV infection, with an annual incidence rate of 13.1 for 100,000 persons in 2009 [19]. On the other hand, our area had an annual incidence rate of 0.7 WNV neuroinvasive diseases for 100,000 population in 2012 [20]. In fact, accordingly to the 2010 UK HPA AoESG etiological case definitions, in our study, TBEV and WNV were recorded as “probable” etiological agents in 3 cases and 1 case of AAMEMs, respectively. Finally, we found a probable case of PVB19 meningitis defined by the presence of serum IgM and IgG antibodies to PVB19 after excluding other etiologies. Since this pathogen is unusual as a neurotropic agent, parvovirus B19 is not reported in the 2010 UK HPA AoESG etiological case definition list. Indeed, only 129 cases of neurological manifestations due to PVB19 infection are reported from 1970 to 2012. The criteria for PVB19 diagnosis were based on the detection of PVB19 DNA or IgM antibodies to PVB19 in CSF or serum, as reported in our case [21].
Our study showed the high proportion of AAMEMs with unknown etiological diagnosis, despite the availability of NAATs and immunometric assays specific to multiple pathogens.
Therefore, we assessed if such unexplained etiology was somehow associated with potential predictors, leading to discover “missing” reservoirs or host characteristics. In order to exclude potential confounding variables, we chose a priori to exclude confirmed bacterial infections. In addition, we speculated that a previous antibiotic therapy might be a potential risk factor for unknown etiological diagnosis in order to exclude some “hidden” bacterial pathogens in our analysis. Our analysis did not show significant difference between the “unknown” and “known” etiological diagnosis categories, suggesting that the high proportion of unknown etiological diagnosis should not be attributed to an unidentified bacterial pathogen. To exclude the presence of opportunistic pathogens causing AAMEM, we selected the presence of immunodeficiency conditions as a potential risk factor for unknown etiological diagnosis. Again, we did not find out a significant difference in the two categories, suggesting that opportunistic pathogens might not be involved in our series. Then, we focused the analysis on the temporal distribution of AAMEMs in relation to the season of illness onset. Once again, the contribution of seasonality as predictor for unknown etiological diagnosis resulted to be not significant, suggesting that an unidentified pathogen with seasonal distribution (i.e., enteroviruses, mosquito, or ticks-borne agents) should not be responsible for the cases with unknown etiological diagnosis.
Finally, although the collection of clinical signs and symptoms at hospital admission was rather heterogeneous, the analysis of such variables did not show significant difference between the “unknown” and “known” etiological diagnosis categories (Table 2). Consequently, this suggests that clinical signs and symptoms at hospital admission might not be useful as a priori predictors of AAMEM with unknown etiological diagnosis.
This study had several limitations. Firstly, the retrospective design of our study forced us to analyze just over half of our cases of AAMEM referring to our hospital, leading to a relatively small sample size. However, the inclusion of clinically ascertained cases and the accuracy in the microbiological diagnosis assessed by the participation of our laboratory to External Quality Assurance programs should have reduced the selection bias in our series. Secondly, the choice of algorithm for diagnosis of each case of AAMEM was at the clinical discretion of the attending physician and the collection of diagnostic tests specific for neurotropic agents resulted to be heterogeneous in our series. However, we applied strict exclusion criteria and disease definitions using a standardized consensus on every single etiological diagnosis according to the 2010 UK HPA AoESG etiological case definition list [10]. Thirdly, data regarding the outcome of AAMEMs are limited to the in-hospital lethality. Lastly, despite our hospital as a referral center for patients with suspected CNS infections belonging to a large geographic area, our data were collected in a single medical center and cannot be generalized.
Notwithstanding these limitations, we confirmed that AAMEMs were uncommon diseases and that only one third of them have been etiologically diagnosed in our series. Next to herpesviruses and enteroviruses, we found some cases due to influenza viruses, TBEV, and WNV which should be tested in epidemic season or endemic areas, respectively. In addition, we recorded a probable infection by PVB19, a rare neurotropic agent which should not be neglected in the diagnosis of AAMEMs. It is hoped that novel molecular tests such as multiplex PCRs or deep sequencing techniques will allow to obtain the identification of most of the current unknown etiologies or yet to be discovered new pathogens in the next future [22]. In-hospital mortality was low and limited to cases due to unidentified etiological agents. Since etiological diagnosis should be pursued in any case, our data suggest that potential infectious agents should be investigated among non-bacterial, non-opportunistic, and non-seasonal organisms.
References
Somand D, Meurer W (2009) Central nervous system infections. Emerg Med Clin North Am 27:89–100
Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, Hartman BJ, Kaplan SL, Scheld WM, Whitley RJ, Infectious Diseases Society of America (2008) The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 47:303–327
Irani DN (2008) Aseptic meningitis and viral myelitis. Neurol Clin 26:635–655
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Höftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Prüss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostásy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15(4):391–404
Marchioni E, Ravaglia S, Montomoli C, Tavazzi E, Minoli L, Baldanti F, Furione M, Alfonsi E, Bergamaschi R, Romani A, Piccolo L, Zardini E, Bastianello S, Pichiecchio A, Ferrante P, Delbue S, Franciotta D, Bono G, Ceroni M (2013) Postinfectious neurologic syndromes: a prospective cohort study. Neurology 80(10):882–889
Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA (2012) The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis 54:899–904
Chen X, Li JM, Liu F, Wang Q, Zhou D, Lai X (2016) Anti-N-methyl-d-aspartate receptor encephalitis: a common cause of encephalitis in the intensive care unit. Neurol Sci 37(12):1993–1998
Granerod J, Tam CC, Crowcroft NS, Davies NWS, Borchert M, Thomas SL (2010) Challenge of the unknown. A systematic review of acute encephalitis in non-outbreak situations. Neurology 75:924–932
Kupila L, Vuorinen T, Vainionpaa R, Hukkanen V, Marttila RJ, Kotilainen P (2006) Etiology of aseptic meningitis and encephalitis in an adult population. Neurology 66:75–80
Granerod J, Cunningham R, Zuckerman M et al (2010) Causality in acute encephalitis: defining aetiologies. Epidemiol Infect 138:783–800
Schwaiger M, Cassinotti P (2003) Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J Clin Virol 27:136–145
ISTAT. http://www.regione.fvg.it/rafvg/cms/RAFVG/GEN/statistica/SCHEDA2. Accessed 26 Aug 2016
Stahl JP, Mailles A, Dacheux L, Morand P (2011) Epidemiology of viral encephalitis in 2011. Med Mal Infect 41:453–464
Frantzidou F, Kamaria F, Dumaidi K, Skoura L, Antoniadis A, Papa A (2008) Aseptic meningitis and encephalitis because of herpesviruses and enteroviruses in an immunocompetent adult population. Eur J Neurol 15:995–997
Ihekwaba UK, Kudesia G, McKendrick MW (2008) Clinical features of viral meningitis in adults: significant differences in cerebrospinal fluid findings among herpes simplex virus, varicella zoster virus, and enterovirus infections. Clin Infect Dis 47:783–789
Beghi E, Nicolosi A, Kurland LT, Mulder DW, Hauser WA, Shuster L (1984) Encephalitis and aseptic meningitis, Olmsted County, Minnesota, 1950-1981: I. Epidemiology. Ann Neurol 16:283–294
Koskiniemi M, Rantalaiho T, Piiparinen H et al (2001) Infections of the central nervous system of suspected viral origin: a collaborative study from Finland. J Neuro-Oncol 7:400–408
Rezza G, Farchi F, Pezzotti P et al (2015) Tick-borne encephalitis in north-east Italy: a 14-year retrospective study, January 2000 to December 2013. Euro Surveill 20(40):30034. https://doi.org/10.2807/1560-7917.ES.2015.20.40.30034
Rizzo C, Napoli C, Venturi G et al (2016) West Nile virus transmission: results from the integrated surveillance system in Italy, 2008 to 2015. Euro Surveill 21(37):30340. https://doi.org/10.2807/1560-7917.ES.2016.21.37.30340
Donoso Mantke O, Escadafal C, Niedrig M et al (2011) Tick-borne encephalitis in Europe, 2007 to 2009. Euro Surveill 16(39):19976. https://doi.org/10.2807/ese.16.39.19976-en
Barah F, Whiteside S, Batista S, Morris J (2014) Neurological aspects of human parvovirus B19 infection: a systematic review. Rev Med Virol 24:154–168
Chan BK, Wilson T, Fischer KF, Kriesel JD (2014) Deep sequencing to identify the causes of viral encephalitis. PLoS One 9(4):e93993
Acknowledgments
We thank Mrs. Elena Samar for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Monticelli, J., Geremia, N., D’Agaro, P. et al. Aseptic central nervous system infections in adults: what predictor for unknown etiological diagnosis?. Neurol Sci 39, 863–870 (2018). https://doi.org/10.1007/s10072-018-3274-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3274-9